Functional Antibodies: Principles for Translational Confidence
A Brief History of the Use of Antibodies in Research
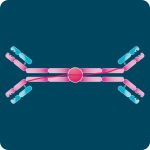
First described in 1890, antibodies, also known as immunoglobulins (Ig), are Y-shaped glycoproteins produced by the immune system to recognize and neutralize foreign antigens such as bacteria, viruses, and toxins. Their remarkable specificity and affinity make them fundamental to immune protection and indispensable tools in biomedical research, diagnostics, and therapeutic development.
Overview of Antibody Structure
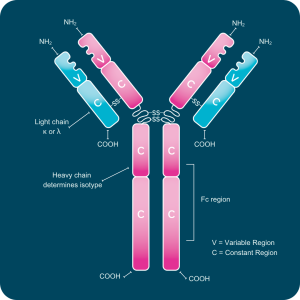 Each antibody molecule consists of two identical heavy chains and two identical light chains. Light chains are categorized as kappa (κ) or lambda (λ) based on sequence differences, while the heavy chain defines the antibody’s isotype.
Each antibody molecule consists of two identical heavy chains and two identical light chains. Light chains are categorized as kappa (κ) or lambda (λ) based on sequence differences, while the heavy chain defines the antibody’s isotype.
Every chain contains one variable (V) domain and one or more constant (C) domains, each composed of roughly 110–130 amino acids. Light chains have a single constant domain, and heavy chains contain three or four. Heavy chains with three constant domains include a flexible hinge region that allows movement of the antigen-binding arms.
A typical light chain has a mass of about 25 kDa, and a heavy chain with three constant domains and a hinge region has a mass near 55 kDa.
Antibody Isotypes and their Subclasses
Antibody isotypes are defined by differences in the heavy-chain constant region, which influence disulfide bonding, glycosylation, and the number of constant domains.
In mammals, five major isotypes exist: IgG, IgM, IgA, IgD, and IgE. In humans and mice, IgG is further divided into subclasses (for example, mouse IgG1, IgG2a, IgG2b, IgG2c, and IgG3). Each isotype contributes distinct biological functions, effector mechanisms, and tissue distributions.
| IgG | IgD | IgE | IgA | IgM |
|---|---|---|---|---|
IgG is the most abundant isotype in serum and exhibits the longest half-life of all immunoglobulins. It includes four subclasses with structural and functional distinctions. IgG1 and IgG3 typically target protein antigens, while IgG2 and IgG4 respond to polysaccharide antigens. Because of its stability and versatility, IgG is the predominant isotype used in research and therapeutic development. | IgD occurs at very low concentrations and has a short half-life. Its exact function remains under investigation, but evidence suggests a role in B-cell activation and regulation. | IgE is the least abundant isotype and has the shortest half-life. It mediates allergic hypersensitivity and provides defense against parasitic infections. Despite its low serum levels, IgE’s ability to engage Fc receptors makes it a potent activator of immune responses. | IgA dominates mucosal immunity. It circulates primarily as a monomer but can form dimers joined by a J-chain and a secretory component that protect it from enzymatic degradation. IgA neutralizes pathogens and toxins and prevents their adherence to epithelial surfaces. | IgM is the first antibody produced during a primary immune response. Individual IgM units assemble into pentamers through disulfide bonds and incorporate a J-chain. Although monomeric IgM has low affinity, the pentameric form achieves high avidity by binding multiple epitopes simultaneously. |
 |  |  |  |  |
F(ab) and Fc Fragment Regions
Each antibody contains two F(ab) regions and one Fc region. The F(ab) regions form the antigen-binding arms, while the Fc region mediates effector functions through receptor and complement interactions.
Papain digestion yields two F(ab) fragments and one Fc fragment. Pepsin digestion produces an intact F(ab′)₂ fragment and a degraded Fc fragment. Because F(ab) and F(ab′)₂ fragments retain antigen-binding capability without Fc-mediated activity, they are useful in imaging, blocking assays, and detection applications.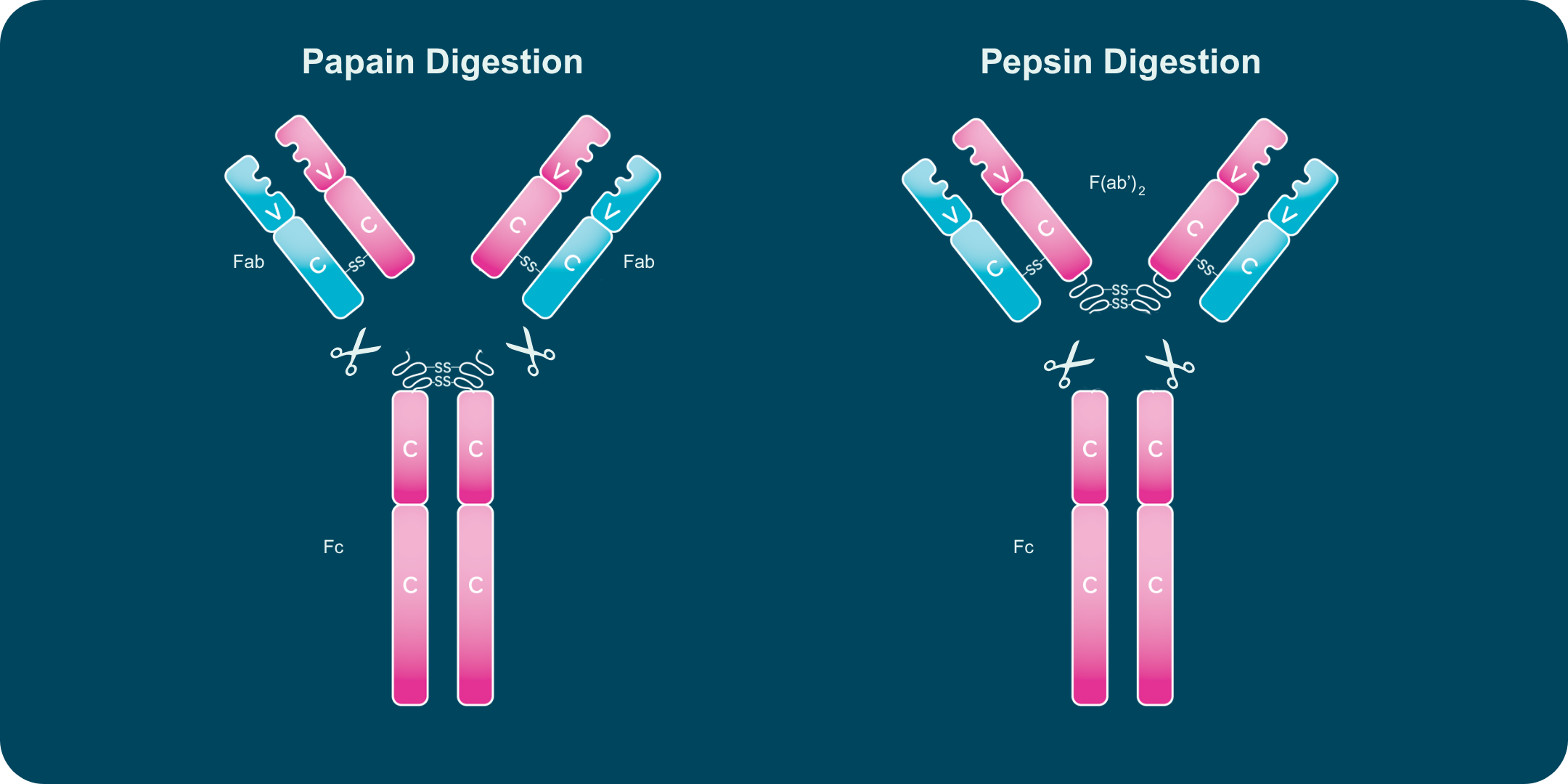
Conclusion
Antibodies are central to both immune defense and experimental biology. Their structural diversity and functional specialization enable precise investigation of signaling, modulation, and therapeutic mechanisms across in vivo, ex vivo, and in vitro systems. These properties make antibodies essential tools for advancing discovery in immunology, oncology, and translational research.
Functional Antibodies: From Detection to Biological Modulation
History of Functional Antibodies
Antibodies are best known for their ability to recognize and bind specific antigens, a property that makes them indispensable in both research and clinical settings. Yet, antibodies are far more than a detection tool. Many antibodies possess functional activity and are actively able to shape biological processes such as cell activation, toxin neutralization, and modulation of immune pathways.
This capacity was first recognized by Paul Ehrlich in the late 19th century, when antibodies were described as “antitoxins” capable of neutralizing harmful agents.1 Today, functional antibodies remain central to disease research and therapeutic development, enabling scientists to probe and manipulate complex biological systems such as in vitro organoids, organs-on-chip, 3D cell and 2D tissue cultures, and in vivo models.
What Are Functional Antibodies?
The term “functional antibody” is commonly used to distinguish antibodies with biological activity from those employed as inert detection reagents. Although the definition is broad, a functional antibody can be understood as one that actively modulates a biological process in a living system.
Mechanisms include:
-
- Activation or inhibition of receptors and pathways
- Neutralization of toxins, cytokines, or pathogens
- Engagement of Fcγ receptor signaling to direct immune responses
The versatility of functional antibodies allows researchers to explore disease mechanisms, create new models, and identify potential therapeutic strategies.2
Takeaway: Functional research requires antibodies validated for biological activity, not just detection.
Functional Antibodies in Action
Agonist and Antagonist Activity
One well-studied example is the use of anti-CD3 antibodies. These can act as agonists, stimulating T cell activation even in immunosuppressed conditions.3 Conversely, inhibitory antibodies such as anti-PD-1 block suppressive checkpoint pathways, reactivating T cells to attack tumor cells.3
Neutralization
Antibodies can neutralize harmful molecules by binding their active sites, preventing downstream interactions. Neutralizing antibodies play roles in blocking toxins, cytokines, and pathogens, and they are critical for vaccine efficacy because they drive post-immunization immune protection.4
Fcγ Receptor–Mediated Signaling
Functional antibodies may exert effects through Fcγ receptors on immune cells. These interactions can promote phagocytosis, upregulate cytokine production, and direct B cell selection and survival.5 Such mechanisms are especially important in cancer immunology, where antibody-driven Fc engagement can amplify anti-tumor responses.
Ensuring Quality in Functional Antibody Studies
-
- Selectivity and specificity: Off-target binding can activate unintended pathways and confound data.1 2
- Purity and formulation: Endotoxins, preservatives, or aggregation may induce non-specific immune effects.3 4
- Reproducibility: Lot-to-lot variability contributes to irreproducibility in preclinical research studies.6
- Pathogen safety: Antibodies contaminated with murine pathogens can compromise animal health, distort immune responses, and undermine study validity.
- Scalability: Consistent quality and reliable supply from milligram to gram quantities ensures that results remain valid as studies progress from early discovery to large-scale in vivo models.
High-quality reagents minimize these risks, ensuring that data accurately reflect underlying biology.
The Bio X Cell Approach to Functional Antibodies
InVivoMAb™ and InVivoPlus™ antibodies are specifically formulated for in vivo studies and in vitro translational studies using organoids, organs-on-chip, and other new approach methodologies (NAMs). Each lot of antibody is ultrapure, features low endotoxins, and is preservative-free. InVivoPlus™ antibodies undergo additional testing and purification to ensure even lower endotoxin levels (greater or equal to 0.5EU/mg). Each primary antibody has a complementary matching isotype control available to help separate target-driven activity from background Fc effects for your studies.
To further support research your research continuity and reduce time loss due to unstable or inconsistent reagents, Bio X Cell antibodies include a one-year shelf-life stability guarantee. This guarantee ensures stable antibody performance throughout your long-term, multi-phase studies.
Setting consistent and reliable standards for functional antibodies helps ensure that antibodies perform as intended, enabling reproducible, reliable results across both in vitro systems and in vivo models.
With 100% PhD-level technical support, available worldwide, our “for scientists, by scientists” approach to catalog target selection, antibody validation, and QC data review helps ensure the antigen and clone you choose are the best possible fit for your experiments. With industry an industry leading citation record, widely cited PD-1 clones for your model, and novel antibody formats such as bispecific antibodies, we are able to support your next set of studies so you can focus on generating impactful results.
Conclusion
Functional antibodies are vital research tools that make it possible to model disease, dissect immune pathways, and identify therapeutic strategies. Their versatility is unmatched, but the reliability of their insights depends on rigorous study design and high-quality reagents.
With thoughtfully validated antibodies such as those in the InVivoMAb™ and InVivoPlus™ product lines, researchers can create experiments that reveal true biology, accelerate discovery, and build a foundation for translational breakthroughs.
translationally meaningful results.
References
- Daëron M. The effector functions of antibodies. Immunol Rev. 2024;328:6–12.
- Uhlén M, et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823–7.
- Menon AP, et al. Modulating T cell responses by targeting CD3. Cancers (Basel). 2023;15(4):1189.
- Zhang A, et al. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat Rev Immunol. 2023;23:381–396.
- Bournazos S, et al. Signaling by antibodies: Recent progress. Annu Rev Immunol. 2017;35:285–311.
- Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015;521:274–276.
Why Functional Antibody Standards Are Unique
Functional antibodies are powerful tools that enable researchers to activate, block, or fine-tune biological pathways in complex in vivo model systems. Their impact depends on more than binding specificity alone. Whether to recapitulate disease in vivo, neutralize targets, block receptors, or probe immune pathways, high-quality antibodies at scale are essential to ensure reproducibility, prevent costly setbacks, and drive translational research toward therapeutic impact. Bio X Cell is the global leader of functional antibodies, with an industry leading 30,000 citation record and a nearly 30 year history in providing researchers with high-quality functional antibodies across in vivo and in vitro model systems.
In addition to their utility in traditional in vivo models, our antibodies also feature a unique formulation which is also optimized for sensitive in vitro systems such as organoids, organs-on-chip, spheroids, 2D/3D cultures and other NAM systems.
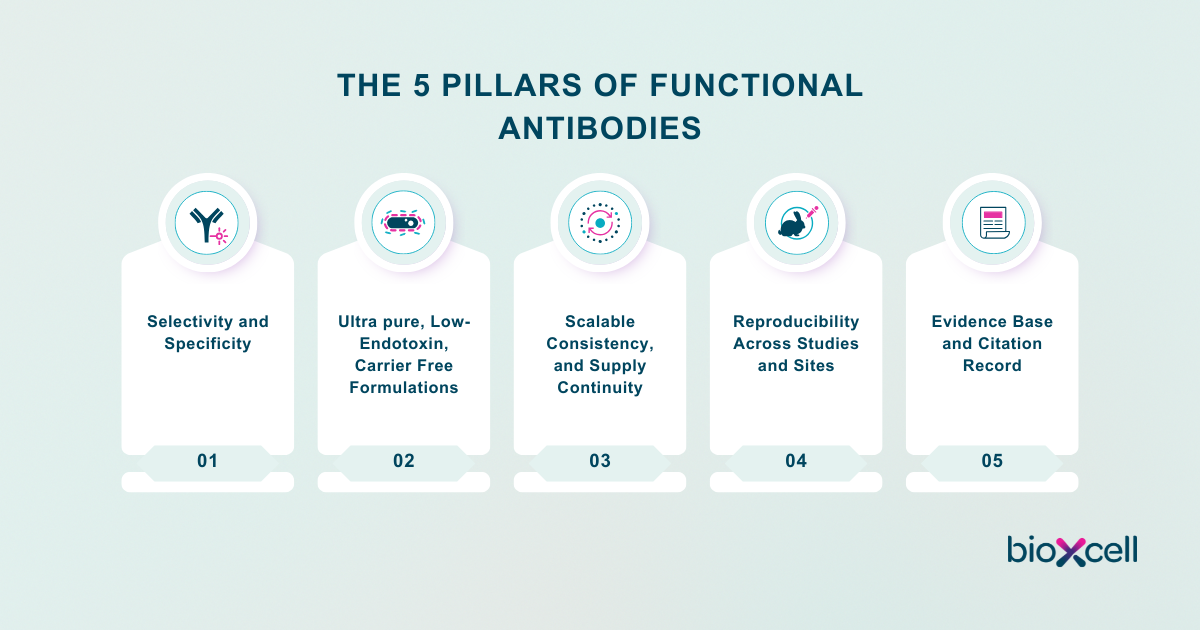
As the global leader of functional antibodies, Bio X Cell has created a comprehensive framework that defines the critical elements of quality in antibody selection for functional studies. This framework, The Five Pillars of Functional Antibodies, describes the role of specificity, purity, scalability, reproducibility, and community validation in the antibody selection process, pushing the definition of quality beyond basic binding validation to encompass performance across living systems, including organoid and other complex translational models.
The 5 Pillars of Functional Antibodies
Functional antibody research requires more than reagent availability. Reliable, translational discovery depends on reproducible results, outcomes that are directly influenced by antibody quality, formulation, and validation.
Antibodies must precisely recognize their intended antigen without cross-reactivity. Off-target binding may result in the activation or inhibition of unintended pathways, creating misleading and difficult to replicate results. High target selectivity and well-characterized antigen specificity are foundational for functional antibody studies.
Best Practices
-
- Verify application-specific validation data
- Confirm species reactivity and cross-blocking studies
- Use RRIDs for traceability across publications
For in vivo and in vitro organoid studies, antibody purity directly impacts study safety and interpretability. Endotoxin contamination provokes cytokine release and background inflammation that can obscure antibody-driven biology 1 2 3. Protein aggregation can cluster receptors and distort signaling, while preservatives or carrier proteins may compromise viability or interfere with downstream assays 4 5. For functional studies, especially in immune-sensitive systems, low endotoxin levels, low aggregation, and carrier-free formulations ensure that results reflect true target engagement and clean pharmacology.
Best Practices
- Select high-purity antibodies with aggregation and murine-pathogen testing
- Verify lot-specific endotoxin data
- Avoid preservatives in live-cell and in vivo systems
- Choose reagents produced under low-endotoxin processes
- Use carrier-free formulations for translational work
Practical Notes on Endotoxin
Endotoxin is measured in endotoxin units (EU) per milligram of protein. Even low levels can trigger cytokine responses in vitro and in vivo; setting stricter thresholds helps ensure results reflect true biology 1 2.
As studies expand from milligram-scale pilot experiments to gram-scale preclinical work, consistent quality and reliable supply are essential. Lot continuity and forward planning reduce repeat experiments, prevent costly setbacks, and protect animal welfare. Bio X Cell antibodies include a one-year shelf-life guarantee, ensuring stable performance throughout long-term, multi-phase studies.
Best Practices
- Plan for lot bridging with retained samples for comparability
- Forecast multi-phase study needs to secure uninterrupted supply
- Select suppliers with proven scalability (e.g., Bio X Cell 2,000 L/month capacity)
Lot Bridging in Practice
When a lot change is unavoidable, running a comparability panel on retained aliquots can confirm activity before scaling up. This minimizes unexpected variation and avoids costly resets.
Reproducibility safeguards both credibility and resources. Inconsistent antibody performance is a leading contributor to irreproducibility in preclinical research 6 7 8. Validation in the intended application, standardized QC, and transparent reporting are critical to ensure dependable results.
Best Practices
- Rely on lot-specific Certificates of Analysis (COAs)
- Check functional validation data in the relevant application
- Use standardized controls and reference protocols across labs
Best Practices
- Favor antibodies with RRIDs and peer-reviewed use
- Verify published performance in the same target, species, and application
- Report RRIDs in methods to support transparency
The World’s Most Cited Functional Antibodies
With nearly 30,000 peer-reviewed citations, Bio X Cell provides a legacy of consistency for global translational research. Far from generic clones or commodity reagents, Bio X Cell antibodies deliver reproducibility, scalability, flexibility, and consistency that researchers rely on to drive discoveries in disease research.
Conclusion
Not all functional antibodies are created equally, even when they share the same clone name, target antigen, or formulation. When selecting a functional antibody supplier, researchers should consider the legacy of consistency which only the best antibody suppliers can provide. Choosing antibodies from manufacturers like Bio X Cell, that generate their own supply, are able to provide scale up solutions for translational studies, and who are consistency able to delivery publication-quality antibodies formulated to be ultra-pure, low endotoxin, carrier free, and optimized for in vivo and in vitro organoid systems allow studies to focus on real experimental outcomes rather than inconsistent reagent effects. With a deep citation base, consistent quality, and dependable supply, Bio X Cell antibodies help ensure that results reflect biology rather than confounding artifacts.
References
- Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76(2-3):97–119. PubMed
- Williams KL. The Biologics Revolution and Endotoxin Test Concerns. 2019. Full Text (PMC)
- Casonato Melo C et al. High Protein Recovery after Endotoxin Removal Using Anti-Lipid A Antibody Microparticles. Int J Mol Sci. 2023;24(18):13971. Open Access
- Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–E507. PubMed
- Ratanji KD et al. Immunogenicity of therapeutic proteins: Influence of aggregation. J Pharm Sci. 2014;103(11):3779–3794. Full Text (PMC)
- Weller MG. Quality Issues of Research Antibodies. Anal Chem Insights. 2016;11:21–27. Open Access
- Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015;521:274–276. Article
- Uhlén M et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823–827. Full Text (PMC)
- Bandrowski A et al. The Resource Identification Initiative: A Cultural Shift in Publishing. J Comp Neurol. 2016;524(1):8–22. Full Text (PMC)
Introduction
Functional antibodies are critical for exploring disease pathways, testing therapeutic strategies, and building translational models. Their power lies in their ability to modulate biology directly. Antibody quality in functional studies determines the reliability of experimental outcomes. Premium antibodies are distinguished by rigorous characterization, high purity, and consistent performance. Choosing well-validated reagents reduces confounding variables, strengthens reproducibility, and saves valuable time.
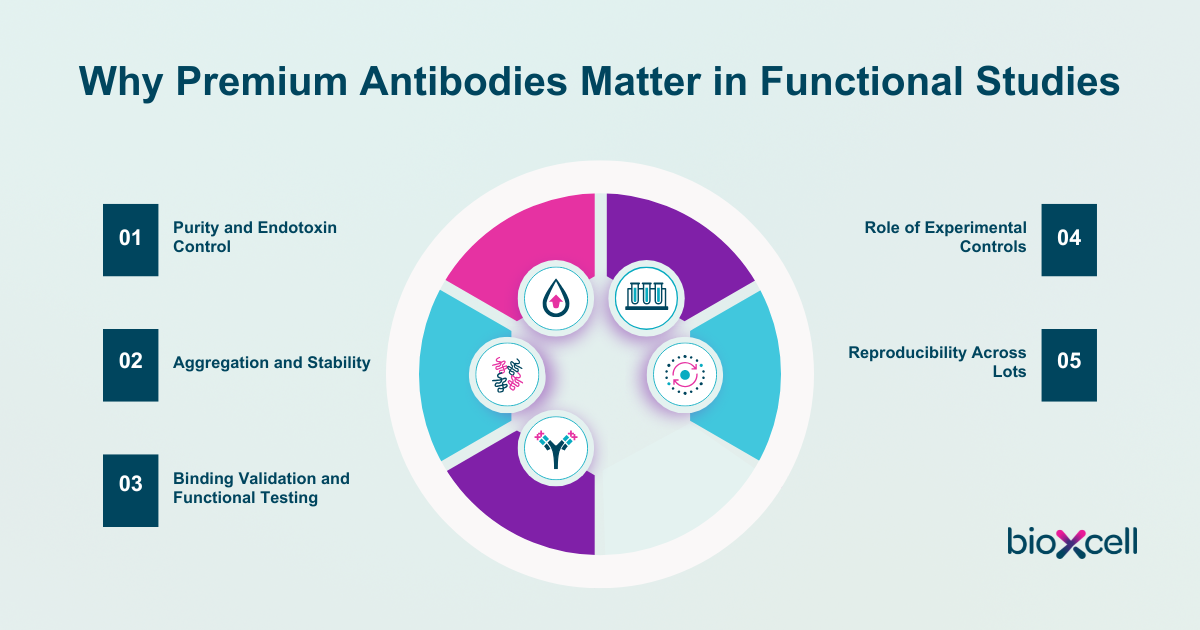
Purity and Endotoxin Control
Reproducible purity is the gold standard of functional antibodies. Even trace contaminants can alter immune readouts or compromise cell viability. Endotoxins are byproducts of bacterial expression systems and can provoke cytokine release and obscure the true activity of an antibody¹. Premium antibodies are formulated to be ultra-pure with low endotoxin levels, ensuring that immune activation reflects the intended biology of the antibody rather than activating off-function effects due to hidden antibody contaminants.
For researchers who work in sensitive preclinical assays, premium-grade functional antibodies provide essential safeguards for studies: comprehensive murine pathogen testing to protect both study integrity and animal health. InVivoPlus™ antibodies from Bio X Cell are premium antibodies which have been rigorously screened for common murine pathogens, including:
Mycoplasma species:
- M. pulmonis, M. arginini, M. fermentans, M. hominis, M. hyorhinis, M. orale, M. pirum, M. salivarium, M. agassizii, M. cynos
- Murine viruses:
- Murine norovirus (MNV)
- Murine parvovirus (MPV 1–5)
- Murine minute virus (MMV/MVM)
- Murine hepatitis virus (MHV)
- Murine reovirus (REO types 1–3)
- Lymphocytic choriomeningitis virus (LCMV)
- Lactate dehydrogenase–elevating virus (LDV)
- Murine rotavirus (MRV/EDIM)
- Theiler’s murine encephalomyelitis virus (TMEV)
- Ectromelia virus (ECTRO)
- Hantavirus (HANTA)
- Polyomavirus (POLY)
- Murine adenovirus (MAD1, MAD2)
- Sendai virus (SEND)
- Pneumonia virus of mice (PVM)
- Murine cytomegalovirus (MCMV 1, 2)
- K virus
This level of screening ensures that InVivoPlus™ antibodies from Bio X Cell meet the most stringent standards for pathogen safety, supporting reproducible results in the most sensitive in vivo and translational studies.
Takeaway: Low-endotoxin formulations are essential for reliable in vivo studies.
Aggregation and Stability
Aggregated antibodies can artificially cluster cell-surface receptors and exaggerate signaling responses³. Aggregation may also accelerate clearance or alter biodistribution in animal models. Premium antibodies are tested for aggregation and stability to support consistent performance across experiments and timeframes.
Binding Validation and Functional Testing
Antibodies validated for detection are not always appropriate for functional use. Functional studies require reagents that are tested for binding specificity and biological activity in the intended context. This includes confirmation that the antibody binds its native target conformation and, when relevant, that Fc activity contributes as expected. Without functional validation, results can be misleading. For example, antibodies validated only in denatured Western blot applications may fail in receptor signaling assays or in vivo immune models⁴.
Role of Experimental Controls
Premium antibodies are often accompanied by matched isotype controls. These controls enable researchers to separate target-specific effects from non-specific background signals, which is especially important in Fc receptor–rich environments. Isotype controls paired with high-purity functional antibodies provide a clearer view of the biology being studied.
Reproducibility Across Lots
Variability between production lots has been cited as a contributor to irreproducibility in preclinical research⁵. Premium suppliers address this by providing lot-specific certificates of analysis and maintaining consistency across production runs. For long-term or translational projects, this level of reproducibility reduces experimental risk and increases confidence in results.
Support for Your Studies
Premium antibodies should come with premium support to ensure experimental success and reduce uncertainty around study design. Bio X Cell products have been selected with PhD-level scientific guidance, application-specific validation data, and transparent documentation to help researchers like you design, troubleshoot, and optimize experiments.
Unlike other antibody reagent providers, Bio X Cell is the only life science company offering 100% PhD-level technical support worldwide, has US based manufacturing, and offers a legacy of nearly 30 years of consistent functional antibody production for both small and large-scale studies. The Bio X Cell approach, “for scientists, by scientists”, extends from catalog target selection and antibody validation to QC data review, ensuring that the antigen and clone you select are the best fit for your experimental question. Our 100% PhD-level technical support is able to help you select the right PD-1 clone for your model, interpret lot-specific QC data, or help to plan your next scale-up study. We help you reduce experimental uncertainty so you can focus on generating reproducible, impactful results.
The World’s Most Cited Functional Antibodies - Only from Bio X Cell
With nearly 30,000 peer-reviewed citations, Bio X Cell is the only functional antibody supplier able to demonstrate such a robust legacy of consistency, enabling global translational research since 1997. Far from generic clones or commodity reagents, Bio X Cell antibodies are the original, industry-leading, functional antibody standard, delivering unmatched reproducibility, scalability, flexibility, and consistency that researchers worldwide rely on to drive breakthrough discoveries in disease research.
Conclusion
Premium antibodies are not a luxury. They are a necessity for functional research. By focusing on purity, stability, functional validation, robust controls, and reproducibility, researchers can minimize confounders and generate results that reflect underlying biology.
References
- Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76:97–119.
- Gorbet MB, Sefton MV. Endotoxin: The uninvited guest. Biomaterials. 2005;26(34):6811–6817.
- Aricescu AR, et al. Aggregation and endotoxin contamination as critical confounders in antibody studies. MAbs. 2016;8(2):280–285.
- Uhlén M, et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823–827.
- Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nature. 2015;518:27–29.
Introduction
Functional antibodies are versatile tools that can activate, inhibit, or fine-tune biological pathways. Their value depends not only on antibody quality but also on the model system used to test them. Choosing an appropriate context reveals true biology and supports translation.
This post outlines how different experimental models, from in vitro organoid/organ-on-chip systems to in vivo syngeneic and humanized mouse models, contribute unique strengths to functional antibody research.
In Vitro Organoid/Organ-on-Chip Systems
In vitro platforms such as 3D tissue culture, organoids, and organ-on-chip systems have emerged as important New Approach Methodologies (NAM) in translational research. Unlike traditional 2D cultures, these new system types replicate aspects of three-dimensional architecture, cellular diversity, and microenvironmental cues which mirror human tissues¹ ² ³.
These NAM systems are powerful tools for:
- Exploring cell–cell and cell–matrix interactions that influence antibody activity
- Modeling patient-specific disease biology with stem cell–derived or patient-derived tissues
- Investigating immune–tumor or immune–barrier interactions in physiologically relevant contexts
- Reducing reliance on animal models during early discovery while supporting human relevance
When NAMs Are Not Enough
Organoid and organ-on-chip systems provide mechanistic insight and human relevance, but they cannot fully replicate a living immune system. They generally lack dynamic circulation, systemic cytokine environments, and whole-body pharmacokinetics.
Syngeneic Mouse Models
Syngeneic mouse models feature intact immune systems that interact naturally with tumors or pathogens. These models are valuable for studying checkpoint blockade and immune activation, evaluating anti-tumor responses in immunocompetent settings, and exploring host–pathogen interactions⁴. They provide insights that cannot be captured in vitro and are essential for understanding how antibodies perform in living organisms.
Humanized Mouse Models
For antibodies that target human-specific proteins, humanized mouse models are indispensable. These systems involve engrafting mice with human immune cells or tissues, enabling study of human-specific checkpoint pathways and evaluation of efficacy and safety in translational contexts⁵ ⁶. Humanized models bridge the gap between preclinical discovery and clinical application.
Beyond Mice: Specialized Models
Although mice dominate preclinical antibody studies, specialized models, including non-human primates or disease-specific engineered systems, can be required for safety testing, pharmacokinetics, and bridging studies prior to clinical translation.
Conclusion
Model choice is as critical as antibody choice in functional research. Organoids and organ-on-chip systems excel at revealing mechanistic insights and support NAM-driven workflows. Syngeneic models capture tumor–immune interactions in a full immune context, while humanized mice allow exploration of human-specific pathways. Specialized models extend this work into translational safety and pharmacology.
Bio X Cell functional antibodies, formulated for use across in vitro and in vivo systems, provide flexibility across these model types. With a suitable model paired to a well-validated antibody, researchers can generate results that are reproducible, translationally relevant, and positioned to accelerate therapeutic development.
References
- Fatehullah A, et al. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246-254.
- Kim J, Koo BK, Knoblich JA. Human organoids: Model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571-584.
- Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA. Organs-on-chips: Into the next decade. Nat Rev Drug Discov. 2021;20:345-361.
- Byrne AT, et al. Interrogating open questions in cancer research using mouse models. Nat Rev Cancer. 2017;17:751-765.
- Shultz LD, et al. Humanized mice in translational biomedical research. Nat Rev Immunol. 2012;12:786-798.
- Rongvaux A, et al. Humanized mice for immune system investigation. Nat Rev Immunol. 2013;13:786-798.
Meet Our Leaders
Learn more about the team behind the gold standard in vivo antibodies.
Discover Bio X Cell
Learn more about our proven expertise and comprehensive antibody solutions.
See Our Impact
Discover the Bio X Cell Fund’s mission to improve the health of our community
Explore Our Latest
See our latest articles and whitepapers for scientific insights and ideas
Consult With Bio X Cell to Enable Your Next Breakthrough Discovery
Whether you need antibody customization or high-volume production, Bio X Cell is committed to advancing your therapeutic innovations.
Contact Us