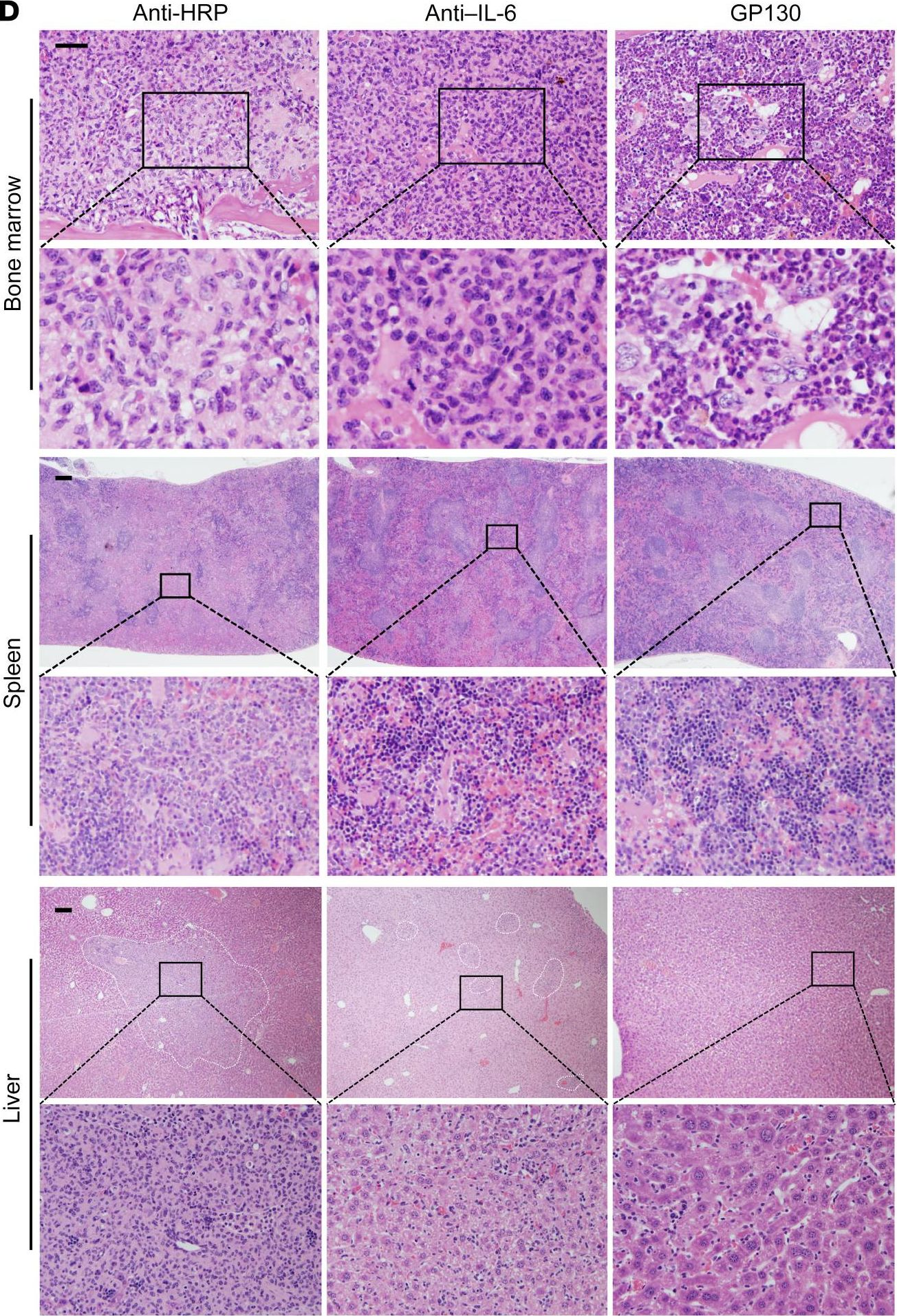
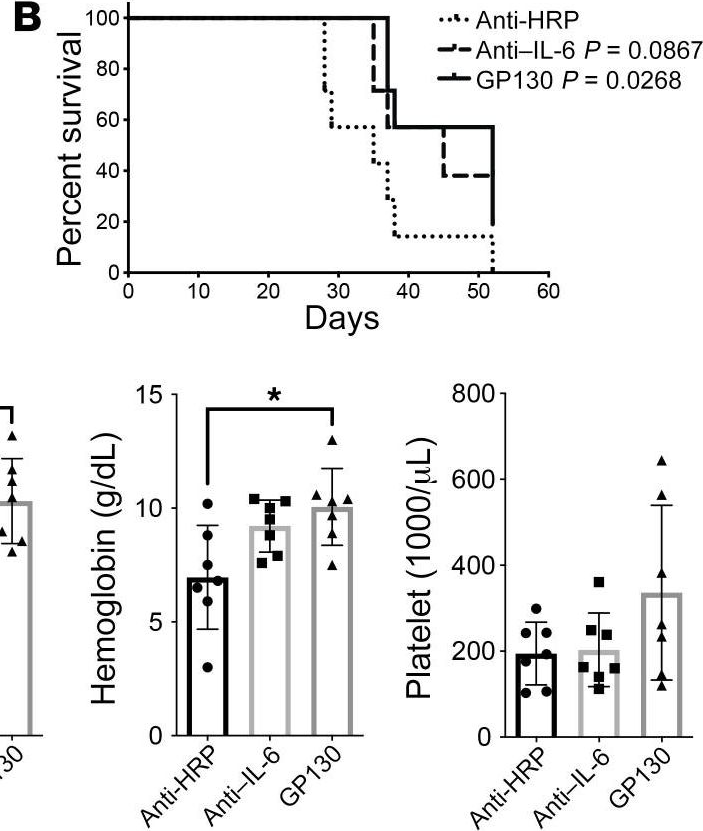
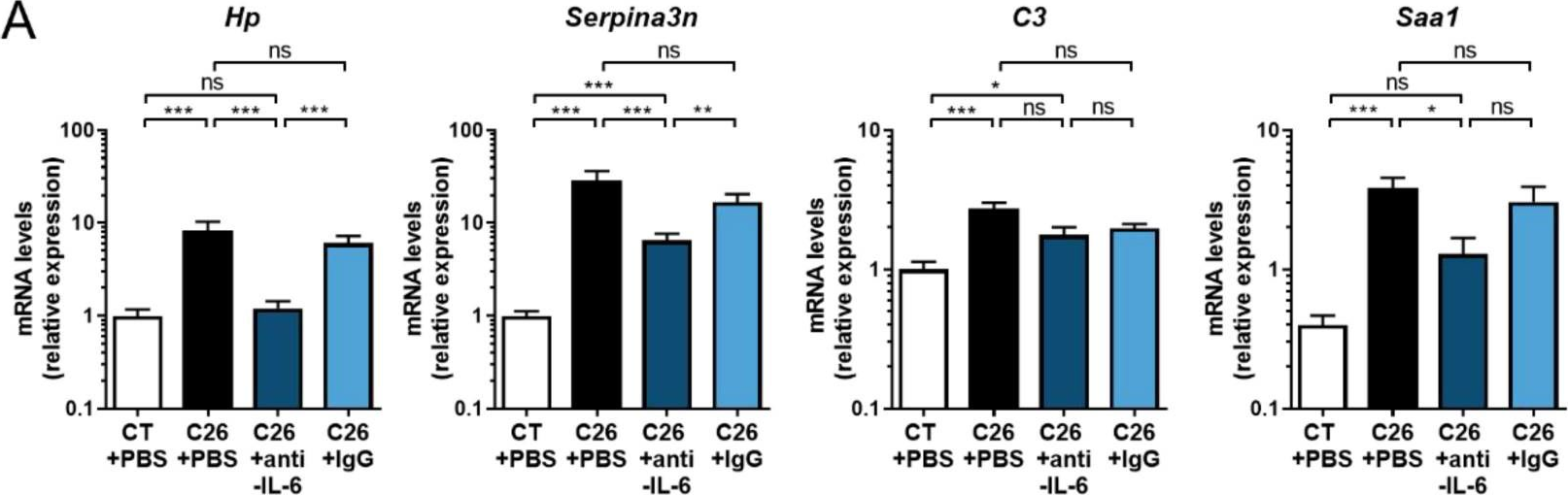
InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107775 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Goschl, L., et al (2018). "A T cell-specific deletion of HDAC1 protects against experimental autoimmune encephalomyelitis" J Autoimmun 86: 51-61.
PubMed
Multiple sclerosis (MS) is a human neurodegenerative disease characterized by the invasion of autoreactive T cells from the periphery into the CNS. Application of pan-histone deacetylase inhibitors (HDACi) ameliorates experimental autoimmune encephalomyelitis (EAE), an animal model for MS, suggesting that HDACi might be a potential therapeutic strategy for MS. However, the function of individual HDAC members in the pathogenesis of EAE is not known. In this study we report that mice with a T cell-specific deletion of HDAC1 (using the Cd4-Cre deleter strain; HDAC1-cKO) were completely resistant to EAE despite the ability of HDAC1cKO CD4(+) T cells to differentiate into Th17 cells. RNA sequencing revealed STAT1 as a prominent upstream regulator of differentially expressed genes in activated HDAC1-cKO CD4(+) T cells and this was accompanied by a strong increase in phosphorylated STAT1 (pSTAT1). This suggests that HDAC1 controls STAT1 activity in activated CD4(+) T cells. Increased pSTAT1 levels correlated with a reduced expression of the chemokine receptors Ccr4 and Ccr6, which are important for the migration of T cells into the CNS. Finally, EAE susceptibility was restored in WT:HDAC1-cKO mixed BM chimeric mice, indicating a cell-autonomous defect. Our data demonstrate a novel pathophysiological role for HDAC1 in EAE and provide evidence that selective inhibition of HDAC1 might be a promising strategy for the treatment of MS.
Clemente-Casares, X., et al (2016). "Expanding antigen-specific regulatory networks to treat autoimmunity" Nature 530(7591): 434-440.
PubMed
Regulatory T cells hold promise as targets for therapeutic intervention in autoimmunity, but approaches capable of expanding antigen-specific regulatory T cells in vivo are currently not available. Here we show that systemic delivery of nanoparticles coated with autoimmune-disease-relevant peptides bound to major histocompatibility complex class II (pMHCII) molecules triggers the generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in different mouse models, including mice humanized with lymphocytes from patients, leading to resolution of established autoimmune phenomena. Ten pMHCII-based nanomedicines show similar biological effects, regardless of genetic background, prevalence of the cognate T-cell population or MHC restriction. These nanomedicines promote the differentiation of disease-primed autoreactive T cells into TR1-like cells, which in turn suppress autoantigen-loaded antigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells, without compromising systemic immunity. pMHCII-based nanomedicines thus represent a new class of drugs, potentially useful for treating a broad spectrum of autoimmune conditions in a disease-specific manner.
Sell, S., et al (2015). "Control of murine cytomegalovirus infection by gammadelta T cells" PLoS Pathog 11(2): e1004481.
PubMed
Infections with cytomegalovirus (CMV) can cause severe disease in immunosuppressed patients and infected newborns. Innate as well as cellular and humoral adaptive immune effector functions contribute to the control of CMV in immunocompetent individuals. None of the innate or adaptive immune functions are essential for virus control, however. Expansion of gammadelta T cells has been observed during human CMV (HCMV) infection in the fetus and in transplant patients with HCMV reactivation but the protective function of gammadelta T cells under these conditions remains unclear. Here we show for murine CMV (MCMV) infections that mice that lack CD8 and CD4 alphabeta-T cells as well as B lymphocytes can control a MCMV infection that is lethal in RAG-1(-/-) mice lacking any T- and B-cells. gammadelta T cells, isolated from infected mice can kill MCMV infected target cells in vitro and, importantly, provide long-term protection in infected RAG-1(-/-) mice after adoptive transfer. gammadelta T cells in MCMV infected hosts undergo a prominent and long-lasting phenotypic change most compatible with the view that the majority of the gammadelta T cell population persists in an effector/memory state even after resolution of the acute phase of the infection. A clonotypically focused Vgamma1 and Vgamma2 repertoire was observed at later stages of the infection in the organs where MCMV persists. These findings add gammadelta T cells as yet another protective component to the anti-CMV immune response. Our data provide clear evidence that gammadelta T cells can provide an effective control mechanism of acute CMV infections, particularly when conventional adaptive immune mechanisms are insufficient or absent, like in transplant patient or in the developing immune system in utero. The findings have implications in the stem cell transplant setting, as antigen recognition by gammadelta T cells is not MHC-restricted and dual reactivity against CMV and tumors has been described.
Grinberg-Bleyer, Y., et al (2015). "Cutting edge: NF-kappaB p65 and c-Rel control epidermal development and immune homeostasis in the skin" J Immunol 194(6): 2472-2476.
PubMed
Psoriasis is an inflammatory skin disease in which activated immune cells and the proinflammatory cytokine TNF are well-known mediators of pathogenesis. The transcription factor NF-kappaB is a key regulator of TNF production and TNF-induced proinflammatory gene expression, and both the psoriatic transcriptome and genetic susceptibility further implicate NF-kappaB in psoriasis etiopathology. However, the role of NF-kappaB in psoriasis remains controversial. We analyzed the function of canonical NF-kappaB in the epidermis using CRE-mediated deletion of p65 and c-Rel in keratinocytes. In contrast to animals lacking p65 or c-Rel alone, mice lacking both subunits developed severe dermatitis after birth. Consistent with its partial histological similarity to human psoriasis, this condition could be prevented by anti-TNF treatment. Moreover, regulatory T cells in lesional skin played an important role in disease remission. Our results demonstrate that canonical NF-kappaB in keratinocytes is essential for the maintenance of skin immune homeostasis and is protective against spontaneous dermatitis.
Park, H. J., et al (2015). "PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells" J Immunol 194(12): 5801-5811.
PubMed
Regulatory T (Treg) cells act as terminators of T cell immuniy during acute phase of viral infection; however, their role and suppressive mechanism in chronic viral infection are not completely understood. In this study, we compared the phenotype and function of Treg cells during acute or chronic infection with lymphocytic choriomeningitis virus. Chronic infection, unlike acute infection, led to a large expansion of Treg cells and their upregulation of programmed death-1 (PD-1). Treg cells from chronically infected mice (chronic Treg cells) displayed greater suppressive capacity for inhibiting both CD8(+) and CD4(+) T cell proliferation and subsequent cytokine production than those from naive or acutely infected mice. A contact between Treg and CD8(+) T cells was necessary for the potent suppression of CD8(+) T cell immune response. More importantly, the suppression required cell-specific expression and interaction of PD-1 on chronic Treg cells and PD-1 ligand on CD8(+) T cells. Our study defines PD-1 upregulated on Treg cells and its interaction with PD-1 ligand on effector T cells as one cause for the potent T cell suppression and proposes the role of PD-1 on Treg cells, in addition to that on exhausted T cells, during chronic viral infection.
Ellis, G. T., et al (2015). "TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection" EMBO Rep 16(9): 1203-1218.
PubMed
Streptococcus pneumoniae coinfection is a major cause of influenza-associated mortality; however, the mechanisms underlying pathogenesis or protection remain unclear. Using a clinically relevant mouse model, we identify immune-mediated damage early during coinfection as a new mechanism causing susceptibility. Coinfected CCR2(-/-) mice lacking monocytes and monocyte-derived cells control bacterial invasion better, show reduced epithelial damage and are overall more resistant than wild-type controls. In influenza-infected wild-type lungs, monocytes and monocyte-derived cells are the major cell populations expressing the apoptosis-inducing ligand TRAIL. Accordingly, anti-TRAIL treatment reduces bacterial load and protects against coinfection if administered during viral infection, but not following bacterial exposure. Post-influenza bacterial outgrowth induces a strong proinflammatory cytokine response and massive inflammatory cell infiltrate. Depletion of neutrophils or blockade of TNF-alpha facilitate bacterial outgrowth, leading to increased mortality, demonstrating that these factors aid bacterial control. We conclude that inflammatory monocytes recruited early, during the viral phase of coinfection, induce TRAIL-mediated lung damage, which facilitates bacterial invasion, while TNF-alpha and neutrophil responses help control subsequent bacterial outgrowth. We thus identify novel determinants of protection versus pathology in influenza-Streptococcus pneumoniae coinfection.
Meisen, W. H., et al (2015). "The Impact of Macrophage- and Microglia-Secreted TNFalpha on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment" Clin Cancer Res 21(14): 3274-3285.
PubMed
PURPOSE: Oncolytic herpes simplex viruses (oHSV) represent a promising therapy for glioblastoma (GBM), but their clinical success has been limited. Early innate immune responses to viral infection reduce oHSV replication, tumor destruction, and efficacy. Here, we characterized the antiviral effects of macrophages and microglia on viral therapy for GBM. EXPERIMENTAL DESIGN: Quantitative flow cytometry of mice with intracranial gliomas (+/-oHSV) was used to examine macrophage/microglia infiltration and activation. In vitro coculture assays of infected glioma cells with microglia/macrophages were used to test their impact on oHSV replication. Macrophages from TNFalpha-knockout mice and blocking antibodies were used to evaluate the biologic effects of TNFalpha on virus replication. TNFalpha blocking antibodies were used to evaluate the impact of TNFalpha on oHSV therapy in vivo. RESULTS: Flow-cytometry analysis revealed a 7.9-fold increase in macrophage infiltration after virus treatment. Tumor-infiltrating macrophages/microglia were polarized toward a M1, proinflammatory phenotype, and they expressed high levels of CD86, MHCII, and Ly6C. Macrophages/microglia produced significant amounts of TNFalpha in response to infected glioma cells in vitro and in vivo. Using TNFalpha-blocking antibodies and macrophages derived from TNFalpha-knockout mice, we discovered TNFalpha-induced apoptosis in infected tumor cells and inhibited virus replication. Finally, we demonstrated the transient blockade of TNFalpha from the tumor microenvironment with TNFalpha-blocking antibodies significantly enhanced virus replication and survival in GBM intracranial tumors. CONCLUSIONS: The results of these studies suggest that FDA approved TNFalpha inhibitors may significantly improve the efficacy of oncolytic virus therapy.
Beug, S. T., et al (2014). "Smac mimetics and innate immune stimuli synergize to promote tumor death" Nat Biotechnol 32(2): 182-190.
PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
DeBerge, M. P., et al (2014). "Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology" J Immunol 192(12): 5839-5851.
PubMed
TNF-alpha is a pleotropic cytokine that has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-alpha is first expressed as a transmembrane protein that is proteolytically processed to release a soluble form. Transmembrane TNF-alpha (memTNF-alpha) and soluble TNF-alpha (solTNF-alpha) have been shown to exert distinct tissue-protective or tissue-pathologic effects in several disease models. However, the relative contributions of memTNF-alpha or solTNF-alpha in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing noncleavable memTNF-alpha or lacking TNF-alpha entirely and examined the outcomes. We found that solTNF-alpha, but not memTNF-alpha, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-alpha, there was a significant increase in the CD8(+) T cell response, including virus-specific CD8(+) T cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-alpha mediates these immunoregulatory effects primarily through TNFR1, because mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-alpha. We also found that solTNF-alpha expression was required early during infection to regulate the magnitude of the CD8(+) T cell response, indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-alpha to release solTNF-alpha is a critical event regulating the immune response during influenza infection.
Walsh, K. B., et al (2014). "Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy" J Virol 88(11): 6281-6293.
PubMed
The cytokine storm is an intensified, dysregulated, tissue-injurious inflammatory response driven by cytokine and immune cell components. The cytokine storm during influenza virus infection, whereby the amplified innate immune response is primarily responsible for pulmonary damage, has been well characterized. Now we describe a novel event where virus-specific T cells induce a cytokine storm. The paramyxovirus pneumonia virus of mice (PVM) is a model of human respiratory syncytial virus (hRSV). Unexpectedly, when C57BL/6 mice were infected with PVM, the innate inflammatory response was undetectable until day 5 postinfection, at which time CD8(+) T cells infiltrated into the lung, initiating a cytokine storm by their production of gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha). Administration of an immunomodulatory sphingosine-1-phosphate (S1P) receptor 1 (S1P1R) agonist significantly inhibited PVM-elicited cytokine storm by blunting the PVM-specific CD8(+) T cell response, resulting in diminished pulmonary disease and enhanced survival. IMPORTANCE: A dysregulated overly exuberant immune response, termed a “cytokine storm,” accompanies virus-induced acute respiratory diseases (VARV), is primarily responsible for the accompanying high morbidity and mortality, and can be controlled therapeutically in influenza virus infection of mice and ferrets by administration of sphingosine-1-phosphate 1 receptor (S1P1R) agonists. Here, two novel findings are recorded. First, in contrast to influenza infection, where the cytokine storm is initiated early by the innate immune system, for pneumonia virus of mice (PVM), a model of RSV, the cytokine storm is initiated late in infection by the adaptive immune response: specifically, by virus-specific CD8 T cells via their release of IFN-gamma and TNF-alpha. Blockading these cytokines with neutralizing antibodies blunts the cytokine storm and protects the host. Second, PVM infection is controlled by administration of an S1P1R agonist.
Perng, O. A., et al (2014). "The degree of CD4+ T cell autoreactivity determines cellular pathways underlying inflammatory arthritis" J Immunol 192(7): 3043-3056.
PubMed
Although therapies targeting distinct cellular pathways (e.g., anticytokine versus anti-B cell therapy) have been found to be an effective strategy for at least some patients with inflammatory arthritis, the mechanisms that determine which pathways promote arthritis development are poorly understood. We have used a transgenic mouse model to examine how variations in the CD4(+) T cell response to a surrogate self-peptide can affect the cellular pathways that are required for arthritis development. CD4(+) T cells that are highly reactive with the self-peptide induce inflammatory arthritis that affects male and female mice equally. Arthritis develops by a B cell-independent mechanism, although it can be suppressed by an anti-TNF treatment, which prevented the accumulation of effector CD4(+) Th17 cells in the joints of treated mice. By contrast, arthritis develops with a significant female bias in the context of a more weakly autoreactive CD4(+) T cell response, and B cells play a prominent role in disease pathogenesis. In this setting of lower CD4(+) T cell autoreactivity, B cells promote the formation of autoreactive CD4(+) effector T cells (including Th17 cells), and IL-17 is required for arthritis development. These studies show that the degree of CD4(+) T cell reactivity for a self-peptide can play a prominent role in determining whether distinct cellular pathways can be targeted to prevent the development of inflammatory arthritis.
Weinlich, R., et al (2013). "Protective roles for caspase-8 and cFLIP in adult homeostasis" Cell Rep 5(2): 340-348.
PubMed
Caspase-8 or cellular FLICE-like inhibitor protein (cFLIP) deficiency leads to embryonic lethality in mice due to defects in endothelial tissues. Caspase-8(-/-) and receptor-interacting protein kinase-3 (RIPK3)(-/-), but not cFLIP(-/-) and RIPK3(-/-), double-knockout animals develop normally, indicating that caspase-8 antagonizes the lethal effects of RIPK3 during development. Here, we show that the acute deletion of caspase-8 in the gut of adult mice induces enterocyte death, disruption of tissue homeostasis, and inflammation, resulting in sepsis and mortality. Likewise, acute deletion of caspase-8 in a focal region of the skin induces local keratinocyte death, tissue disruption, and inflammation. Strikingly, RIPK3 ablation rescues both phenotypes. However, acute loss of cFLIP in the skin produces a similar phenotype that is not rescued by RIPK3 ablation. TNF neutralization protects from either acute loss of caspase-8 or cFLIP. These results demonstrate that caspase-8-mediated suppression of RIPK3-induced death is required not only during development but also for adult homeostasis. Furthermore, RIPK3-dependent inflammation is dispensable for the skin phenotype.
Mohr, E., et al (2010). "IFN-{gamma} produced by CD8 T cells induces T-bet-dependent and -independent class switching in B cells in responses to alum-precipitated protein vaccine" Proc Natl Acad Sci U S A 107(40): 17292-17297.
PubMed
Alum-precipitated protein (alum protein) vaccines elicit long-lasting neutralizing antibody responses that prevent bacterial exotoxins and viruses from entering cells. Typically, these vaccines induce CD4 T cells to become T helper 2 (Th2) cells that induce Ig class switching to IgG1. We now report that CD8 T cells also respond to alum proteins, proliferating extensively and producing IFN-gamma, a key Th1 cytokine. These findings led us to question whether adoptive transfer of antigen-specific CD8 T cells alters the characteristic CD4 Th2 response to alum proteins and the switching pattern in responding B cells. To this end, WT mice given transgenic ovalbumin (OVA)-specific CD4 (OTII) or CD8 (OTI) T cells, or both, were immunized with alum-precipitated OVA. Cotransfer of antigen-specific CD8 T cells skewed switching patterns in responding B cells from IgG1 to IgG2a and IgG2b. Blocking with anti-IFN-gamma antibody largely inhibited this altered B-cell switching pattern. The transcription factor T-bet is required in B cells for IFN-gamma-dependent switching to IgG2a. By contrast, we show that this transcription factor is dispensable in B cells both for IFN-gamma-induced switching to IgG2b and for inhibition of switching to IgG1. Thus, T-bet dependence identifies distinct transcriptional pathways in B cells that regulate IFN-gamma-induced switching to different IgG isotypes.
Product Citations
-
-
Immunology and Microbiology
-
Genetics
mRNA vaccine expressing enterovirus D68 virus-like particles induces potent neutralizing antibodies and protects against infection.
In Mol Ther Nucleic Acids on 9 December 2025 by Kunishima, Y., Senpuku, K., et al.
PubMed
Enterovirus D68 (EV-D68) causes respiratory illness in children. It also causes severe paralysis called acute flaccid myelitis (AFM), which has become a global health threat. Here, we generated an mRNA vaccine expressing virus-like particles (VLPs) of EV-D68. We found that the mRNA vaccine elicited potent neutralizing antibodies against EV-D68 in the blood, and the neutralizing titer was superior to that of the inactivated whole virion (IWV) vaccine. The mRNA vaccine showed protective effects against intranasal challenge with EV-D68, and antisera from the vaccinated mice prevented the paralysis caused by EV-D68 infection in neonatal mice. Moreover, the mRNA vaccine induced neutralizing antibodies in the respiratory tract, which is the entry site for EV-D68. Additionally, it attenuated infection with coxsackievirus B3 (CVB3), which belongs to another enterovirus group, via CD8+ T cell responses. In conclusion, our results suggest that this mRNA vaccine is a promising candidate for EV-D68 prevention.
-
-
-
Immunology and Microbiology
-
Cancer Research
CBX6 induces CD8+ T cell exhaustion and tumor development in esophageal squamous cell carcinoma through SMARCD1-mediated CCL8 secretion and lactate efflux.
In Cell Biol Toxicol on 12 November 2025 by Wang, L., Liu, G., et al.
PubMed
This study investigates the functions of chromobox 6 (CBX6) in esophageal squamous cell carcinoma (ESCC) and delves into its functional mechanisms. The bioinformatics insights suggested that CBX6 was overexpressed in ESCC and linked to dismal prognosis. Cbx6 knockdown was induced in mouse mEC25 cells. This procedure curbed the proliferation and migration of mEC25 cells and reduced exhaustion of the co-cultured CD8+ T cells. In vivo, Cbx6 knockdown in mEC25 cells reduced tumorigenesis while enhancing immune activity in mice. Further experiments showed that CBX6 reduced CD8+ T cell cytotoxicity by secreting C-C motif chemokine ligand 8 (CCL8) and promoting monocarboxylate transporter 4 (MCT4)-mediated lactate transport. Regarding the mechanism, CBX6 regulated the expression of SWI/SNF related BAF chromatin remodeling complex subunit D1 (Smarcd1) to modulate chromatin remodeling, thus promoting transcription of Ccl8 and Slc16a3 (encoding MCT4). Smarcd1 overexpression restored metabolic activity in mEC25 cells, reduced activity of co-cultured CD8+ T cells, and promoted tumorigenesis in vivo. Tissue microarrays analysis suggested that CBX6 and SMARCD1 were linked to immunosuppression and poor prognosis in clinical samples. In conclusion, this study suggests that CBX6 induces CD8+ T cell exhaustion and tumor development in ESCC through SMARCD1-mediated CCL8 secretion and lactate efflux.
-
-
-
Cancer Research
-
Immunology and Microbiology
VEGFR2 blockade converts thermally ablative focused ultrasound into a potent driver of T cell-dependent anti-tumor immunity
In bioRxiv on 24 October 2025 by Schwartz, M. R., Anwar, N. Z., et al.
-
-
-
Immunology and Microbiology
-
Neuroscience
IL-6 Inhibition Partially Ameliorates Maternal Immune Activation-Induced Autism-Like Behavioral Abnormalities in Mice.
In Curr Issues Mol Biol on 16 October 2025 by Zhang, X., Luo, W., et al.
PubMed
Prenatal maternal immune activation (MIA) has been implicated in autism spectrum disorder (ASD) pathogenesis, with interleukin-6 (IL-6) identified as a key inflammatory mediator. We investigated the therapeutic potential of IL-6 inhibition in an MIA mouse model induced by Toxoplasma gondii soluble tachyzoite antigen (STAg). Adult MIA offspring received systemic administration of the IL-6-neutralizing antibody (MP5-20F3) or isotype control, followed by behavioral assessments one week later. Open field and elevated plus maze tests revealed heightened anxiety-like behaviors in the STAg offspring, which were largely reversed by IL-6 inhibition. Reciprocal social interaction tests showed diminished sociability in the STAg offspring, which was partially restored by IL-6 inhibition. However, core ASD-like features, including impaired social preference and recognition in the three-chamber test, as well as increased repetitive behaviors, remained resistant to IL-6 inhibition. These findings demonstrate that STAg-induced MIA elicits anxiety-like and ASD-like phenotypes in adult offspring, with IL-6 playing an important role in anxiety-like behaviors and social interaction deficits. Systemic IL-6 inhibition partially ameliorates behavioral abnormalities. This study suggests that IL-6-targeted therapies may address a subset of ASD-related symptoms, and comprehensive strategies are needed for broader efficacy.
-
-
-
Cancer Research
-
Flow cytometry/Cell sorting
Single-cell profiling of ERBB family receptors identifies ERBB3 as a key regulator in head and neck squamous cell carcinoma progression.
In Discov Oncol on 9 October 2025 by Luo, Y., Li, Y., et al.
PubMed
The ERBB receptor family is widely implicated in epithelial malignancies, yet the functional and immunological significance of ERBB3 in head and neck squamous cell carcinoma (HNSCC) remains insufficiently characterized. In this study, we employed single-cell and bulk transcriptomic analyses to comprehensively map the expression patterns and prognostic value of ERBB family members in HNSCC, identifying ERBB3 as a tumor-specific marker predominantly enriched in malignant epithelial clusters. Notably, high ERBB3 expression was paradoxically associated with favorable overall survival, prompting further mechanistic investigation. Functional assays in SCC9 cells demonstrated that ERBB3 promotes proliferation, colony formation, and invasion. Immune profiling revealed that ERBB3-high tumors displayed enhanced communication with B cell subsets, particularly involving immunosuppressive signals such as TNFRSF13B and IL4R. Flow cytometry analysis in a 4NQO-induced mouse model showed that ERBB3 inhibition reduced CCDC50⁺ B cells while restoring MHC-II expression, indicating a shift toward immune activation. These findings highlight a dual role of ERBB3 in HNSCC, acting both as an oncogenic contributor and an immune-modulatory regulator, and position ERBB3 as a promising context-dependent therapeutic target.
-
-
Synergistic exacerbation of oral mucositis caused by IL-23 deficiency and oral Candida albicans exposure.
In MBio on 8 October 2025 by Dillon, J. T., Hickey, M. T., et al.
PubMed
Targeted head and neck irradiation (HNI) used for cancer therapy causes mucosal damage and immune dysregulation, leaving cancer patients highly susceptible to infections of the oral mucosa. Oropharyngeal candidiasis (OPC) is an opportunistic infection caused by Candida albicans, a commensal fungus found in up to 80% of the population at any given time. High colonization levels of C. albicans are known to worsen damage caused by HNI. The interleukin-23 (IL-23)-T-helper 17 (Th17) axis is a central and non-redundant mediator of immunity to OPC, and anti-cytokine biologics targeting IL-23 have come into widespread clinical use for treating various autoimmune conditions. Here, we sought to understand the consequences of IL-23 deficiency in the setting of HNI, taking advantage of a mouse model of radiation-induced oral mucositis (OM) which we combined with fungal infection. Surprisingly, mice lacking IL-23 did not show increased signs of injury to the oral mucosa when subjected to HNI. However, in mice subjected to HNI and exposed to oral C. albicans, damage to the oral mucosa was markedly exacerbated, accompanied by substantially increased fungal susceptibility when IL-23 was absent. Thus, IL-23-driven control of fungal infections is needed to mitigate susceptibility to OM in the high proportion of individuals who carry C. albicans in the mouth.IMPORTANCEIL-23 plays key roles in expanding the T-helper 17 (Th17) cell subset during differentiation and promoting expression of cytokines, including the subset-defining cytokine IL-17 (IL-17A). While the effector cytokines produced by Th17 cells are required for host defense against extracellular microbes, especially Candida albicans, these can also be pathogenic during inflammatory and autoimmune disorders including psoriasis, psoriatic arthritis, and ankylosing spondylitis, among others. While IL-23 and IL-17 are similarly required for protection against oropharyngeal candidiasis (OPC), they can exert divergent functions in other forms of immune-mediated inflammation; for example, anti-IL-17 blockade or Il17ra gene deficiency is linked to inflammatory bowel disease, whereas loss of IL-23 is protective in this setting. We previously showed that head and neck irradiation (HNI) induces IL-17 expression in oral tissue and that IL-17 receptor (IL-17R) signaling is required for resistance against mucosal damage and OPC. In contrast, we show here that loss of IL-23 does not affect oral mucosal injury caused by HNI. However, lack of IL-23 magnifies HNI-induced susceptibility to OPC due to insufficient levels of antimicrobial peptides. Clinically, these findings suggest that patients receiving treatments that target IL-23 may not need to discontinue therapy should they require HNI, but that screening for oral C. albicans may be useful to help limit the risk of developing severe OM and its attendant adverse events.
-
-
Immunology and Microbiology
TIM-3 ameliorates host responses to Salmonella infection by controlling iron driven CD4+ T cell differentiation and interleukin-10 formation.
In EBioMedicine on 1 October 2025 by Pfeifhofer-Obermair, C., Brigo, N., et al.
PubMed
Iron loading increases infection risk in being a nutrient for invading siderophilic bacteria and by modulating immune functions including the expression of the immune checkpoint regulator T-cell immunoglobulin-and-mucin-containing-domain-3 (TIM-3). TIM-3 affects specific immune cell functions including T-helper cell differentiation but also T cell dysfunction, and immune exhaustion. Given the prevalence of iron overload specifically in patients at higher risk for infection such as those suffering from hemo-oncological diseases, we investigated TIM-3's role in immune control of bacterial sepsis.
-
-
TCF1 and LEF1 promote B-1a cell homeostasis and regulatory function.
In Nature on 1 October 2025 by Shen, Q., Wang, H., et al.
PubMed
B-1 cells are innate-like immune cells abundant in serosal cavities with antibodies enriched in bacterial recognition, yet their existence in humans has been controversial1-3. The CD5+ B-1a subset expresses anti-inflammatory molecules including IL-10, PDL1 and CTLA4 and can be immunoregulatory4-6. Unlike conventional B cells that are continuously replenished, B-1a cells are produced early in life and maintained through self-renewal7. Here we show that the transcription factors TCF1 and LEF1 are critical regulators of B-1a cells. LEF1 expression is highest in fetal and bone marrow B-1 progenitors, whereas the levels of TCF1 are higher in splenic and peritoneal B-1 cells than in B-1 progenitors. TCF1-LEF1 double deficient mice have reduced B-1a cells and defective B-1a cell maintenance. These transcription factors promote MYC-dependent metabolic pathways and induce a stem-like population upon activation, partly via IL-10 production. In the absence of TCF1 and LEF1, B-1 cells proliferate excessively and acquire an exhausted phenotype with reduced IL-10 and PDL1 expression. Furthermore, adoptive transfer of B-1 cells lacking TCF1 and LEF1 fails to suppress brain inflammation. These transcription factors are also expressed in human chronic lymphocytic leukaemia B cells and in a B-1-like population that is abundant in pleural fluid and circulation of some patients with pleural infection. Our findings define a TCF1-LEF1-driven transcriptional program that integrates stemness and regulatory function in B-1a cells.
-
-
Cancer Research
Early adipose tissue wasting in a preclinical model of human lung cancer cachexia.
In Cell Rep on 23 September 2025 by Snoke, D. B., van der Velden, J. L., et al.
PubMed
Cancer cachexia (CC), a syndrome of skeletal muscle and adipose wasting, reduces responsiveness to therapies and increases mortality. There are no approved treatments for CC, which may relate to discordance between preclinical models and human CC. To address the need for clinically relevant models of lung CC, we generated inducible, lung epithelial cell-specific KrasG12D/+ (G12D) mice. G12D mice develop CC over a protracted time course and phenocopy tissue and tumor, cellular, mutational, transcriptomic, and metabolic characteristics of human lung CC. G12D mice demonstrate early loss of adipose, a phenotype that was apparent across numerous models of CC and translates to patients with lung cancer. Tumor-released factors promote adipocyte lipolysis, a driver of adipose wasting in CC, and adipose wasting was inversely related to tumor burden. Thus, G12D mice model key features of human lung CC and highlight a role for early tumor metabolic reprogramming of adipose tissue in CC.
-
-
Macrophages orchestrate elimination of Shigella from the intestinal epithelial cell niche via TLR-induced IL-12 and IFN-γ.
In Cell Host Microbe on 10 September 2025 by Eislmayr, K. D., Nichols, C. A., et al.
PubMed
Bacteria of the genus Shigella replicate in intestinal epithelial cells and cause shigellosis, a severe diarrheal disease that resolves spontaneously in most healthy individuals. During shigellosis, neutrophils are abundantly recruited to the gut and have long been thought to be central to Shigella control and pathogenesis. However, how shigellosis resolves remains poorly understood due to the longstanding lack of a tractable and physiological animal model. Here, using our newly developed Nlrc4-/-Casp11-/- mouse model of shigellosis, we unexpectedly find no major role for neutrophils in limiting Shigella or in disease pathogenesis. Instead, we uncover an essential role for macrophages in the host control of Shigella. Macrophages respond to Shigella via Toll-like receptors (TLRs) to produce IL-12, which then induces IFN-γ, a cytokine that is essential to control Shigella replication in intestinal epithelial cells. Collectively, our findings reshape our understanding of the innate immune response to Shigella.
-
-
Pathology
-
Immunology and Microbiology
Interleukin-10 limits immune-mediated pathology in chronic subclinical plasmodial infection.
In PLoS Negl Trop Dis on 1 September 2025 by Silva, L. S., Monks, B. G., et al.
PubMed
Subclinical parasitemia constitutes the predominant proportion of Plasmodium spp. infections in hyperendemic regions of the world. Elevated levels of serum interleukin-10 (IL-10) are observed in both acute symptomatic and chronic subclinical Plasmodium spp. infections. The role of IL-10 in acute infection has been extensively studied; however, the role of sustained elevated levels of IL-10 in chronic subclinical plasmodial infections remains to be determined. We investigated the role of IL-10 in a long-term subclinical and patent Plasmodium chabaudi chabaudi-AS (Pc) infection using mice lacking humoral immunity (µMT-/- mice). Pc-infected µMT-/- mice exhibit a long-term (99 days) chronic infection, with microscopic levels of parasitemia and without any outward signs of disease. We found that chronically infected mice have slightly elevated levels of tumor necrosis factor α (TNFα) and interferon-γ (IFNγ), and high levels of IL-10 in the circulation. The source of IL-10 was CD4+ T cells. We found that elevated IL-10 levels were mechanistically linked to subclinical Plasmodium infection by blocking IL-10 signaling. Anti-IL-10R resulted in a marked, albeit transient, reduction of the parasitemia that was accompanied by a robust pro-inflammatory response and death of chronically infected µMT-/- mice. A similar outcome was observed in infected µMT-/- mice after CD4+ T cell depletion with anti-CD4 antibody. CD4-depleted infected µMT-/- mice exhibited reduced IL-10 and rapid weight loss, succumbing to infection by day 6 after CD4 neutralization. Our results showed that IL-10 from CD4+ T cells limits immune-mediated pathology in chronic subclinical Pc infection in µMT-/- mice by protecting against excessive inflammatory responses to blood-stage parasites.
-
-
-
Immunology and Microbiology
Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity.
In Cell Rep Med on 19 August 2025 by Ahn, J. J., Dudics, S., et al.
PubMed
Wild-type interleukin (IL)-2 induces anti-tumor immunity and toxicity, predominated by vascular leak syndrome (VLS) leading to edema, hypotension, organ toxicity, and regulatory T cell (Treg) expansion. Efforts to uncouple IL-2 toxicity from its potency have failed in the clinic. We hypothesize that IL-2 toxicity is driven by cytokine release syndrome (CRS) followed by VLS and that coupling IL-2 with IL-10 will ameliorate toxicity. Our data, generated using human primary cells, mouse models, and non-human primates, suggest that coupling of these cytokines prevents toxicity while retaining cytotoxic T cell activation and limiting Treg expansion. In syngeneic murine tumor models, DK210 epidermal growth factor receptor (EGFR), an IL-2/IL-10 fusion molecule targeted to EGFR via an anti-EGFR single-chain variable fragment (scFV), potently activates T cells and natural killer (NK) cells and elicits interferon (IFN)γ-dependent anti-tumor function without peripheral inflammatory toxicity or Treg accumulation. Therefore, combining IL-2 with IL-10 uncouples toxicity from immune activation, leading to a balanced and pleiotropic anti-tumor immune response.
-
-
-
Cancer Research
-
Immunology and Microbiology
TRIM6 ablation reverses ICB resistance in MSS gastric cancer by unleashing cGAS-STING-dependent antitumor immunity.
In J Exp Clin Cancer Res on 15 August 2025 by Niu, Y., Ding, C., et al.
PubMed
Gastric cancers are classified into four molecular subtypes according to The Cancer Genome Atlas (TCGA) classification: Epstein-Barr virus-positive (EBV-positive), microsatellite instability-high (MSI-H), chromosomal instability (CIN), and genomically stable (GS). Unlike MSI-H gastric cancer, GS and CIN subtypes exhibit immunologically inert microenvironments and demonstrate minimal response to immune checkpoint blockade (ICB), necessitating novel strategies to overcome immunotherapy resistance.
-
-
-
Immunology and Microbiology
Foxp3+ Regulatory T Cells Restrain Th1 Response Shielding the Brain from Lethal Inflammatory Damage during Cryptococcal Meningoencephalitis
In Research Square on 30 July 2025 by Olszewski, M., Li, H., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Macrophages suppress CD8 + T cell cytotoxic function in triple negative breast cancer via VISTA.
In Br J Cancer on 1 July 2025 by Abudula, M., Astuti, Y., et al.
PubMed
Immunotherapy targeting negative immune checkpoint regulators to enhance the anti-tumour immune response holds promise in the treatment of TNBC. V-domain Ig suppressor of T-cell activation (VISTA) is an immune checkpoint molecule, known to be upregulated and involved in modulating tumour immunity in TNBC. However, how VISTA affects immune response and its therapeutic potential in TNBC remains unclear.
-
-
-
Cancer Research
Fc-optimized anti-CTLA-4 antibodies increase tumor-associated high endothelial venules and sensitize refractory tumors to PD-1 blockade.
In Cell Rep Med on 17 June 2025 by Blanchard, L., Vina, E., et al.
PubMed
The lack of T cells in tumors is a major hurdle to successful immune checkpoint therapy (ICT). Therefore, therapeutic strategies promoting T cell recruitment into tumors are warranted to improve the treatment efficacy. Here, we report that Fc-optimized anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies are potent remodelers of tumor vasculature that increase tumor-associated high endothelial venules (TA-HEVs), specialized blood vessels supporting lymphocyte entry into tumors. Mechanistically, this effect is dependent on the Fc domain of anti-CTLA-4 antibodies and CD4+ T cells and involves interferon gamma (IFNγ). Unexpectedly, we find that the human anti-CTLA-4 antibody ipilimumab fails to increase TA-HEVs in a humanized mouse model. However, increasing its Fc effector function rescues the modulation of TA-HEVs, promotes CD4+ and CD8+ T cell infiltration into tumors, and sensitizes recalcitrant tumors to programmed cell death protein 1 (PD-1) blockade. Our findings suggest that Fc-optimized anti-CTLA-4 antibodies could be used to reprogram tumor vasculature in poorly immunogenic cold tumors and improve the efficacy of ICT.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Genetics
-
Immunology and Microbiology
Enhanced durability of a Zika virus self-amplifying RNA vaccine through combinatorial OX40 and 4-1BB agonism.
In JCI Insight on 22 May 2025 by Lu, H. H., dos Santos Alves, R. P., et al.
PubMed
The SARS-CoV-2 pandemic highlighted the potential of mRNA vaccines in rapidly responding to emerging pathogens. However, immunity induced by conventional mRNA vaccines wanes quickly, requiring frequent boosters. Self-amplifying RNA (saRNA) vaccines, which extend antigen expression via self-replication, offer a promising strategy to induce more durable immune responses. In this study, we developed an saRNA vaccine encoding Zika virus (ZIKV) membrane and envelope proteins and evaluated its efficacy in mice. A single vaccination elicited strong humoral and cellular immune responses and reduced viral loads but only for 28 days. By day 84, antibody titers and T cell responses had significantly declined, resulting in reduced efficacy. To address this, we evaluated agonist antibodies targeting the T cell costimulatory molecules OX40 and 4-1BB. Coadministration of agonist antibodies enhanced CD8+ T cell responses to vaccination, resulting in sustained immunity and reduced viral loads at day 84. Depletion and passive transfer studies verified that long-term antiviral immunity was primarily CD8+ T cell dependent, with minimal contributions from antibody responses. These findings suggest that agonists targeting members of the tumor necrosis receptor superfamily, such as OX40 and 4-1BB, might enhance the durability of saRNA vaccine-induced protection, addressing a key limitation of current mRNA vaccine platforms.
-
-
-
Cancer Research
Hepatic iNKT cells facilitate colorectal cancer metastasis by inducing a fibrotic niche in the liver.
In iScience on 16 May 2025 by Nater, M., Brügger, M., et al.
PubMed
The liver is an important metastatic organ that contains many innate immune cells, yet little is known about their role in anti-metastatic defense. We investigated how invariant natural killer T (iNKT) cells influence colorectal cancer-derived liver metastasis using different models in immunocompetent mice. We found that hepatic iNKT cells promote metastasis by creating a supportive niche for disseminated cancer cells. Mechanistically, iNKT cells respond to disseminating cancer cells by producing the fibrogenic cytokines interleukin-4 (IL-4) and IL-13 in a T cell receptor-independent manner. Selective abrogation of IL-4 and IL-13 sensing in hepatic stellate cells prevented their transdifferentiation into extracellular matrix-producing myofibroblasts, which hindered metastatic outgrowth of disseminated cancer cells. This study highlights a novel tumor-promoting axis driven by iNKT cells in the initial stages of metastasis.
-
-
-
Cancer Research
-
Immunology and Microbiology
Development of PVTX-405 as a potent and highly selective molecular glue degrader of IKZF2 for cancer immunotherapy.
In Nat Commun on 1 May 2025 by Chen, Z., Dhruv, H., et al.
PubMed
IKZF2 (Helios) is a transcription factor that is selectively expressed by Tregs and is essential for preserving the function and stability of Tregs in the tumor microenvironment (TME), where it suppresses the anti-tumor immune response. Targeted IKZF2 degradation by small molecules represents a promising strategy for the development of a new class of cancer immunotherapy. Herein, we describe the discovery of PVTX-405, a potent, effective, highly selective, and orally efficacious IKZF2 molecular glue degrader. PVTX-405 degrades IKZF2 (DC50 = 0.7 nM and Dmax = 91%) while sparing other CRBN neo-substrates. Degradation of IKZF2 by PVTX-405 increases production of inflammatory cytokine IL-2 and reduces the suppressive activity of Tregs, leading to an increase in Teff cell proliferation. Once-daily oral administration of PVTX-405 as single agent significantly delays the growth of MC38 tumors in a syngeneic tumor model using humanized CRBN mice. PVTX-405 in combination with anti-PD1 or anti-LAG3 significantly increases animal survival compared to anti-PD1 or anti-LAG3 alone. Together, these results demonstrate that PVTX-405 is a promising IKZF2 degrader for clinical development for the treatment of human cancers.
-
-
-
Immunology and Microbiology
B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine.
In Nat Immunol on 1 May 2025 by Cembellin-Prieto, A., Luo, Z., et al.
PubMed
The rapid onset of innate immune defenses is critical for early control of viral replication in an infected host and yet it can also lead to irreversible tissue damage, especially in the respiratory tract. Sensitive regulators must exist that modulate inflammation, while controlling the infection. In the present study, we identified acetylcholine (ACh)-producing B cells as such early regulators. B cells are the most prevalent ACh-producing leukocyte population in the respiratory tract demonstrated with choline acetyltransferase (ChAT)-green fluorescent protein (GFP) reporter mice, both before and after infection with influenza A virus. Mice lacking ChAT in B cells, disabling their ability to generate ACh (ChatBKO), but not those lacking ChAT in T cells, significantly, selectively and directly suppressed α7-nicotinic-ACh receptor-expressing interstitial, but not alveolar, macrophage activation and their ability to secrete tumor necrosis factor (TNF), while better controlling virus replication at 1 d postinfection. Conversely, TNF blockade via monoclonal antibody treatment increased viral loads at that time. By day 10 of infection, ChatBKO mice showed increased local and systemic inflammation and reduced signs of lung epithelial repair despite similar viral loads and viral clearance. Thus, B cells are key participants of an immediate early regulatory cascade that controls lung tissue damage after viral infection, shifting the balance toward reduced inflammation at the cost of enhanced early viral replication.
-