RecombiMAb human IgG4 (S228P) isotype control, anti-hen egg lysozyme
Product Description
Note: This product was previously sold as catalog number BE0349 and is identical to the product previously sold as BE0349.
Specifications
| Isotype | Human IgG4, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
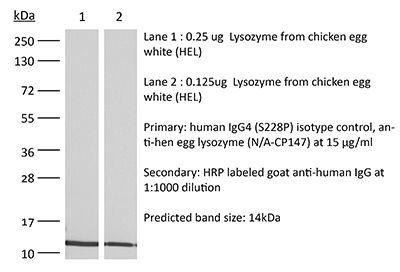
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Mutations | S228P |
| Immunogen | Hen egg lysozyme (HEL) |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from CHO cell supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894768 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Product Citations
-
-
Immunology and Microbiology
Inflammatory arthritis immune related adverse events represent a unique autoimmune disease entity primarily driven by T cells, but likely not autoantibodies
In medRxiv on 6 June 2025 by Zhu, X., Yu, Y., et al.
-
-
-
Cancer Research
Reprograming immunosuppressive microenvironment by eIF4G1 targeting to eradicate pancreatic ductal adenocarcinoma.
In Cell Rep Med on 15 October 2024 by He, L., Zhang, X., et al.
PubMed
Current therapies against pancreatic ductal adenocarcinoma (PDAC) have limited clinical benefits owing to tumor heterogeneity and their unique immunosuppressive microenvironments. The eukaryotic initiation factor (eIF) 4F complex is involved in regulating translation and various downstream carcinogenic signaling pathways. We report that eIF4G1, one of the subunits of eIF4F, is overexpressed in cancer cells and cancer-associated fibroblasts, and this correlates with poor prognosis in patients with PDAC. In PDAC mice, eIF4G1 inhibition limits tumor progression and prolongs overall survival, especially when combined with PD1/PDL1 antagonists and gemcitabine. Mechanistically, eIF4G1 inhibition hinders the production of cytokines and chemokines that promote fibrosis and inhibit cytotoxic T cell chemotaxis. Moreover, eIF4G1 inhibition impairs integrinβ1 protein translation and exerts tumor suppression effects through the FAK-ERK/AKT signaling pathway. These findings highlight the effects of eIF4G1 on tumor immune dependence and independence and identify eIF4G1 as a promising therapeutic target for PDAC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting PD-L1 in solid cancer with myeloid cells expressing a CAR-like immune receptor.
In Front Immunol on 10 May 2024 by Myers Chen, K., Grun, D., et al.
PubMed
Solid cancers Myeloid cells are prevalent in solid cancers, but they frequently exhibit an anti-inflammatory pro-tumor phenotype that contribute to the immunosuppressive tumor microenvironment (TME), which hinders the effectiveness of cancer immunotherapies. Myeloid cells' natural ability of tumor trafficking makes engineered myeloid cell therapy an intriguing approach to tackle the challenges posed by solid cancers, including tumor infiltration, tumor cell heterogenicity and the immunosuppressive TME. One such engineering approach is to target the checkpoint molecule PD-L1, which is often upregulated by solid cancers to evade immune responses.
-
-
-
Cancer Research
-
Immunology and Microbiology
ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis.
In iScience on 19 April 2024 by Deng, R., Tian, R., et al.
PubMed
Hepatitis B virus (HBV) infection generally elicits weak type-I interferon (IFN) immune response in hepatocytes, covering the regulatory effect of IFN-stimulated genes. In this study, low level of IFN-stimulated gene 12a (ISG12a) predicted malignant transformation and poor prognosis of HBV-associated hepatocellular carcinoma (HCC), whereas high level of ISG12a indicated active NK cell phenotypes. ISG12a interacts with TRIM21 to inhibit the phosphorylation activation of protein kinase B (PKB, also known as AKT) and β-catenin, suppressing PD-L1 expression to block PD-1/PD-L1 signaling, thereby enhancing the anticancer effect of NK cells. The suppression of PD-1-deficient NK-92 cells on HBV-associated tumors was independent of ISG12a expression, whereas the anticancer effect of PD-1-expressed NK-92 cells on HBV-associated tumors was enhanced by ISG12a and treatments of atezolizumab and nivolumab. Thus, tumor intrinsic ISG12a promotes the anticancer effect of NK cells by regulating PD-1/PD-L1 signaling, presenting the significant role of innate immunity in defending against HBV-associated HCC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting PD-L1 in solid cancer with myeloid cells expressing a CAR-like immune receptor
In bioRxiv on 1 February 2024 by Chen, K. M., Grun, D., et al.
-
-
-
Immunology and Microbiology
Modulation of urelumab glycosylation separates immune stimulatory activity from organ toxicity.
In Front Immunol on 18 October 2022 by Reitinger, C., Ipsen-Escobedo, A., et al.
PubMed
Checkpoint control and immunomodulatory antibodies have become important tools for modulating tumor or self-reactive immune responses. A major issue preventing to make full use of the potential of these immunomodulatory antibodies are the severe side-effects, ranging from systemic cytokine release syndrome to organ-specific toxicities. The IgG Fc-portion has been demonstrated to contribute to both, the desired as well as the undesired antibody activities of checkpoint control and immunomodulatory antibodies via binding to cellular Fcγ-receptors (FcγR). Thus, choosing IgG subclasses, such as human IgG4, with a low ability to interact with FcγRs has been identified as a potential strategy to limit FcγR or complement pathway dependent side-effects. However, even immunomodulatory antibodies on the human IgG4 background may interact with cellular FcγRs and show dose limiting toxicities. By using a humanized mouse model allowing to study the immunomodulatory activity of human checkpoint control antibodies in vivo, we demonstrate that deglycosylation of the CD137-specific IgG4 antibody urelumab results in an amelioration of liver toxicity, while maintaining T cell stimulatory activity. In addition, our results emphasize that antibody dosing impacts the separation of side-effects of urelumab from its therapeutic activity via IgG deglycosylation. Thus, glycoengineering of human IgG4 antibodies may be a possible approach to limit collateral damage by immunomodulatory antibodies and allow for a greater therapeutic window of opportunity.
-