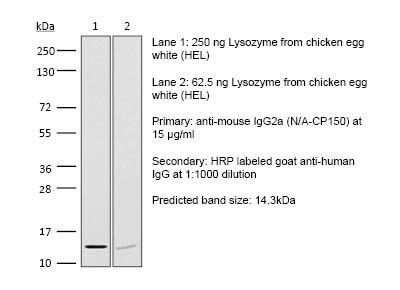
Catalog #CP150
RecombiMAb mouse IgG2a (D265A) isotype control, anti-hen egg lysozyme
Product Citations
2
Isotype
Mouse IgG2a