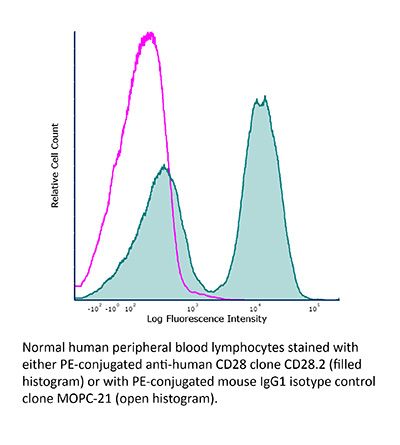
InVivoMAb mouse IgG1 isotype control, unknown specificity
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107784 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Faraco, G., et al (2018). "Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response" Nat Neurosci 21(2): 240-249.
PubMed
A diet rich in salt is linked to an increased risk of cerebrovascular diseases and dementia, but it remains unclear how dietary salt harms the brain. We report that, in mice, excess dietary salt suppresses resting cerebral blood flow and endothelial function, leading to cognitive impairment. The effect depends on expansion of TH17 cells in the small intestine, resulting in a marked increase in plasma interleukin-17 (IL-17). Circulating IL-17, in turn, promotes endothelial dysfunction and cognitive impairment by the Rho kinase-dependent inhibitory phosphorylation of endothelial nitric oxide synthase and reduced nitric oxide production in cerebral endothelial cells. The findings reveal a new gut-brain axis linking dietary habits to cognitive impairment through a gut-initiated adaptive immune response compromising brain function via circulating IL-17. Thus, the TH17 cell-IL-17 pathway is a putative target to counter the deleterious brain effects induced by dietary salt and other diseases associated with TH17 polarization.
Macal, M., et al (2018). "Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection" Immunity 48(4): 730-744 e735.
PubMed
Although characterization of T cell exhaustion has unlocked powerful immunotherapies, the mechanisms sustaining adaptations of short-lived innate cells to chronic inflammatory settings remain unknown. During murine chronic viral infection, we found that concerted events in bone marrow and spleen mediated by type I interferon (IFN-I) and Toll-like receptor 7 (TLR7) maintained a pool of functionally exhausted plasmacytoid dendritic cells (pDCs). In the bone marrow, IFN-I compromised the number and the developmental capacity of pDC progenitors, which generated dysfunctional pDCs. Concurrently, exhausted pDCs in the periphery were maintained by self-renewal via IFN-I- and TLR7-induced proliferation of CD4(-) subsets. On the other hand, pDC functional loss was mediated by TLR7, leading to compromised IFN-I production and resistance to secondary infection. These findings unveil the mechanisms sustaining a self-perpetuating pool of functionally exhausted pDCs and provide a framework for deciphering long-term exhaustion of other short-lived innate cells during chronic inflammation.
Sell, S., et al (2015). "Control of murine cytomegalovirus infection by gammadelta T cells" PLoS Pathog 11(2): e1004481.
PubMed
Infections with cytomegalovirus (CMV) can cause severe disease in immunosuppressed patients and infected newborns. Innate as well as cellular and humoral adaptive immune effector functions contribute to the control of CMV in immunocompetent individuals. None of the innate or adaptive immune functions are essential for virus control, however. Expansion of gammadelta T cells has been observed during human CMV (HCMV) infection in the fetus and in transplant patients with HCMV reactivation but the protective function of gammadelta T cells under these conditions remains unclear. Here we show for murine CMV (MCMV) infections that mice that lack CD8 and CD4 alphabeta-T cells as well as B lymphocytes can control a MCMV infection that is lethal in RAG-1(-/-) mice lacking any T- and B-cells. gammadelta T cells, isolated from infected mice can kill MCMV infected target cells in vitro and, importantly, provide long-term protection in infected RAG-1(-/-) mice after adoptive transfer. gammadelta T cells in MCMV infected hosts undergo a prominent and long-lasting phenotypic change most compatible with the view that the majority of the gammadelta T cell population persists in an effector/memory state even after resolution of the acute phase of the infection. A clonotypically focused Vgamma1 and Vgamma2 repertoire was observed at later stages of the infection in the organs where MCMV persists. These findings add gammadelta T cells as yet another protective component to the anti-CMV immune response. Our data provide clear evidence that gammadelta T cells can provide an effective control mechanism of acute CMV infections, particularly when conventional adaptive immune mechanisms are insufficient or absent, like in transplant patient or in the developing immune system in utero. The findings have implications in the stem cell transplant setting, as antigen recognition by gammadelta T cells is not MHC-restricted and dual reactivity against CMV and tumors has been described.
Manlove, L. S., et al (2015). "Adaptive Immunity to Leukemia Is Inhibited by Cross-Reactive Induced Regulatory T Cells" J Immunol .
PubMed
BCR-ABL+ acute lymphoblastic leukemia patients have transient responses to current therapies. However, the fusion of BCR to ABL generates a potential leukemia-specific Ag that could be a target for immunotherapy. We demonstrate that the immune system can limit BCR-ABL+ leukemia progression although ultimately this immune response fails. To address how BCR-ABL+ leukemia escapes immune surveillance, we developed a peptide: MHC class II tetramer that labels endogenous BCR-ABL-specific CD4+ T cells. Naive mice harbored a small population of BCR-ABL-specific T cells that proliferated modestly upon immunization. The small number of naive BCR-ABL-specific T cells was due to negative selection in the thymus, which depleted BCR-ABL-specific T cells. Consistent with this observation, we saw that BCR-ABL-specific T cells were cross-reactive with an endogenous peptide derived from ABL. Despite this cross-reactivity, the remaining population of BCR-ABL reactive T cells proliferated upon immunization with the BCR-ABL fusion peptide and adjuvant. In response to BCR-ABL+ leukemia, BCR-ABL-specific T cells proliferated and converted into regulatory T (Treg) cells, a process that was dependent on cross-reactivity with self-antigen, TGF-beta1, and MHC class II Ag presentation by leukemic cells. Treg cells were critical for leukemia progression in C57BL/6 mice, as transient Treg cell ablation led to extended survival of leukemic mice. Thus, BCR-ABL+ leukemia actively suppresses antileukemia immune responses by converting cross-reactive leukemia-specific T cells into Treg cells.
Leon, B., et al (2014). "FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability" Nat Commun 5: 3495.
PubMed
Here, we test the role of FoxP3(+) regulatory T cells (Tregs) in controlling T follicular helper (Tfh) and germinal centre (GC) B-cell responses to influenza. In contrast to the idea that Tregs suppress T-cell responses, we find that Treg depletion severely reduces the Tfh cell response to influenza virus. Furthermore, Treg depletion prevents the accumulation of influenza-specific GCs. These effects are not due to alterations in TGFbeta availability or a precursor-progeny relationship between Tregs and Tfh cells, but are instead mediated by increased availability of IL-2, which suppresses the differentiation of Tfh cells and as a consequence, compromises the GC B response. Thus, Tregs promote influenza-specific GC responses by preventing excessive IL-2 signalling, which suppresses Tfh cell differentiation.
Beug, S. T., et al (2014). "Smac mimetics and innate immune stimuli synergize to promote tumor death" Nat Biotechnol 32(2): 182-190.
PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
Perng, O. A., et al (2014). "The degree of CD4+ T cell autoreactivity determines cellular pathways underlying inflammatory arthritis" J Immunol 192(7): 3043-3056.
PubMed
Although therapies targeting distinct cellular pathways (e.g., anticytokine versus anti-B cell therapy) have been found to be an effective strategy for at least some patients with inflammatory arthritis, the mechanisms that determine which pathways promote arthritis development are poorly understood. We have used a transgenic mouse model to examine how variations in the CD4(+) T cell response to a surrogate self-peptide can affect the cellular pathways that are required for arthritis development. CD4(+) T cells that are highly reactive with the self-peptide induce inflammatory arthritis that affects male and female mice equally. Arthritis develops by a B cell-independent mechanism, although it can be suppressed by an anti-TNF treatment, which prevented the accumulation of effector CD4(+) Th17 cells in the joints of treated mice. By contrast, arthritis develops with a significant female bias in the context of a more weakly autoreactive CD4(+) T cell response, and B cells play a prominent role in disease pathogenesis. In this setting of lower CD4(+) T cell autoreactivity, B cells promote the formation of autoreactive CD4(+) effector T cells (including Th17 cells), and IL-17 is required for arthritis development. These studies show that the degree of CD4(+) T cell reactivity for a self-peptide can play a prominent role in determining whether distinct cellular pathways can be targeted to prevent the development of inflammatory arthritis.
Kerzerho, J., et al (2013). "Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity" J Allergy Clin Immunol 131(4): 1048-1057, 1057 e1041-1042.
PubMed
BACKGROUND: Asthma is defined as a chronic inflammatory disease of the airways; however, the underlying physiologic and immunologic processes are not fully understood. OBJECTIVE: The aim of this study was to determine whether TH9 cells develop in vivo in a model of chronic airway hyperreactivity (AHR) and what factors control this development. METHOD: We have developed a novel chronic allergen exposure model using the clinically relevant antigen Aspergillus fumigatus to determine the time kinetics of TH9 development in vivo. RESULTS: TH9 cells were detectable in the lungs after chronic allergen exposure. The number of TH9 cells directly correlated with the severity of AHR, and anti-IL-9 treatment decreased airway inflammation. Moreover, we have identified programmed cell death ligand (PD-L) 2 as a negative regulator of TH9 cell differentiation. Lack of PD-L2 was associated with significantly increased TGF-beta and IL-1alpha levels in the lungs, enhanced pulmonary TH9 differentiation, and higher morbidity in the sensitized mice. CONCLUSION: Our findings suggest that PD-L2 plays a pivotal role in the regulation of TH9 cell development in chronic AHR, providing novel strategies for modulating adaptive immunity during chronic allergic responses.
Myles, I. A., et al (2013). "Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus" Nat Immunol 14(8): 804-811.
PubMed
Staphylococcus aureus causes most infections of human skin and soft tissue and is a major infectious cause of mortality. Host defense mechanisms against S. aureus are incompletely understood. Interleukin 19 (IL-19), IL-20 and IL-24 signal through type I and type II IL-20 receptors and are associated with inflammatory skin diseases such as psoriasis and atopic dermatitis. We found here that those cytokines promoted cutaneous infection with S. aureus in mice by downregulating IL-1beta- and IL-17A-dependent pathways. We noted similar effects of those cytokines in human keratinocytes after exposure to S. aureus, and antibody blockade of the IL-20 receptor improved outcomes in infected mice. Our findings identify an immunosuppressive role for IL-19, IL-20 and IL-24 during infection that could be therapeutically targeted to alter susceptibility to infection.
Vokaer, B., et al (2013). "IL-17A and IL-2-expanded regulatory T cells cooperate to inhibit Th1-mediated rejection of MHC II disparate skin grafts" PLoS One 8(10): e76040.
PubMed
Several evidences suggest that regulatory T cells (Treg) promote Th17 differentiation. Based on this hypothesis, we tested the effect of IL-17A neutralization in a model of skin transplantation in which long-term graft survival depends on a strong in vivo Treg expansion induced by transient exogenous IL-2 administration. As expected, IL-2 supplementation prevented rejection of MHC class II disparate skin allografts but, surprisingly, not in IL-17A-deficient recipients. We attested that IL-17A was not required for IL-2-mediated Treg expansion, intragraft recruitment or suppressive capacities. Instead, IL-17A prevented allograft rejection by inhibiting Th1 alloreactivity independently of Tregs. Indeed, T-bet expression of naive alloreactive CD4+ T cells and the subsequent Th1 immune response was significantly enhanced in IL-17A deficient mice. Our results illustrate for the first time a protective role of IL-17A in CD4+-mediated allograft rejection process.
Lamere, M. W., et al (2011). "Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance" J Virol 85(10): 5027-5035.
PubMed
Seasonal influenza epidemics recur due to antigenic drift of envelope glycoprotein antigens and immune evasion of circulating viruses. Additionally, antigenic shift can lead to influenza pandemics. Thus, a universal vaccine that protects against multiple influenza virus strains could alleviate the continuing impact of this virus on human health. In mice, accelerated clearance of a new viral strain (cross-protection) can be elicited by prior infection (heterosubtypic immunity) or by immunization with the highly conserved internal nucleoprotein (NP). Both heterosubtypic immunity and NP-immune protection require antibody production. Here, we show that systemic immunization with NP readily accelerated clearance of a 2009 pandemic H1N1 influenza virus isolate in an antibody-dependent manner. However, human immunization with trivalent inactivated influenza virus vaccine (TIV) only rarely and modestly boosted existing levels of anti-NP IgG. Similar results were observed in mice, although the reaction could be enhanced with adjuvants, by adjusting the stoichiometry among NP and other vaccine components, and by increasing the interval between TIV prime and boost. Importantly, mouse heterosubtypic immunity that had waned over several months could be enhanced by injecting purified anti-NP IgG or by boosting with NP protein, correlating with a long-lived increase in anti-NP antibody titers. Thus, current immunization strategies poorly induce NP-immune antibody that is nonetheless capable of contributing to long-lived cross-protection. The high conservation of NP antigen and the known longevity of antibody responses suggest that the antiviral activity of anti-NP IgG may provide a critically needed component of a universal influenza vaccine.
Product Citations
-
-
Cancer Research
KRAS Inhibition Activates an Actionable CD24 Do Not Eat Me Signal in Pancreatic Cancer.
In Cancer Res on 1 December 2025 by Wei, Y., Liu, M., et al.
PubMed
KRASG12C inhibitors (G12Ci) have produced encouraging, albeit modest and transient, clinical benefit in pancreatic ductal adenocarcinoma (PDAC). Identifying and targeting resistance mechanisms to G12Ci treatment are therefore crucial. To better understand the function of KRASG12C and possible G12Ci bypass mechanisms, we developed an autochthonous KRASG12C-driven PDAC model. Compared with the classical KRASG12D PDAC model, the G12C model exhibits slower tumor growth, yet similar histopathologic and molecular features. Aligned with clinical experience, G12Ci treatment of KRASG12C tumors produced modest impact despite stimulating a "hot" tumor immune microenvironment. Immunoprofiling revealed that CD24, a "do not eat me" signal, is significantly upregulated on cancer cells upon G12Ci treatment. Blocking CD24 enhanced macrophage phagocytosis of cancer cells and significantly sensitized tumors to G12Ci treatment. Similar findings were observed in KRASG12D-driven PDAC. Together, this study reveals common and distinct oncogenic KRAS allele-specific biology and identifies a clinically actionable adaptive mechanism that may improve the efficacy of oncogenic KRAS inhibitor therapy in PDAC.
-
-
-
Immunology and Microbiology
-
Cancer Research
IL33-induced lipid droplet formation in mature low-density neutrophils drives colorectal cancer liver metastasis.
In Cell Mol Immunol on 1 December 2025 by Zhang, Y., Yu, S., et al.
PubMed
The microenvironment of distant organs affects the colonization and growth of disseminated tumor cells. It remains unclear how tumor-associated neutrophils are influenced by the microenvironment of distant organs. Here, we demonstrate that mature low-density neutrophils in colorectal cancer patients abnormally accumulate neutral lipids and induce the reactivation of dormant tumor cells, a process regulated by hepatic stellate cells. Mechanistically, activated hepatic stellate cells increased DGAT1/2-dependent lipid droplet synthesis in low-density neutrophils through the secretion of IL33, thereby maintaining the survival and immunosuppressive function of these neutrophils. The uptake of lipids from lipid-laden low-density neutrophils drives dormant tumor cell reactivation through the potentiation of β-oxidation and the stimulation of protumorigenic eicosanoid synthesis. In mouse models, targeting IL33 blocked neutrophil lipid synthesis, decreased the colonization of colorectal cancer cells in the liver, and enhanced the efficacy of immunotherapy. Overall, our study revealed that lipid accumulation in mature low-density neutrophils regulates the growth of dormant tumor cells and antitumor immunity to facilitate colorectal cancer liver metastasis. Targeting IL33 could be a promising therapeutic approach for colorectal cancer liver metastases.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
The transcription factor Blimp-1 is suppressed by SLAMF1 and drives Treg cell-mediated immune evasion in non-small cell lung cancer.
In BJC Rep on 21 October 2025 by Finotto, S., Trufa, D. I., et al.
PubMed
Immune checkpoint inhibitors targeting the interaction between PD1 and PDL1 are effective for immunotherapy in non-small cell lung cancer (NSCLC). However, only subgroups of patients respond to therapy, suggesting the existence of resistance mechanisms.
-
-
Eosinophil-derived interleukin-24 compromises epithelial integrity and aggravates airway remodeling in mouse models of allergic asthma.
In Nat Commun on 20 October 2025 by Wu, Y. R., Hsing, C. H., et al.
PubMed
Asthma is a heterogeneous disease characterized by infiltration of immune cells that interact with epithelial cells and release various factors driving chronic inflammation and airway remodeling. Although monoclonal antibody-based biologics alleviate inflammation, their efficacy in suppressing airway remodeling is limited. Interleukin-24 (IL-24) has been implicated in neutrophilic asthma, but its role in eosinophilic asthma remains unclear. Here, we show that IL-24 is mainly secreted by infiltrating eosinophils in mice with OVA- and HDM-induced asthma-like lung disease models. IL-24 knockout mice exhibit reduced inflammatory responses, alleviated pulmonary fibrosis, improved epithelial integrity, and decreased mucus hypersecretion. Mechanistically, IL-24 activates the CXCL5/CXCR1/CXCR2 axis, enhancing eosinophil recruitment to the lungs. IL-24 disrupts epithelial tight junction integrity, contributing to increased permeability. Furthermore, IL-24 targets airway epithelial cells, promoting EMT-like changes and the secretion of profibrotic mediators, which leads to bronchial wall thickening and pulmonary fibrosis. Therefore, targeting IL-24 holds promise for anti-asthmatic interventions by suppressing inflammation and pathological remodeling.
-
-
Immunology and Microbiology
EP300 compromises antitumor immunity by increasing SOCS1 expression.
In J Immunother Cancer on 15 October 2025 by Zeng, Y., Zhou, Y., et al.
PubMed
Beyond supporting cancer cell proliferation, tumor growth relies on the ability of cancer cells to evade immune surveillance. Identifying novel molecules that promote tumor immune escape may help develop more effective immunotherapeutic strategies. The histone acetyltransferase E1A-binding protein p300 (EP300) is a key epigenetic regulator that modulates gene transcription through chromatin remodeling and acetylation of histones and transcription factors. However, its role in regulating immune evasion remains incompletely understood. This study investigates the impact of EP300 on tumor immune escape and suggests its potential as an immunotherapeutic target.
-
-
-
Immunology and Microbiology
-
Cancer Research
IL-17A blockade alleviates immune checkpoint inhibitor-associated psoriasiform rash: preclinical and clinical responses.
In Cancer Biol Med on 9 October 2025 by Ruan, Y., Shi, M., et al.
PubMed
-
-
-
Cancer Research
FXR shapes an immunosuppressive microenvironment in PD-L1lo/- non-small cell lung cancer by upregulating HVEM.
In JCI Insight on 23 September 2025 by Xu, X., Shang, B., et al.
PubMed
Immune checkpoint therapy has changed cancer treatment, including non-small cell lung cancer (NSCLC). The unresponsiveness of PD-L1lo/- tumors to anti-PD-1/PD-L1 immunotherapy is attributed to alternative immune evasion mechanisms that remain elusive. We previously reported that farnesoid X receptor (FXR) was increased in PD-L1lo/- NSCLC. Herein, we found that immune checkpoint HVEM was positively correlated with FXR but inversely correlated with PD-L1 in NSCLC. HVEM was highly expressed in FXRhiPD-L1lo NSCLC. Consistently, clinically relevant FXR antagonist dose-dependently inhibited HVEM expression in NSCLC. FXR inhibited cytokine production and cytotoxicity of cocultured CD8+ T cells in vitro, and it shaped an immunosuppressive tumor microenvironment (TME) in mouse tumors in vivo through the HVEM/BTLA pathway. Clinical investigations show that the FXR/HVEM axis was associated with immunoevasive TME and inferior survival outcomes in patients with NSCLC. Mechanistically, FXR upregulated HVEM via transcriptional activation, intracellular Akt, Erk1/2 and STAT3 signals, and G1/S cycle progression in NSCLC cells. In vivo treatment experiments demonstrated that anti-BTLA immunotherapy reinvigorated antitumor immunity in TME, resulting in enhanced tumor inhibition and survival improvement in FXRhiPD-L1lo mouse Lewis lung carcinomas. In summary, our findings establish the FXR/HVEM axis as an immune evasion mechanism in PD-L1lo/- NSCLC, providing translational implications for future immunotherapy in this subgroup of patients.
-
-
-
Immunology and Microbiology
Identification and validation of intratumoral microbiome associated with sensitization to immune checkpoint inhibitors.
In Cell Rep Med on 16 September 2025 by Chen, J., Gao, Y., et al.
PubMed
As a part of the commensal microbiome, the regulatory role of the intratumoral microbiome in tumor immunity is gradually revealed. However, the relationship between the intratumoral microbiome and the efficacy of immune checkpoint inhibitors (ICIs) clinical treatment remains unclear. Here, we collect RNA sequencing (RNA-seq) data and clinical information from publicly available ICIs therapy cohorts. By developing an improved bioinformatics pipeline to identify the intratumoral microbiome and performing a comprehensive association analysis, we find that the intratumoral microbiome is associated with response to ICIs and characteristics of the tumor microenvironment (TME). In vivo experiments demonstrate that intratumoral injection of Burkholderia cepacia, Priestia megaterium, or Corynebacterium kroppenstedtii, which were selected from our analysis results, would synergize with anti-PD-1 therapy to inhibit tumor growth and enhance antitumor immunity. Our findings highlight the essential role of the intratumoral microbiome in the clinical effectiveness differences of ICIs, suggesting its potential in future ICIs combination therapy.
-
-
-
Cardiovascular biology
Splenic erythrophagocytosis is regulated by ALX/FPR2 signaling.
In Haematologica on 11 September 2025 by Asplund, H., Dreyer, H. H., et al.
PubMed
Maintaining a healthy pool of circulating red blood cells (RBCs) is essential for adequate perfusion, as even minor changes in the population can impair oxygen delivery, resulting in serious health complications including tissue ischemia and organ dysfunction. This responsibility largely falls to specialized macrophages in the spleen, known as red pulp macrophages, which efficiently take up and recycle damaged RBCs. However, questions remain regarding how these macrophages are acutely activated to accommodate increased demand. Proresolving lipid mediators stimulate macrophage phagocytosis and efferocytosis but their role in erythrophagocytosis has only recently been described. To investigate the role of lipid mediators on red pulp macrophage function, we targeted the ALX/FPR2 signaling pathway, as this receptor binds multiple lipid mediator ligands eliciting potent macrophage responses. We found that mice with Fpr2 deletion exhibited disrupted erythrocyte homeostasis resulting in an aged RBC pool, decreased markers of splenic RBC turnover, and altered splenic macrophage phenotype characterized by changes in heme metabolism. Upon activation of ondemand erythrophagocytosis, production of the ALX/FPR2 ligand, lipoxin A4 (LXA4), was induced in the spleen while receptor-deficient animals were unable to efficiently clear damaged RBCs, a defect that was conserved in mice with myeloid-specific FPR2 deletion. Similarly, mice lacking the LXA4 biosynthetic enzyme displayed defective erythrophagocytosis that was rescued with LXA4 administration. These results indicate that the ALX/FPR2 signaling axis is necessary for maintenance of RBC homeostasis and LXA4 activation is a critical aspect of the red pulp macrophage response to acute erythroid stress.
-
-
-
Immunology and Microbiology
Oropouche virus disrupts neurodevelopment and is vertically transmitted
In Research Square on 9 September 2025 by Jurado, K., Bannerman, C., et al.
-
-
-
Immunology and Microbiology
-
COVID-19
IL-4 and TGF-β regulate inflammatory cytokines and cellular infiltration in the lung and systemic IL-6 in mouse-adapted SARS-CoV-2 infection.
In Immunohorizons on 25 August 2025 by Taye Sima, S., Puebla-Clark, L., et al.
PubMed
The pathology of severe COVID-19 is due to a hyperinflammatory immune response persisting after viral clearance. To understand how the immune response to SARS-CoV-2 is regulated to avoid severe COVID-19, we tested relevant immunoregulatory cytokines. Transforming growth factor β (TGF-β), interleukin (IL)-10, and IL-4 were neutralized upon infection with mouse-adapted SARS-CoV-2 (CMA3p20), a model of mild disease; lung inflammation was quantified by histology and flow cytometry at early and late time points. Mild weight loss and lung inflammation including consolidation and alveolar thickening were evident 3 d postinfection (dpi), and inflammation persisted to 7 dpi. Coinciding with early monocytic infiltrates, CCL2 and granulocyte colony-stimulating factor were transiently produced 3 dpi, while IL-12 and CCL5 persisted to 7 dpi, modeling viral and inflammatory phases of disease. Neutralization of TGF-β, but not IL-10 or IL-4, significantly increased lung inflammatory monocytes and elevated serum but not lung IL-6. Neutralization of IL-4 prolonged weight loss and increased early perivascular infiltration without changing viral titer. Anti-IL-4 reduced expression of Arg1, a gene associated with alternative activation of macrophages. Neutralizing TGF-β and IL-4 had differential effects on pathology after virus control. Lung perivascular infiltration was reduced 7 dpi by neutralization of IL-4 or TGF-β, and periairway inflammation was affected by anti-TGF-β, while alveolar infiltrates were not affected by either. Anti-IL-4 prolonged IL-12 to 7 dpi along with reduced IL-10 in lungs. Overall, the immunoregulatory cytokines TGF-β and IL-4 dampen initial inflammation in this mouse-adapted SARS-CoV-2 infection, suggesting that promotion of immunoregulation could help patients in early stages of disease.
-
-
-
Cancer Research
CARG-2020 targets IL-12, IL-17, and PD-L1 pathways to effectively treat melanoma and breast cancer.
In Sci Rep on 13 August 2025 by Ahmadi, E., Chiari, C., et al.
PubMed
Cancer immunotherapy has recently achieved a breakthrough status, however, it is not effective in all cancer types. Genetically engineered oncolytic viruses (OVs) with immunomodulators are promising new therapeutic modalities for cancer. CARG-2020 is an engineered trivalent oncolytic viral construct that specifically expresses three immune modulators that inhibit IL-17RA signaling and regulate PD-L1 expression by shRNAs, along with the cytokine IL-12 which activates multiple tumoricidal pathways. Previous work showed that intratumoral (i.t.) injection of CARG-2020 exhibits robust efficacy against established colorectal cancer (CRC). In this study, we report that systemic delivery of CARG-2020 via the intravenous (i.v.) route can successfully control CRC growth. To further expand the scope of CARG-2020 as a pan-cancer candidate, we also show that CARG-2020 works in two additional mouse models of melanoma and triple-negative breast cancer. Administration of CARG-2020 resulted in increased accumulation of CD8+ T lymphocytes in the tumors, and depletion of these T cells results in poor tumor regression mediated by CARG-2020. Overall, our study shows a broad-spectrum efficacy of CARG-2020 in solid tumors and demonstrates the potential of CARG-2020 to be developed as a clinical candidate for the treatment of multiple human cancers that are surgically accessible.
-
-
Integrin-mediated mTOR signaling drives TGF-β overactivity and myxomatous mitral valve degeneration in hypomorphic fibrillin-1 mice.
In J Clin Invest on 15 July 2025 by Gao, F., Chen, Q., et al.
PubMed
Mitral valve prolapse is often benign, but progression to mitral regurgitation may require invasive intervention and there is no specific medical therapy. An association of mitral valve prolapse with Marfan syndrome resulting from pathogenic FBN1 variants supports the use of hypomorphic fibrillin-1 mgR mice to investigate mechanisms and therapy for mitral valve disease. mgR mice developed severe myxomatous mitral valve degeneration with mitral regurgitation by 12 weeks of age. Persistent activation of TGF-β and mTOR signaling along with macrophage recruitment preceded histological changes at 4 weeks of age. Short-term mTOR inhibition with rapamycin from 4 to 5 weeks of age prevented TGF-β overactivity and leukocytic infiltrates, while long-term inhibition of mTOR or TGF-β signaling from 4 to 12 weeks of age rescued mitral valve leaflet degeneration. Transcriptomic analysis identified integrins as key receptors in signaling interactions, and serologic neutralization of integrin signaling or a chimeric integrin receptor altering signaling prevented mTOR activation. We confirmed increased mTOR signaling and a conserved transcriptome signature in human specimens of sporadic mitral valve prolapse. Thus, mTOR activation from abnormal integrin-dependent cell-matrix interactions drives TGF-β overactivity and myxomatous mitral valve degeneration, and mTOR inhibition may prevent disease progression of mitral valve prolapse.
-
The gut microbiome controls reactive astrocytosis during Aβ amyloidosis via propionate-mediated regulation of IL-17.
In J Clin Invest on 1 July 2025 by Chandra, S., Popović, J., et al.
PubMed
Accumulating evidence implicates the gut microbiome (GMB) in the pathogenesis and progression of Alzheimer's disease (AD). We recently showed that the GMB regulates reactive astrocytosis and Aβ plaque accumulation in a male APPPS1-21 AD mouse model. Yet, the mechanism(s) by which GMB perturbation alters reactive astrocytosis in a manner that reduces Aβ deposition remain unknown. Here, we performed metabolomics on plasma from mice treated with antibiotics (ABX) and identified a significant increase in plasma propionate, a gut-derived short-chain fatty acid, only in male mice. Administration of sodium propionate reduced reactive astrocytosis and Aβ plaques in APPPS1-21 mice, phenocopying the ABX-induced phenotype. Astrocyte-specific RNA-Seq on ABX- and propionate-treated mice showed reduced expression of proinflammatory and increased expression of neurotrophic genes. Next, we performed flow cytometry experiments, in which we found that ABX and propionate decreased peripheral RAR-related orphan receptor-γ+ (Rorγt+) CD4+ (Th17) cells and IL-17 secretion, which positively correlated with reactive astrocytosis. Last, using an IL-17 mAb to deplete IL-17, we found that propionate reduced reactive astrocytosis and Aβ plaques in an IL-17-dependent manner. Together, these results suggest that gut-derived propionate regulates reactive astrocytosis and Aβ amyloidosis by decreasing peripheral Th17 cells and IL-17 release. Thus, propionate treatment or strategies boosting propionate production may represent novel therapeutic strategies for the treatment of AD.
-
-
Cardiovascular biology
-
Immunology and Microbiology
Interleukin-17A-Related Inflammation Mediates Cardiac Injury in Chronic Relapsing Psoriasis-Like Mouse Model.
In Exp Dermatol on 1 July 2025 by Hu, M., Cao, H., et al.
PubMed
Psoriasis is an inflammatory disease characterised by chronic recurrent relapses. Previous observational studies have shown that patients with psoriasis are predisposed to cardiovascular comorbidities, but few studies have investigated the impact of psoriasis-related chronic inflammation on cardiac function. In this study, we used imiquimod (IMQ) to establish psoriasis-like mouse models with short-term inflammation (IMQ-ST) or long-term repeated inflammation (IMQ-LT), to mimic acute or chronic recurrent pathophysiology of psoriasis inflammation. The inflammatory pattern in the hearts of IMQ-ST mice and IMQ-LT mice was similar to that in the skin, characterised by increased level of interleukin (IL)-17A and proportion of IL-17A-producing γδT cells. However, only IMQ-LT mice showed declined cardiac function, significant myocardial tissue necrosis, and decreased expression of genes encoding structural and functional proteins in cardiomyocytes. Furthermore, IL-17A neutralisation markedly alleviated myocardial injury and improved cardiac function in IMQ-LT mice. In conclusion, we demonstrated that IL-17A-mediated inflammation was present in the skin and heart of acute and chronic psoriasis-like mouse models. However, only IMQ-LT mice developed myocardial injury and declined cardiac function, which could be prevented by IL-17A neutralisation.
-
-
-
Immunology and Microbiology
Th1 differentiation and function are inhibited in neonates following human metapneumovirus infection.
In J Immunol on 1 July 2025 by Brown, E., Lan, J., et al.
PubMed
Human metapneumovirus (HMPV) is a leading cause of lower respiratory tract infection in children accounting for 7% of acute care visits and hospitalizations. In particular, neonates and infants have worse outcomes with HMPV infection. The neonatal immune system is regulated to favor anti-inflammatory and tolerogenic responses compared to adults, including prior work demonstrating epigenetic factors in neonatal CD4+ T cells promoting Th2 formation rather than antiviral Th1 differentiation. To interrogate the neonatal immune response to HMPV, 4-to-6 day-old mice or adult 6-to-8 week-old mice were infected with HMPV. Neonates had a decreased Th1 population and increased Th2 and regulatory T-cell (Treg) populations compared to adults. Neonatal Th1 function, but not cell number, was restrained by surface PD-1 expression. To assess if neonatal Th1 formation was intrinsically inhibited after HMPV, neonatal and adult CD4s were transferred into immunocompetent or immunodeficient neonates. Both adult and neonatal CD4s demonstrated reduced Th1 differentiation in the immunocompetent neonates, but robust Th1 differentiation in immunodeficient neonates and immunocompetent adults, suggesting an extrinsic mechanism. Loss of neonatal Tregs led to increased Th1 differentiation after HMPV infection. Neonatal Tregs had increased TGF-β production compared to adult Tregs, and disruption of TGF-β signaling increased Th1 induction. These data demonstrate Tregs provide extrinsic regulation of Th1 formation in the context of respiratory viral infections, rather than an intrinsic limitation of neonatal CD4s. Collectively, these findings identify a nuanced neonatal response to respiratory viruses limiting Th1 formation and function.
-
-
-
Immunology and Microbiology
Mucosal unadjuvanted booster vaccines elicit local IgA responses by conversion of pre-existing immunity in mice.
In Nat Immunol on 1 June 2025 by Kwon, D. I., Mao, T., et al.
PubMed
Mucosal delivery of vaccine boosters induces robust local protective immune responses even without any adjuvants. Yet, the mechanisms by which antigen alone induces mucosal immunity in the respiratory tract remain unclear. Here we show that an intranasal booster with an unadjuvanted recombinant SARS-CoV-2 spike protein, after intramuscular immunization with 1 μg of mRNA-LNP vaccine encoding the full-length SARS-CoV-2 spike protein (Pfizer/BioNTech BNT162b2), elicits protective mucosal immunity by retooling the lymph node-resident immune cells. On intranasal boosting, peripheral lymph node-primed B cells rapidly migrated to the lung through CXCR3-CXCL9 and CXCR3-CXCL10 signaling and differentiated into antigen-specific IgA-secreting plasma cells. Memory CD4+ T cells in the lung served as a natural adjuvant for developing mucosal IgA by inducing the expression of chemokines CXCL9 and CXCL10 for memory B cell recruitment. Furthermore, CD40 and TGFβ signaling had important roles in mucosal IgA development. Repeated mucosal boosting with an unadjuvanted protein amplified anamnestic IgA responses in both the upper and the lower respiratory tracts. These findings help explain why nasal boosters do not require an adjuvant to induce robust mucosal immunity at the respiratory mucosa and can be used to design safe and effective vaccines against respiratory pathogens.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Cancer Research
NECTIN4 regulates the cell surface expression of CD155 in non-small cell lung cancer cells and induces tumor resistance to PD-1 inhibitors.
In Cancer Immunol Immunother on 20 May 2025 by Mizusaki, S., Yoneshima, Y., et al.
PubMed
The development of immune checkpoint inhibitors has changed treatment strategies for some patients with non-small cell lung cancer (NSCLC). However, resistance remains a major problem, requiring the elucidation of resistance mechanisms, which might aid the development of novel therapeutic strategies. The upregulation of CD155, a primary ligand of the immune checkpoint receptor TIGIT, has been implicated in a mechanism of resistance to PD-1/PD-L1 inhibitors, and it is therefore important to characterize the mechanisms underlying the regulation of CD155 expression in tumor cells. The aim of this study was to identify a Nectin that might regulate CD155 expression in NSCLC and affect anti-tumor immune activity. In this study, we demonstrated that NECTIN4 regulated the cell surface expression and stabilization of CD155 by interacting and co-localizing with CD155 on the cell surface. In a syngeneic mouse model, NECTIN4-overexpressing cells exhibited increased cell surface CD155 and resistance to anti-PD-1 antibodies. Of note, combination therapy with anti-PD-1 and anti-TIGIT antibodies significantly suppressed tumor growth. These findings provide new insights into the mechanisms of resistance to anti-PD-1 antibodies and suggest that NECTIN4 could serve as a valuable marker in therapeutic strategies targeting TIGIT.
-
-
-
Immunology and Microbiology
Type I interferon drives T cell cytotoxicity by upregulation of interferon regulatory factor 7 in autoimmune kidney diseases in mice.
In Nat Commun on 20 May 2025 by Wang, H., Engesser, J., et al.
PubMed
In anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) and systemic lupus erythematosus (SLE), glomerulonephritis is a severe kidney complication driven by immune cells, including T cells. However, the mechanisms underlying T cell activation in these contexts remain elusive. Here we report that in patients with AAV and SLE, type I interferon (IFN-I) induces T cell differentiation into interferon-stimulated genes-expressing T (ISG-T) cells, which are characterized by an elevated IFN-I signature, an immature phenotype, and cytotoxicity in inflamed tissue. Mechanistically, IFN-I stimulates the expression of interferon regulatory factor 7 (IRF7) in T cells, which in turn induces granzyme B production. In mice, blocking IFN-I signaling reduces IRF7 and granzyme B expression in T cells, thus ameliorating glomerulonephritis. In parallel, spatial transcriptomic analyses of kidney biopsies from patients with AAV or SLE reveal an elevated ISG signature and the presence of ISG-T cells in close proximity to plasmacytoid dendritic cells, the primary producers of IFN-I. Our results from both patients and animal models thus suggest that IFN-I production in inflamed tissue may drive ISG-T cell differentiation to expand the pool of cytotoxic T cells in autoimmune diseases.
-