Quality Control
Bio X Cell is committed to providing translational and preclinical researchers with the highest-quality in vivo research tools, including monoclonal, bispecific, and biosimilar antibodies, as well as associated buffers. All Bio X Cell products are rigorously tested for quality, ensuring consistent performance, outstanding reliability, and exceptional lot-to-lot consistency.
Headquartered in the United States, we have over 25 years of antibody manufacturing experience. With a global distribution network, including supply centers in China and Germany, Bio X Cell is able to scale up with research needs wherever a lab is based. We have over 25,000 product citations in peer-reviewed journals, allowing you to trust that antibodies from Bio X Cell deliver consistent performance, resulting in publication-quality data.
The Bio X Cell One-Year Shelf-Life Guarantee
We stand by the quality of our products and provide a no-hassle replacement or credit if an antibody does not perform as indicated on the datasheet. Please contact our technical support team or complete the Technical Service Request Form within 12 months of product receipt.
Precision and Care in Every Product
Each lot we produce is tested in various assays to ensure lot-to-lot consistency. Lot-specific quality control data is available upon request by contacting technical support.
Purity
Each lot of each antibody is checked for purity and integrity to ensure the purity level is ≥95%.
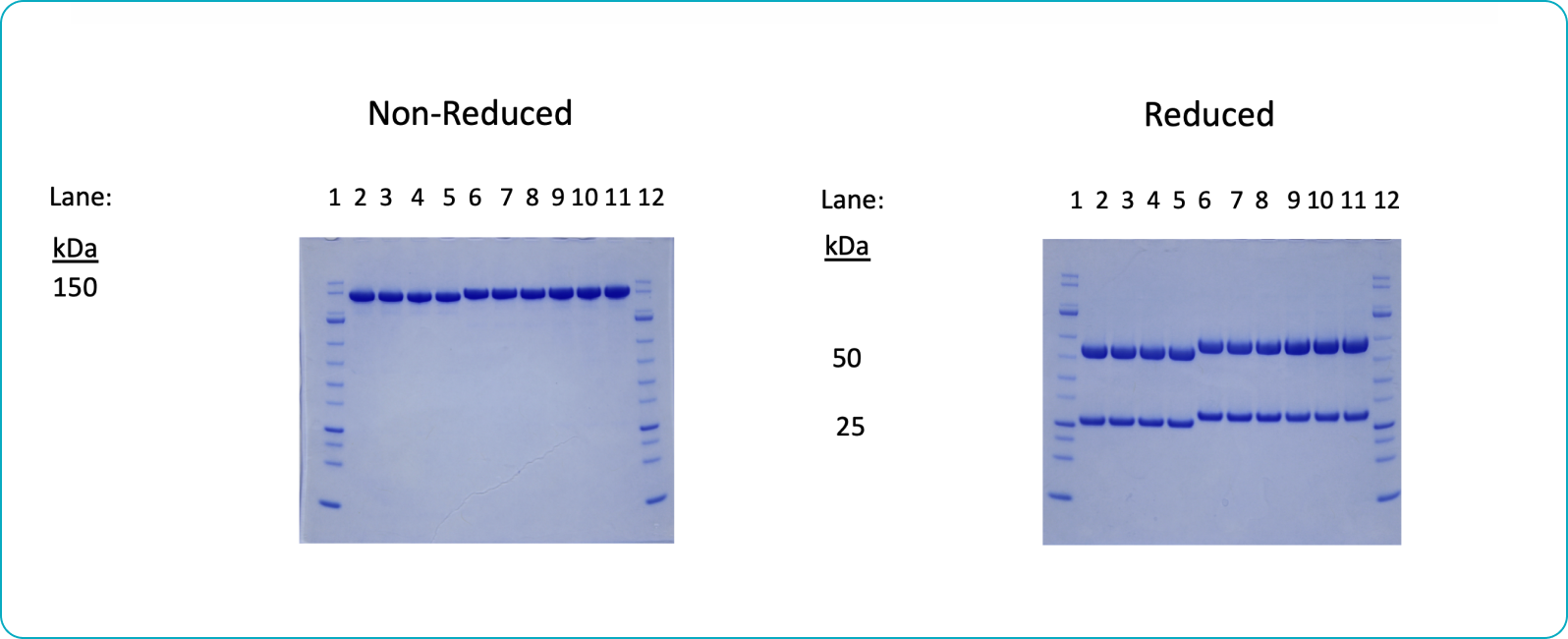
SDS-PAGE Antibody Purity Assessment Example
Binding Validation
Each lot of each applicable antibody is screened for binding specificity against its antigen and a negative control antigen via immunoblot. For select products, we also ensure binding using flow cytometry.
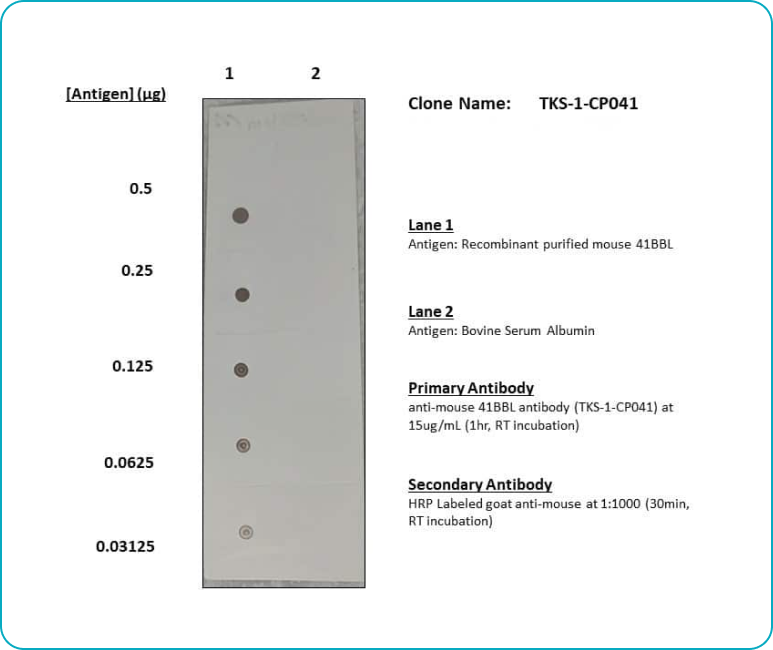
Immunoblot Antibody Binding Validation Example
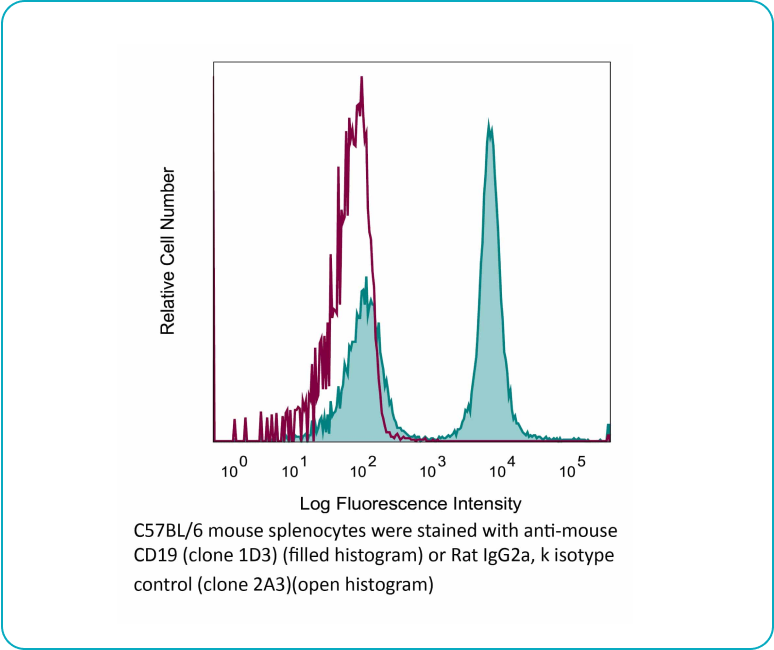
Flow cytometry Antibody Binding Validation Example
Antibody Aggregation Analysis
Each lot of each applicable antibody is analyzed using size exclusion chromatography (SEC) to determine the aggregate level and ensure a monomer content of ≥95%.
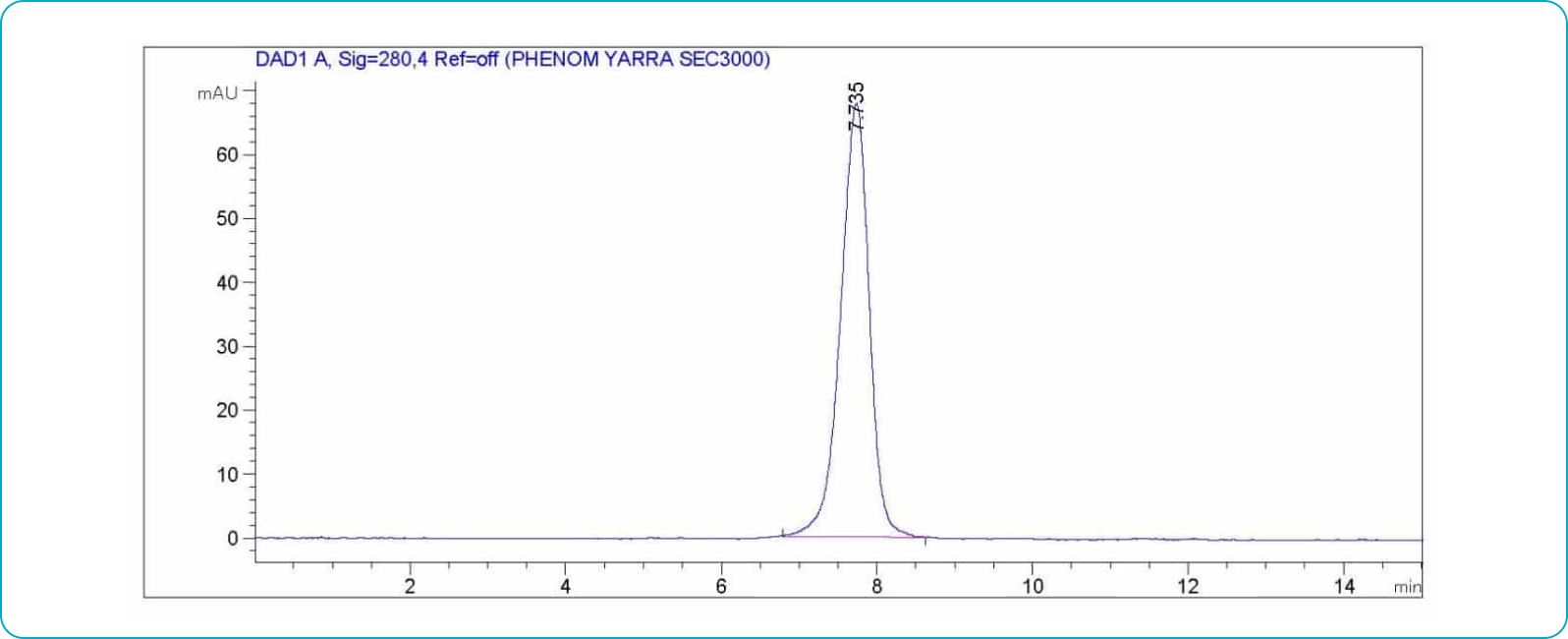
SEC Antibody Aggregation Assessment Example
Bio X Cell's Pathogen Testing Panel
Each lot of every applicable antibody is screened for an extensive panel of contaminants using a highly specific, ultra-sensitive real-time PCR-based assay.
Isotype Confirmation
Each antibody lot is isotyped using a rapid lateral flow antibody isotyping assay, ensuring that the antibody is the correct host species, isotype class, subtype class, and light-chain identity.
Endotoxin Testing
Each lot of every antibody is screened and ensured to be ultra-low in bacterial endotoxin using the Limulus Amebocyte Lysate (LAL) gel clotting assay.
Detailed Breakdown of Quality Control Assays Included for each Product Line
Meet Our Leaders
Learn more about the team behind the gold standard in vivo antibodies.
Discover Bio X Cell
Learn more about our proven expertise and comprehensive antibody solutions.
See Our Impact
Discover the Bio X Cell Fund’s mission to improve the health of our community
Explore Our Latest
See our latest articles and whitepapers for scientific insights and ideas
Consult With Bio X Cell to Enable Your Next Breakthrough Discovery
Whether you need antibody customization or high-volume production, Bio X Cell is committed to advancing your therapeutic innovations.
Contact Us