RecombiMAb human IgG4 (S228P/R409K) isotype control, anti-hen egg lysozyme
Product Description
Specifications
| Isotype | Human IgG4, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Mutations | S228P/R409K |
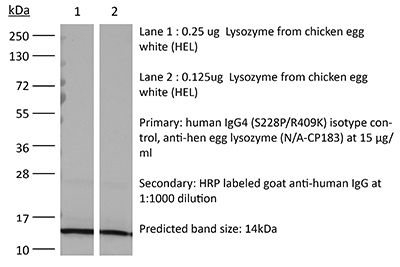
| Immunogen | Hen egg lysozyme (HEL) |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from CHO cell supernatant in an animal-free facility |
| Purification | Protein A |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Namisaki H, Saito S, Hiraishi K, Haba T, Tanaka Y, Yoshida H, Iida S, Takahashi N (2020). "R409K mutation prevents acid-induced aggregation of human IgG4" PLoS One 15(3):e0229027.
PubMed
Human immunoglobulin G isotype 4 (IgG4) antibodies are suitable for use in either the antagonist or agonist format because their low effector functions prevent target cytotoxicity or unwanted cytokine secretion. However, while manufacturing therapeutic antibodies, they are exposed to low pH during purification, and IgG4 is more susceptible to low-pH-induced aggregation than IgG1. Therefore, we investigated the underlying mechanisms of IgG4 aggregation at low pH and engineered an IgG4 with enhanced stability. By swapping the constant regions of IgG1 and IgG4, we determined that the constant heavy chain (CH3) domain is critical for aggregate formation, but a core-hinge-stabilizing S228P mutation in IgG4 is insufficient for preventing aggregation. To identify the aggregation-prone amino acid, we substituted the CH3 domain of IgG4 with that of IgG1, changing IgG4 Arg409 to a Lys, thereby preventing the aggregation of the IgG4 variant as effectively as in IgG1. A stabilizing effect was also recorded with other variable-region variants. Analysis of thermal stability using differential scanning calorimetry revealed that the R409K substitution increased the Tm value of CH3, suggesting that the R409K mutation contributed to the structural strengthening of the CH3-CH3 interaction. The R409K mutation did not influence the binding to antigens/human Fcγ receptors; whereas, the concurrent S228P and R409K mutations in IgG4 suppressed Fab-arm exchange drastically and as effectively as in IgG1, in both in vitro and in vivo in mice models. Our findings suggest that the IgG4 R409K variant represents a potential therapeutic IgG for use in low-effector-activity format that exhibits increased stability.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Interferon-α and thymosin-α1 plus tislelizumab enhance CD8+ T cell cytotoxicity toward pancreatic ductal adenocarcinoma.
In iScience on 15 August 2025 by Deng, S., Deng, R., et al.
PubMed
The strong immunosuppression and immune evasion of pancreatic ductal adenocarcinoma (PDAC) result in poor efficacy of immune checkpoint blockade. In this study, the PD-1 level on CD8+ T cells in the peripheral blood of patients with PDAC was significantly greater than that in the peripheral blood of healthy individuals. To enhance the anticancer activity of adoptive CD8+ T cells toward PDAC, interferon-α (IFN-α) and thymosin-α1 (Tα1) plus tislelizumab were preclinically explored. Compared with those of tislelizumab monotherapy, the proliferation and cytokine secretion of CD8+ T cells and the cytotoxic activity toward PDAC cells were significantly greater with the combination treatment of IFN-α and Tα1 plus tislelizumab. Moreover, the growth of PDAC tumors was inhibited by CD8+ T cells with high efficacy under the combination treatment. Thus, IFN-α and Tα1 plus tislelizumab enhance the anticancer activity of CD8+ T cells toward PDAC, representing an alternative strategy for improving cancer immunotherapy.
-