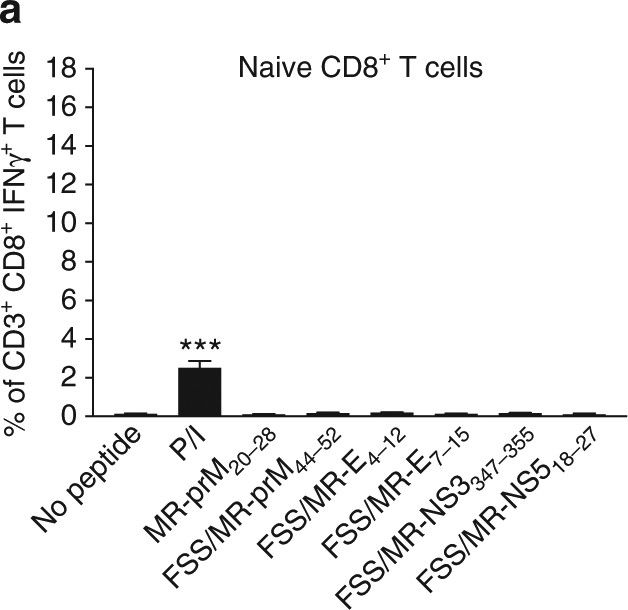
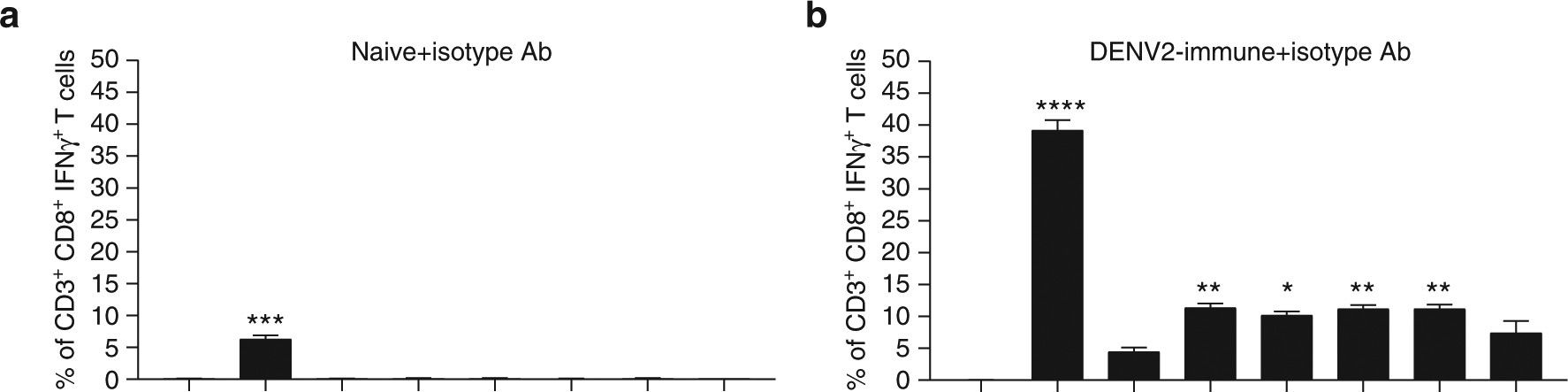
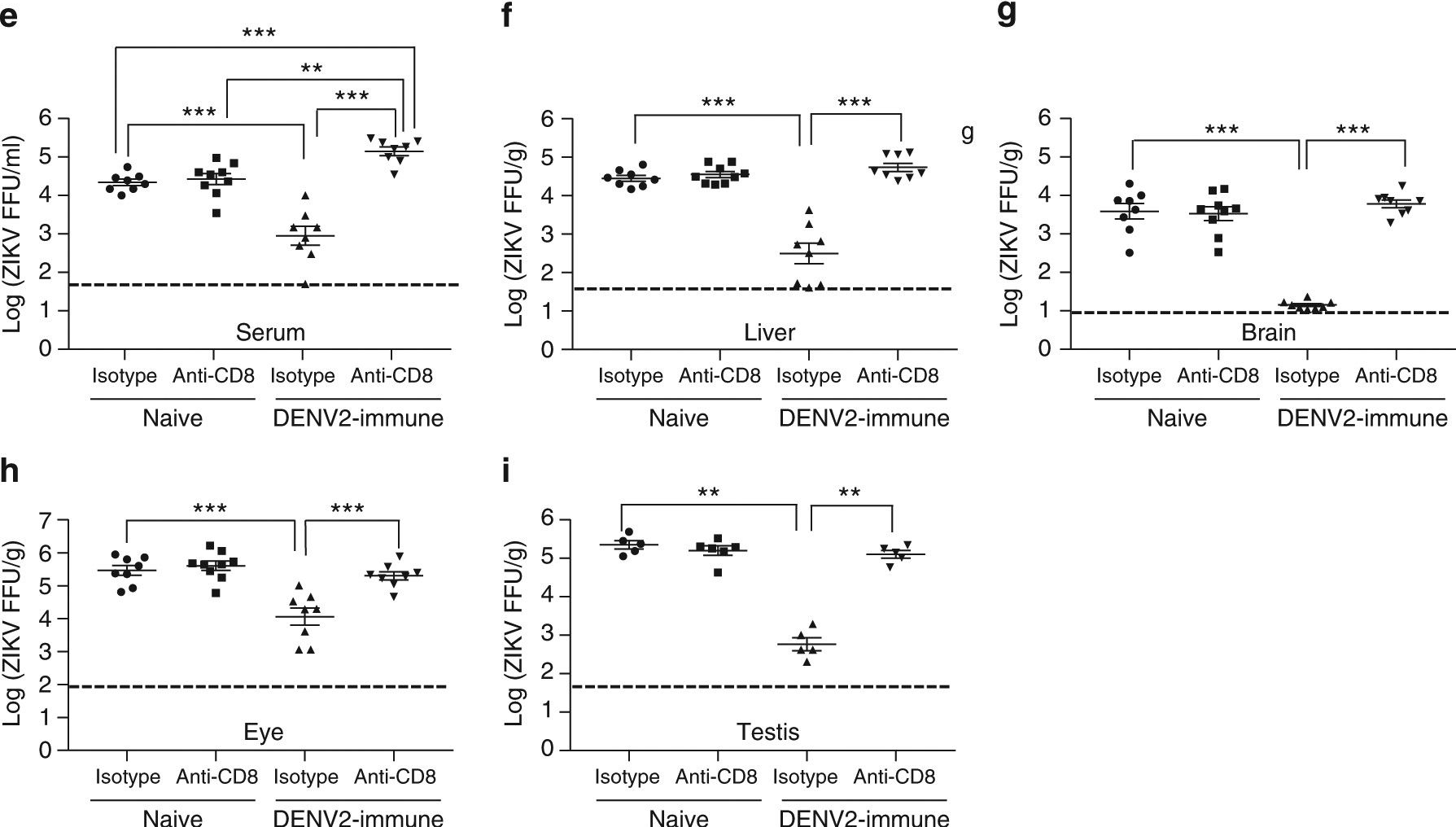
InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107780 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Bauche, D., et al (2018). "LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis" Immunity 49(2): 342-352 e345.
PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3(+) regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1beta production from intestinal-resident CX3CR1(+) macrophages but not CD103(+) dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1(+) macrophage production of IL-23 and IL-1beta. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1(+) tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function.
Triplett, T. A., et al (2018). "Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme" Nat Biotechnol 36(8): 758-764.
PubMed
Increased tryptophan (Trp) catabolism in the tumor microenvironment (TME) can mediate immune suppression by upregulation of interferon (IFN)-gamma-inducible indoleamine 2,3-dioxygenase (IDO1) and/or ectopic expression of the predominantly liver-restricted enzyme tryptophan 2,3-dioxygenase (TDO). Whether these effects are due to Trp depletion in the TME or mediated by the accumulation of the IDO1 and/or TDO (hereafter referred to as IDO1/TDO) product kynurenine (Kyn) remains controversial. Here we show that administration of a pharmacologically optimized enzyme (PEGylated kynureninase; hereafter referred to as PEG-KYNase) that degrades Kyn into immunologically inert, nontoxic and readily cleared metabolites inhibits tumor growth. Enzyme treatment was associated with a marked increase in the tumor infiltration and proliferation of polyfunctional CD8(+) lymphocytes. We show that PEG-KYNase administration had substantial therapeutic effects when combined with approved checkpoint inhibitors or with a cancer vaccine for the treatment of large B16-F10 melanoma, 4T1 breast carcinoma or CT26 colon carcinoma tumors. PEG-KYNase mediated prolonged depletion of Kyn in the TME and reversed the modulatory effects of IDO1/TDO upregulation in the TME.
Aloulou, M., et al (2016). "Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells" Nat Commun 7: 10579.
PubMed
T follicular regulatory (Tfr) cells are a subset of Foxp3(+) regulatory T (Treg) cells that form in response to immunization or infection, which localize to the germinal centre where they control the magnitude of the response. Despite an increased interest in the role of Tfr cells in humoral immunity, many fundamental aspects of their biology remain unknown, including whether they recognize self- or foreign antigen. Here we show that Tfr cells can be specific for the immunizing antigen, irrespective of whether it is a self- or foreign antigen. We show that, in addition to developing from thymic derived Treg cells, Tfr cells can also arise from Foxp3(-) precursors in a PD-L1-dependent manner, if the adjuvant used is one that supports T-cell plasticity. These findings have important implications for Tfr cell biology and for improving vaccine efficacy by formulating vaccines that modify the Tfr:Tfh cell ratio.
Twyman-Saint Victor, C., et al (2015). "Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer" Nature 520(7547): 373-377.
PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
Park, H. J., et al (2015). "PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells" J Immunol 194(12): 5801-5811.
PubMed
Regulatory T (Treg) cells act as terminators of T cell immuniy during acute phase of viral infection; however, their role and suppressive mechanism in chronic viral infection are not completely understood. In this study, we compared the phenotype and function of Treg cells during acute or chronic infection with lymphocytic choriomeningitis virus. Chronic infection, unlike acute infection, led to a large expansion of Treg cells and their upregulation of programmed death-1 (PD-1). Treg cells from chronically infected mice (chronic Treg cells) displayed greater suppressive capacity for inhibiting both CD8(+) and CD4(+) T cell proliferation and subsequent cytokine production than those from naive or acutely infected mice. A contact between Treg and CD8(+) T cells was necessary for the potent suppression of CD8(+) T cell immune response. More importantly, the suppression required cell-specific expression and interaction of PD-1 on chronic Treg cells and PD-1 ligand on CD8(+) T cells. Our study defines PD-1 upregulated on Treg cells and its interaction with PD-1 ligand on effector T cells as one cause for the potent T cell suppression and proposes the role of PD-1 on Treg cells, in addition to that on exhausted T cells, during chronic viral infection.
Finkin, S., et al (2015). "Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma" Nat Immunol. doi : 10.1038/ni.3290.
PubMed
Ectopic lymphoid-like structures (ELSs) are often observed in cancer, yet their function is obscure. Although ELSs signify good prognosis in certain malignancies, we found that hepatic ELSs indicated poor prognosis for hepatocellular carcinoma (HCC). We studied an HCC mouse model that displayed abundant ELSs and found that they constituted immunopathological microniches wherein malignant hepatocyte progenitor cells appeared and thrived in a complex cellular and cytokine milieu until gaining self-sufficiency. The egress of progenitor cells and tumor formation were associated with the autocrine production of cytokines previously provided by the niche. ELSs developed via cooperation between the innate immune system and adaptive immune system, an event facilitated by activation of the transcription factor NF-kappaB and abolished by depletion of T cells. Such aberrant immunological foci might represent new targets for cancer therapy.
Zhang, J., et al (2015). "Micro-RNA-155-mediated control of heme oxygenase 1 (HO-1) is required for restoring adaptively tolerant CD4+ T-cell function in rodents" Eur J Immunol 45(3): 829-842.
PubMed
T cells chronically stimulated by a persistent antigen often become dysfunctional and lose effector functions and proliferative capacity. To identify the importance of micro-RNA-155 (miR-155) in this phenomenon, we analyzed mouse miR-155-deficient CD4(+) T cells in a model where the chronic exposure to a systemic antigen led to T-cell functional unresponsiveness. We found that miR-155 was required for restoring function of T cells after programmed death receptor 1 blockade. Heme oxygenase 1 (HO-1) was identified as a specific target of miR-155 and inhibition of HO-1 activity restored the expansion and tissue migration capacity of miR-155(-/-) CD4(+) T cells. Moreover, miR-155-mediated control of HO-1 expression in CD4(+) T cells was shown to sustain in vivo antigen-specific expansion and IL-2 production. Thus, our data identify HO-1 regulation as a mechanism by which miR-155 promotes T-cell-driven inflammation.
Erickson, J. J., et al (2014). "Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection" J Immunol 193(10): 5108-5117.
PubMed
Reinfections with respiratory viruses are common and cause significant clinical illness, yet precise mechanisms governing this susceptibility are ill defined. Lung Ag-specific CD8(+) T cells (T(CD8)) are impaired during acute viral lower respiratory infection by the inhibitory receptor programmed death-1 (PD-1). To determine whether PD-1 contributes to recurrent infection, we first established a model of reinfection by challenging B cell-deficient mice with human metapneumovirus (HMPV) several weeks after primary infection, and found that HMPV replicated to high titers in the lungs. A robust secondary effector lung TCD8 response was generated during reinfection, but these cells were more impaired and more highly expressed the inhibitory receptors PD-1, LAG-3, and 2B4 than primary T(CD8). In vitro blockade demonstrated that PD-1 was the dominant inhibitory receptor early after reinfection. In vivo therapeutic PD-1 blockade during HMPV reinfection restored lung T(CD8) effector functions (i.e., degranulation and cytokine production) and enhanced viral clearance. PD-1 also limited the protective efficacy of HMPV epitope-specific peptide vaccination and impaired lung T(CD8) during heterotypic influenza virus challenge infection. Our results indicate that PD-1 signaling may contribute to respiratory virus reinfection and evasion of vaccine-elicited immune responses. These results have important implications for the design of effective vaccines against respiratory viruses.
Rutigliano, J. A., et al (2014). "Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells" J Virol 88(3): 1636-1651.
PubMed
One question that continues to challenge influenza A research is why some strains of virus are so devastating compared to their more mild counterparts. We approached this question from an immunological perspective, investigating the CD8(+) T cell response in a mouse model system comparing high- and low-pathological influenza virus infections. Our findings reveal that the early (day 0 to 5) viral titer was not the determining factor in the outcome of disease. Instead, increased numbers of antigen-specific CD8(+) T cells and elevated effector function on a per-cell basis were found in the low-pathological infection and correlated with reduced illness and later-time-point (day 6 to 10) viral titer. High-pathological infection was associated with increased PD-1 expression on influenza virus-specific CD8(+) T cells, and blockade of PD-L1 in vivo led to reduced virus titers and increased CD8(+) T cell numbers in high- but not low-pathological infection, though T cell functionality was not restored. These data show that high-pathological acute influenza virus infection is associated with a dysregulated CD8(+) T cell response, which is likely caused by the more highly inflamed airway microenvironment during the early days of infection. Therapeutic approaches specifically aimed at modulating innate airway inflammation may therefore promote efficient CD8(+) T cell activity. We show that during a severe influenza virus infection, one type of immune cell, the CD8 T cell, is less abundant and less functional than in a more mild infection. This dysregulated T cell phenotype correlates with a lower rate of virus clearance in the severe infection and is partially regulated by the expression of a suppressive coreceptor called PD-1. Treatment with an antibody that blocks PD-1 improves T cell functionality and increases virus clearance.
Steel, C. D., et al (2014). "Role of peripheral immune response in microglia activation and regulation of brain chemokine and proinflammatory cytokine responses induced during VSV encephalitis" J Neuroimmunol 267(1-2): 50-60.
PubMed
We report herein that neuroinvasion by vesicular stomatitis virus (VSV) activates microglia and induces a peripheral dendritic cell (DC)-dependent inflammatory response in the central nervous system (CNS). VSV neuroinvasion rapidly induces multiple brain chemokine and proinflammatory cytokine mRNAs that display bimodal kinetics. Peripheral DC ablation or T cell depletion suppresses the second wave of this response demonstrating that infiltrating T cells are primarily responsible for the bimodal characteristics of this response. The robust infiltrate associated with VSV encephalitis likely depends on sustained production of brain CCL19 and CCR7 expression on infiltrating inflammatory cells.
Sledzinska, A., et al (2013). "TGF-beta signalling is required for CD4(+) T cell homeostasis but dispensable for regulatory T cell function" PLoS Biol 11(10): e1001674.
PubMed
TGF-beta is widely held to be critical for the maintenance and function of regulatory T (T(reg)) cells and thus peripheral tolerance. This is highlighted by constitutive ablation of TGF-beta receptor (TR) during thymic development in mice, which leads to a lethal autoimmune syndrome. Here we describe that TGF-beta-driven peripheral tolerance is not regulated by TGF-beta signalling on mature CD4(+) T cells. Inducible TR2 ablation specifically on CD4(+) T cells did not result in a lethal autoinflammation. Transfer of these TR2-deficient CD4(+) T cells to lymphopenic recipients resulted in colitis, but not overt autoimmunity. In contrast, thymic ablation of TR2 in combination with lymphopenia led to lethal multi-organ inflammation. Interestingly, deletion of TR2 on mature CD4(+) T cells does not result in the collapse of the T(reg) cell population as observed in constitutive models. Instead, a pronounced enlargement of both regulatory and effector memory T cell pools was observed. This expansion is cell-intrinsic and seems to be caused by increased T cell receptor sensitivity independently of common gamma chain-dependent cytokine signals. The expression of Foxp3 and other regulatory T cells markers was not dependent on TGF-beta signalling and the TR2-deficient T(reg) cells retained their suppressive function both in vitro and in vivo. In summary, absence of TGF-beta signalling on mature CD4(+) T cells is not responsible for breakdown of peripheral tolerance, but rather controls homeostasis of mature T cells in adult mice.
van der Merwe, M., et al (2013). "Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplant
PubMed
We showed previously that nonmyeloablative total lymphoid irradiation/rabbit anti-thymocyte serum (TLI/ATS) conditioning facilitates potent donor-recipient immune tolerance following bone marrow transplantation (BMT) across MHC barriers via recipient invariant NKT (iNKT) cell-derived IL-4-dependent expansion of donor Foxp3(+) naturally occurring regulatory T cells (nTregs). In this study, we report a more specific mechanism. Wild-type (WT) BALB/c (H-2(d)) hosts were administered TLI/ATS and BMT from WT or STAT6(-/-) C57BL/6 (H-2(b)) donors. Following STAT6(-/-) BMT, donor nTregs demonstrated no loss of proliferation in vivo, indicating that an IL-4-responsive population in the recipient, rather than the donor, drives donor nTreg proliferation. In graft-versus-host disease (GVHD) target organs, three recipient CD11b(+) cell subsets (Gr-1(high)CD11c(-), Gr-1(int)CD11c(-), and Gr-1(low)CD11c(+)) were enriched early after TLI/ATS + BMT versus total body irradiation/ATS + BMT. Gr-1(low)CD11c(+) cells induced potent H-2K(b+)CD4(+)Foxp3(+) nTreg proliferation in vitro in 72-h MLRs. Gr-1(low)CD11c(+) cells were reduced significantly in STAT6(-/-) and iNKT cell-deficient Jalpha18(-/-) BALB/c recipients after TLI/ATS + BMT. Depletion of CD11b(+) cells resulted in severe acute GVHD, and adoptive transfer of WT Gr-1(low)CD11c(+) cells to Jalpha18(-/-) BALB/c recipients of TLI/ATS + BMT restored day-6 donor Foxp3(+) nTreg proliferation and protection from CD8 effector T cell-mediated GVHD. Blockade of programmed death ligand 1 and 2, but not CD40, TGF-beta signaling, arginase 1, or iNOS, inhibited nTreg proliferation in cocultures of recipient-derived Gr-1(low)CD11c(+) cells with donor nTregs. Through iNKT-dependent Th2 polarization, myeloid-derived immunomodulatory dendritic cells are expanded after nonmyeloablative TLI/ATS conditioning and allogeneic BMT, induce PD-1 ligand-dependent donor nTreg proliferation, and maintain potent graft-versus-host immune tolerance.
Kearl, T. J., et al (2013). "Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma" J Immunol 190(11): 5620-5628.
PubMed
Early phase clinical trials targeting the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers. This pathway appears to play an important role in the failure of immune reactivity to malignant plasma cells in multiple myeloma patients, as the tumor cells express relatively high levels of PD-L1, and T cells show increased PD-1 expression. In the current study, we demonstrate that PD-1/PD-L1 blockade with a PD-L1-specific Ab elicits rejection of a murine myeloma when combined with lymphodepleting irradiation. This particular combined approach by itself has not previously been shown to be efficacious in other tumor models. The antitumor effect of lymphodepletion/anti-PD-L1 therapy was most robust when tumor Ag-experienced T cells were present either through cell transfer or survival after nonmyeloablative irradiation. In vivo depletion of CD4 or CD8 T cells completely eliminated antitumor efficacy of the lymphodepletion/anti-PD-L1 therapy, indicating that both T cell subsets are necessary for tumor rejection. Elimination of myeloma by T cells occurs relatively quickly as tumor cells in the bone marrow were nearly nondetectable by 5 d after the first anti-PD-L1 treatment, suggesting that antimyeloma reactivity is primarily mediated by preactivated T cells, rather than newly generated myeloma-reactive T cells. Anti-PD-L1 plus lymphodepletion failed to improve survival in two solid tumor models, but demonstrated significant efficacy in two hematologic malignancy models. In summary, our results support the clinical testing of lymphodepletion and PD-1/PD-L1 blockade as a novel approach for improving the survival of patients with multiple myeloma.
Willimsky, G., et al (2013). "Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance" J Clin Invest 123(3): 1032-1043.
PubMed
T cell surveillance is often effective against virus-associated tumors because of their high immunogenicity. It is not clear why surveillance occasionally fails, particularly against hepatitis B virus- or hepatitis C virus-associated hepatocellular carcinoma (HCC). We established a transgenic murine model of virus-induced HCC by hepatocyte-specific adenovirus-induced activation of the oncogenic SV40 large T antigen (TAg). Adenovirus infection induced cytotoxic T lymphocytes (CTLs) targeted against the virus and TAg, leading to clearance of the infected cells. Despite the presence of functional, antigen-specific T cells, a few virus-infected cells escaped immune clearance and progressed to HCC. These cells expressed TAg at levels similar to HCC isolated from neonatal TAg-tolerant mice, suggesting that CTL clearance does not select for cells with low immunogenicity. Virus-infected mice revealed significantly greater T cell infiltration in early-stage HCC compared with that in late-stage HCC, demonstrating progressive local immune suppression through inefficient T cell infiltration. Programmed cell death protein-1 (PD-1) and its ligand PD-L1 were expressed in all TAg-specific CD8+ T cells and HCC, respectively, which contributed to local tumor-antigen-specific tolerance. Thus, we have developed a model of virus-induced HCC that may allow for a better understanding of human HCC.
Coers, J., et al (2011). "Compensatory T cell responses in IRG-deficient mice prevent sustained Chlamydia trachomatis infections" PLoS Pathog 7(6): e1001346.
PubMed
The obligate intracellular pathogen Chlamydia trachomatis is the most common cause of bacterial sexually transmitted diseases in the United States. In women C. trachomatis can establish persistent genital infections that lead to pelvic inflammatory disease and sterility. In contrast to natural infections in humans, experimentally induced infections with C. trachomatis in mice are rapidly cleared. The cytokine interferon-gamma (IFNgamma) plays a critical role in the clearance of C. trachomatis infections in mice. Because IFNgamma induces an antimicrobial defense system in mice but not in humans that is composed of a large family of Immunity Related GTPases (IRGs), we questioned whether mice deficient in IRG immunity would develop persistent infections with C. trachomatis as observed in human patients. We found that IRG-deficient Irgm1/m3((-/-)) mice transiently develop high bacterial burden post intrauterine infection, but subsequently clear the infection more efficiently than wildtype mice. We show that the delayed but highly effective clearance of intrauterine C. trachomatis infections in Irgm1/m3((-/-)) mice is dependent on an exacerbated CD4(+) T cell response. These findings indicate that the absence of the predominant murine innate effector mechanism restricting C. trachomatis growth inside epithelial cells results in a compensatory adaptive immune response, which is at least in part driven by CD4(+) T cells and prevents the establishment of a persistent infection in mice.
Product Citations
-
-
Immunology and Microbiology
-
Genetics
mRNA vaccine expressing enterovirus D68 virus-like particles induces potent neutralizing antibodies and protects against infection.
In Mol Ther Nucleic Acids on 9 December 2025 by Kunishima, Y., Senpuku, K., et al.
PubMed
Enterovirus D68 (EV-D68) causes respiratory illness in children. It also causes severe paralysis called acute flaccid myelitis (AFM), which has become a global health threat. Here, we generated an mRNA vaccine expressing virus-like particles (VLPs) of EV-D68. We found that the mRNA vaccine elicited potent neutralizing antibodies against EV-D68 in the blood, and the neutralizing titer was superior to that of the inactivated whole virion (IWV) vaccine. The mRNA vaccine showed protective effects against intranasal challenge with EV-D68, and antisera from the vaccinated mice prevented the paralysis caused by EV-D68 infection in neonatal mice. Moreover, the mRNA vaccine induced neutralizing antibodies in the respiratory tract, which is the entry site for EV-D68. Additionally, it attenuated infection with coxsackievirus B3 (CVB3), which belongs to another enterovirus group, via CD8+ T cell responses. In conclusion, our results suggest that this mRNA vaccine is a promising candidate for EV-D68 prevention.
-
-
-
Cell Biology
-
Immunology and Microbiology
-
Biochemistry and Molecular biology
FOXO1-driven metabolic reprogramming of hematomal CD8+ T cells drives the expansion of perihematomal edema following intracerebral hemorrhage.
In Cell Mol Immunol on 1 December 2025 by Lin, J., Ren, H., et al.
PubMed
Intracerebral hemorrhage (ICH) causes hematoma formation, leading to PHE, which is associated with leukocyte mobilization and increased inflammation at the site of brain injury. However, the fate of accumulated leukocytes within the hematoma and their impact on PHE expansion remain unknown. We performed single-cell immune profiling of hematoma cells from patients with acute ICH and reported a distinct phenotypic transformation of CD8+ T cells within the hematoma during the first 24 h after onset. In addition to enhanced IFN-γ production and migration capacity, these CD8+ T cells displayed remarkable glycolytic signatures. The metabolic fitness and functional reprogramming of hematomal CD8+ T cells are associated with the transcription factor FOXO1. Single-cell profiling of brain-infiltrating CD8+ T cells within the perihematomal tissues of ICH patients and cell culture assays revealed their capacity to activate microglia via the production of IFN-γ. Furthermore, the removal of hematomal CD8+ T cells reduced neuroinflammation, PHE expansion and neurological deficits in ICH mice. Thus, CD8+ T cells undergo metabolic and functional reprogramming within the hematoma during the acute phase of ICH, which contributes to PHE formation and neurological deterioration.
-
-
-
Cancer Research
KRAS Inhibition Activates an Actionable CD24 Do Not Eat Me Signal in Pancreatic Cancer.
In Cancer Res on 1 December 2025 by Wei, Y., Liu, M., et al.
PubMed
KRASG12C inhibitors (G12Ci) have produced encouraging, albeit modest and transient, clinical benefit in pancreatic ductal adenocarcinoma (PDAC). Identifying and targeting resistance mechanisms to G12Ci treatment are therefore crucial. To better understand the function of KRASG12C and possible G12Ci bypass mechanisms, we developed an autochthonous KRASG12C-driven PDAC model. Compared with the classical KRASG12D PDAC model, the G12C model exhibits slower tumor growth, yet similar histopathologic and molecular features. Aligned with clinical experience, G12Ci treatment of KRASG12C tumors produced modest impact despite stimulating a "hot" tumor immune microenvironment. Immunoprofiling revealed that CD24, a "do not eat me" signal, is significantly upregulated on cancer cells upon G12Ci treatment. Blocking CD24 enhanced macrophage phagocytosis of cancer cells and significantly sensitized tumors to G12Ci treatment. Similar findings were observed in KRASG12D-driven PDAC. Together, this study reveals common and distinct oncogenic KRAS allele-specific biology and identifies a clinically actionable adaptive mechanism that may improve the efficacy of oncogenic KRAS inhibitor therapy in PDAC.
-
-
-
Immunology and Microbiology
Targeting hepatocytic TβRI ameliorates liver metastatic outcomes by revitalizing stem-like CD8+ Tex subsets.
In Nat Commun on 27 November 2025 by Wang, H., Zhou, Y., et al.
PubMed
Stem-like CD8⁺ exhausted T cells (Tex) sustain antitumor immunity, whereas TGFβ signaling acts as a major immunosuppressive pathway. In patients with colorectal liver metastases, we observe that elevated TβRI expression in peri-metastatic hepatocytes correlates with poor prognosis. We therefore investigate whether disrupting hepatocytic TGFβ signaling can reinvigorate stem-like CD8⁺ Tex cells to restrict liver metastasis. In support of this hypothesis, mice with hepatocyte-specific TβRI depletion exhibit reduced liver metastatic burden across multiple tumor models. Mechanistically, hepatocytic TβRI blockade suppresses Galectin-9 secretion, which reshapes the transcriptional program of intra-tumoral CD8⁺ T cells. This reprogramming promotes a phenotypic transition from terminal exhaustion toward stem-like and effector states, yielding T cell subsets with enhanced metastasis-control capacity. Importantly, this axis functions independently of macrophages and CD4⁺ T cells. Furthermore, therapeutic delivery of Galunisertib using choline-modified lipid nanoparticles synergizes with αPD-1, fostering the conversion of exhausted CD8⁺ T cells into responsive Ly108⁺CX3CR1⁺ subsets and suppressing liver metastases. Collectively, our results identify hepatocyte TGFβ signaling as a targetable checkpoint against liver metastases.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting tumor-intrinsic BCL9 reverses immunotherapy resistance by eliciting macrophage-mediated phagocytosis and antigen presentation.
In Nat Commun on 17 November 2025 by Wu, S. Y., Zhu, Y. Y., et al.
PubMed
Immune checkpoint inhibitors (ICI) benefit some cancer patients but de novo resistance remains poorly understood. Analyzing transcriptional data from two clinical trial cohorts, GO30140 and IMbrave150, we find B cell lymphoma 9 (BCL9), a Wnt/β-catenin co-factor, associated with resistance. We develop a BCL9-targeting peptide, hsBCL9Z96, which suppresses tumor growth in combination with anti-PD-L1 ab in preclinical hepatocellular carcinoma (HCC) mouse models. Multi-omics analyses implicate targeting BCL9 inhibits BMP4 secretion and downregulates CD24 on tumor cells, reprogramming macrophages toward a tumor-suppressive phenotype and promoting macrophage phagocytosis. This in turn rejuvenates T cell immunity via enhanced macrophage-mediated antigen presentation. Our data extend our understanding of how tumor-derived Wnt/β-catenin signaling impedes the innate and adaptive immune responses in the tumor microenvironment and provide preliminary evidence that targeting BCL9 is a promising preclinical strategy to mitigate ICI resistance in HCC.
-
-
-
Cancer Research
-
Immunology and Microbiology
TAMs-mediated resistance to oncolytic virus M1 in solid tumors.
In J Immunother Cancer on 13 November 2025 by Liang, X., Li, J., et al.
PubMed
Oncolytic virus M1 (OVM), a naturally occurring alphavirus, has demonstrated potent antitumor activity in various solid tumor models by inducing immunogenic cell death and activating CD8+ T cells. However, its in vivo efficacy varies widely, and resistance mechanisms remain poorly understood. Tumor-associated macrophages (TAMs), key immunosuppressive cells within the tumor microenvironment, may limit OVM therapeutic potential.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Neuroscience
Brain tumors induce widespread disruption of calvarial bone and alteration of skull marrow immune landscape.
In Nat Neurosci on 1 November 2025 by Dubey, A., Yamashita, E., et al.
PubMed
The skull marrow niche has recently been identified as a reservoir that supplies the brain with monocytes and neutrophils in the context of disease and injury, but its role in brain cancers remains unknown. Here we show that glioblastoma, the most malignant type of brain tumor, induces calvarial bone abnormalities in murine models and patients with glioblastoma, altering osteoclast activities and increasing the number of skull channels in mice. Single-cell RNA sequencing revealed glioblastoma-mediated alterations in the immune landscape of skull marrow and femoral bone marrow, including expansion of neutrophils and deterioration of various B cell subsets. In vivo inhibition of bone resorption reduced bone abnormalities, but promoted tumor progression in mesenchymal subtype tumors. This also abolished the survival benefit of the checkpoint inhibitor anti-PD-L1, by reducing activated T cell and increasing inflammatory neutrophil numbers. Together, these data provide insight into how brain tumors affect skull bone and the immune environment.
-
-
-
Neuroscience
BST2 expression at astrocyte borders promotes microglial recruitment via the C3/C3aR signaling.
In Neuron on 24 October 2025 by Zhang, S., Yuan, M., et al.
PubMed
Following central nervous system injury, astrocytes form borders that were traditionally regarded as physical barriers. Emerging evidence demonstrates their capacity to regulate inflammation and repair; however, the specific characteristics of these border astrocytes and their interactions with immune cells remain insufficiently characterized. Using single-cell sequencing and spatial transcriptomics, we identified astrocytes expressing the interferon-inducible protein bone marrow stromal cell antigen 2 (BST2) enriched at injury boundaries that promote microglial recruitment via C3/C3aR signaling. Astrocyte-specific Bst2 knockout reduced astrocyte-microglia interactions and attenuated border formation, correlating with early neurological improvement after stroke. Mechanistically, BST2 enhanced C3 expression through protein kinase C-βII (PKCβII) phosphorylation. Moreover, treatment with a BST2 monoclonal antibody diminished astrocyte-microglia interactions and improved neurological function. Together, these findings highlight the pivotal role of astrocyte-microglia interactions in lesion border formation and suggest that BST2 may represent a therapeutic target to modulate these interactions and reduce early brain injury after stroke.
-
-
-
Cancer Research
-
Immunology and Microbiology
Munc13-4 mediates tumor immune evasion by regulating the sorting and secretion of PD-L1 via exosomes.
In Nat Commun on 13 October 2025 by Liu, C., Liu, D., et al.
PubMed
Tumor-derived exosomes carry programmed death-ligand 1 (PD-L1), which binds programmed cell death protein 1 (PD-1) on T cells, suppressing immune responses locally and systemically. However, the mechanisms governing exosomal PD-L1 sorting and secretion remain elusive. Here, we identify Munc13-4 as a crucial regulator of this process. Deletion of Munc13-4 in breast tumors enhances T cell-mediated anti-tumor immunity, suppresses tumor growth, and improves the efficacy of immune checkpoint inhibitors. Mechanistically, Munc13-4 collaborates with hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), Rab27, and SNAREs to facilitate PD-L1 sorting and secretion via exosomes. Cryogenic electron microscopy (cryo-EM) analysis of the Munc13-4-Rab27a complex provide structural insights into exosome secretion. Importantly, PD-L1 sorting relies on a ternary complex composed of Munc13-4, PD-L1 and HRS, which is regulated by interferon gamma (IFNγ) signaling. A designed peptide that disrupts Munc13-4-PD-L1 interaction impedes PD-L1 sorting, enhances antitumor immunity, and suppresses tumor growth, highlighting the therapeutic potential of targeting this pathway.
-
-
-
Immunology and Microbiology
Ligand-receptor interactions induce and mediate regulatory functions of BATF3+ B cells.
In Sci Adv on 10 October 2025 by Yan, H., Wang, R., et al.
PubMed
B cells express many protein ligands, yet their regulatory functions are incompletely understood. We profiled ligand expression across murine B sublineage cells, including those activated by defined receptor signals, and assessed their regulatory capacities and specificities through in silico analysis of ligand-receptor interactions. Consequently, we identified a B cell subset that expressed cytokine interleukin-27 (IL-27) and chemokine CXCL10. Through the IL-27-IL-27 receptor interaction, these IL-27/CXCL10-producing B cells targeted CD40-activated B cells in vitro and, upon induction by immunization and viral infection, optimized antibody responses and antiviral immunity in vivo. Also present in breast cancer tumors and retained there through CXCL10-CXCR3 interaction-mediated self-targeting, these cells promoted B cell PD-L1 expression and immune evasion. Mechanistically, Il27 and Cxcl10 transcription was induced by synergizing Toll-like receptor (TLR) and CD40 signals and driven by coinduced transcription factor BATF3, which directly targeted these genes. By applying a discovery framework focusing on regulatory cells, our findings expand the recognized scope of B cell regulatory functions.
-
-
-
Immunology and Microbiology
Simultaneous STING and lymphotoxin-β receptor activation induces B cell responses in tertiary lymphoid structures to potentiate antitumor immunity.
In Nat Immunol on 1 October 2025 by Sawada, J., Kikuchi, Y., et al.
PubMed
B cell-rich tertiary lymphoid structures (TLS) are associated with favorable prognosis and positive response to immunotherapy in cancer. Here we show that simultaneous activation of innate immune effectors, STING and lymphotoxin-β receptor (LTβR), results in CD8+ T cell-dependent tumor suppression while inducing high endothelial venule development and germinal center-like B cell responses in tumors to generate functional TLS in a T cell-dependent manner. In a neoadjuvant setting, activation of STING and LTβR by their agonists effectively immunized mice against tumor recurrence, leading to long-term survival. STING activation alone was insufficient for inducing B cell-containing TLS or eliciting long-term therapeutic effects. However, when combined with LTβR activation, it improved the fitness of TLS with B cell expansion and maturation to IgG-producing long-lived plasma cells and memory cells, increased CD4+ T cell recruitment and memory CD8+ T cell expansion, and shifted the TH2/TH17 balance, resulting in the potentiation of humoral and cellular immunity against tumors. These findings suggest a therapeutic approach of simultaneously activating STING and lymphotoxin pathways.
-
-
-
Immunology and Microbiology
Monocyte/macrophage-derived interleukin-15 mediates the pro-inflammatory phenotype of CD226+ B cells in type 1 diabetes.
In EBioMedicine on 1 October 2025 by Li, J., Liang, X., et al.
PubMed
Type 1 diabetes (T1D) is characterised by the autoimmune-mediated destruction of pancreatic β-cells. Although traditionally viewed as a disease dominated by T cells, recent studies have emphasised the crucial role of B cells in the development of T1D. Genome-wide association studies (GWAS) have revealed that CD226 is related to susceptibility to several autoimmune diseases, including T1D. Our recent work identified a pathogenic role of CD226+ CD8+ T cells in T1D. However, the involvement of CD226+ B cells in T1D development remains unclear.
-
-
-
Cancer Research
FK228 reshapes tumor microenvironment to enhance anti-PD-L1 efficacy.
In Oncogene on 1 October 2025 by Gong, L., Tian, L., et al.
PubMed
The lack of a favorable tumor immune microenvironment (TIME) results in limited response rates to immune checkpoint blockade (ICB) across human solid tumors, necessitating the development of novel combination strategies. In this study, we repurposed FK228, an US FDA-approved histone deacetylase inhibitor that is used clinically in non-solid tumor treatment, as a novel ICB sensitizer in solid tumors and revealed the diverse regulatory functions of FK228 in the TIME. FK228 serves as a novel necroptosis inducer in cancer cells by triggering endoplasmic reticulum stress. This in turn enhances the immunogenicity of cancer cells and increases the infiltration of tumor-killing immunocytes, including CD8+ T and natural killer cells, particularly activating tumor-infiltrated CD8+ T cells. Meanwhile, FK228 treatment shifts macrophages toward the pro-inflammatory phenotype. Moreover, the combined use of FK228 and a PD-L1 inhibitor significantly delay tumor growth and extend the survival of tumor bearing mice. Overall, our findings reveal new possibilities for the clinical application of FK228 in solid tumors and underscore the critical role of histone deacetylases in maintaining the immune-unfavorable TIME.
-
-
-
Immunology and Microbiology
-
Cancer Research
NOTCH1 reverses immune suppression in small cell lung cancer through reactivation of STING.
In J Clin Invest on 16 September 2025 by Kim, Y. S., Nabet, B. Y., et al.
PubMed
Downregulation of antigen presentation and lack of immune infiltration are defining features of small cell lung cancer (SCLC), limiting response to immune checkpoint blockade (ICB). While a high-MHC class I, immune-inflamed subset benefits from ICB, underlying mechanisms of immune response in SCLC have yet to be elucidated. Here we show that in the IMpower133 clinical trial, high, but not low, NOTCH1 expression was significantly associated with longer survival with the addition of ICB to chemotherapy among approximately 80% of SCLC patients with NE-enriched tumors (ASCL1-enriched, HR 0.39, P = 0.0012; NEUROD1-enriched, HR 0.44, P = 0.024). Overexpression or pharmacologic activation of NOTCH1 in ASCL1 and NEUROD1 SCLC cell lines dramatically upregulated MHC class I through epigenetic reactivation of STING. In syngeneic mouse models, Notch1 activation reprogrammed SCLC tumors from immune-excluded to immune-inflamed, facilitating durable, complete responses with ICB combined with a STING agonist. STING1 expression was significantly enriched in high- compared with low-NOTCH1-expressing tumors in IMpower133, validating our proposed mechanism. Our data reveal a previously undiscovered role for NOTCH1 as a critical driver of SCLC immunogenicity and a potential predictive biomarker for ICB in SCLC. NOTCH1 activation may be a therapeutic strategy to unleash antitumor immune responses in SCLC and other neuroendocrine cancers in which NOTCH1 is typically suppressed.
-
-
-
Immunology and Microbiology
Zeaxanthin augments CD8+ effector T cell function and immunotherapy efficacy.
In Cell Rep Med on 16 September 2025 by Zhang, F. Q., Li, J., et al.
PubMed
The detailed mechanisms underlying the regulatory significance of dietary components in modulating anti-tumor immunity remain largely unknown. Here, we apply a co-culture-based screening approach using a blood nutrient compound library and identify zeaxanthin (ZEA), a dietary carotenoid pigment found in many fruits and vegetables and known for its role in eye health, as an immunomodulator that enhances the cytotoxicity of CD8+ T cells against tumor cells. Oral supplementation with ZEA, but not its structural isomer lutein (LUT), enhances anti-tumor immunity in vivo. Integrated multi-omics mechanistic studies reveal that ZEA promotes T cell receptor (TCR) stimulation on the CD8+ T cell surface, leading to improved intracellular TCR signaling for effector T cell function. Hence, ZEA treatment augments the efficacy of anti-PD1 immune checkpoint inhibitor in vivo and the cytotoxicity of human TCR gene-engineered CD8+ T cells in vitro. Our findings uncover a previously unknown immunoregulatory function of ZEA, which has translational potential as a dietary element in bolstering immunotherapy.
-
-
-
Immunology and Microbiology
-
Cancer Research
Double-positive T cells form heterotypic clusters with circulating tumor cells to foster cancer metastasis.
In J Clin Invest on 16 September 2025 by Scholten, D., El-Shennawy, L., et al.
PubMed
The immune ecosystem is central to maintaining effective defensive responses. However, it remains largely understudied how immune cells in the peripheral blood interact with circulating tumor cells (CTCs) in metastasis. Here, blood analysis of patients with advanced breast cancer revealed that over 75% of CTC-positive blood specimens contained heterotypic CTC clusters with CD45+ white blood cells (WBCs), which correlates with breast cancer subtypes, racial groups, and decreased survival. CTC-WBC clusters included overrepresented T cells and underrepresented neutrophils. Specifically, a rare subset of CD4 and CD8 double-positive T (DPT) cells was 140-fold enriched in CTC clusters versus their frequency in WBCs. DPT cells shared properties with CD4+ and CD8+ T cells but exhibited unique features of T cell exhaustion and immune suppression. Mechanistically, the integrin heterodimer α4β1, also named very late antigen 4 (VLA-4), in DPT cells and its ligand, VCAM1, in tumor cells are essential mediators of DPT-CTC clusters. Neoadjuvant administration of anti-VLA-4 neutralizing antibodies markedly blocked CTC-DPT clusters, inhibited metastasis, and extended mouse survival. These findings highlight a pivotal role of rare DPT cells in fostering cancer dissemination through CTC clustering. It lays a foundation for developing innovative biomarker-guided therapeutic strategies to prevent and target cancer metastasis.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Immunology and Microbiology
An age-related decrease in leptin contributes to CD8+ T cell aging in the tumor microenvironment.
In Cell Rep Med on 16 September 2025 by Wang, F., Bao, R., et al.
PubMed
T cell dysfunction with age underlies an increased incidence of cancer in elderly individuals; however, how T cell aging is triggered in the tumor microenvironment is unclear. Here, we show that an age-associated reduction in adipocyte-derived leptin contributes to the accumulation of tumor-infiltrating senescent CD8+ T cells. Single-cell profiling of human and mouse cancer tissues reveals that the frequency of intratumoral senescent CD8+ T cells increases with age, leading to a weak antitumor effect. Moreover, decreased levels of adipocyte-derived leptin are an indispensable factor for CD8+ T cell aging. Leptin signaling prevents p38-dependent CD8+ T cell senescence. Furthermore, plasma leptin levels are negatively related to intratumoral CD8+ T cell senescence in cancer patients. Our findings identify an unappreciated interplay between metabolic perturbation and T cell aging and suggest that modulating adipocyte-derived leptin levels may be a promising therapeutic strategy for older cancer patients.
-
-
-
Cancer Research
Selective Alanine Transporter Utilization Is a Therapeutic Vulnerability in ARID1A-Mutant Ovarian Cancer.
In Cancer Res on 15 September 2025 by Nie, H., Liao, L., et al.
PubMed
Subunits of the SWI/SNF chromatin remodeling complex are altered in ∼20% of human cancers. Exemplifying the alterations is the ARID1A mutation that occurs in ∼50% of ovarian clear-cell carcinoma (OCCC), a disease with limited therapeutic options. In this study, we showed that ARID1A mutations create a dependence on alanine by regulating alanine transporters to increase intracellular alanine levels. ARID1A directly repressed the alanine importer SLC38A2 and simultaneously promoted the alanine exporter SLC7A8. ARID1A inactivation increased alanine utilization predominantly in protein synthesis and passively through the tricarboxylic acid cycle. Indeed, ARID1A-mutant OCCCs were hypersensitive to the inhibition of SLC38A2. In addition, SLC38A2 inhibition enhanced chimeric antigen receptor T-cell assault in vitro and synergized with immune checkpoint blockade using an anti-PD-L1 antibody in a genetically engineered mouse model of OCCC driven by conditional Arid1a inactivation in a CD8+ T-cell-dependent manner. These findings suggest that targeting alanine transport alone or in combination with immunotherapy may represent an effective therapeutic strategy for ARID1A-mutant cancers.
-
-
-
Immunology and Microbiology
-
Cell Biology
Enhancing immunotherapy efficacy in NSCLC through the combined use of phenelzine and Akkermansia muciniphila to regulate gut microbial metabolite 5-HIAA.
In J Immunother Cancer on 10 September 2025 by Sun, S., Wang, L., et al.
PubMed
Improving the efficacy of anti-programmed death 1 (PD-1) monoclonal antibody (mAb) therapy remains a major challenge for cancer immunotherapy in non-small cell lung cancer (NSCLC). Gut microbial metabolites can influence immunotherapy efficacy.
-
-
-
Immunology and Microbiology
Next-generation Candida albicans recombinant Als3p and Hyr1p dual antigen vaccine for invasive Candida infections
In Research Square on 5 September 2025 by Singh, S., Youssef, E. G., et al.
-