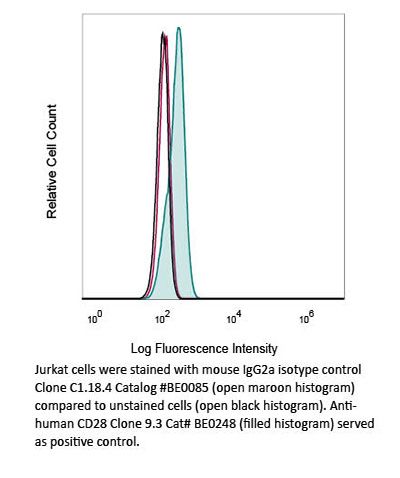
InVivoMAb mouse IgG2a isotype control, unknown specificity
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107771 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Carmi, Y., et al (2015). "Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity" Nature 521(7550): 99-104.
PubMed
Whereas cancers grow within host tissues and evade host immunity through immune-editing and immunosuppression, tumours are rarely transmissible between individuals. Much like transplanted allogeneic organs, allogeneic tumours are reliably rejected by host T cells, even when the tumour and host share the same major histocompatibility complex alleles, the most potent determinants of transplant rejection. How such tumour-eradicating immunity is initiated remains unknown, although elucidating this process could provide the basis for inducing similar responses against naturally arising tumours. Here we find that allogeneic tumour rejection is initiated in mice by naturally occurring tumour-binding IgG antibodies, which enable dendritic cells (DCs) to internalize tumour antigens and subsequently activate tumour-reactive T cells. We exploited this mechanism to treat autologous and autochthonous tumours successfully. Either systemic administration of DCs loaded with allogeneic-IgG-coated tumour cells or intratumoral injection of allogeneic IgG in combination with DC stimuli induced potent T-cell-mediated antitumour immune responses, resulting in tumour eradication in mouse models of melanoma, pancreas, lung and breast cancer. Moreover, this strategy led to eradication of distant tumours and metastases, as well as the injected primary tumours. To assess the clinical relevance of these findings, we studied antibodies and cells from patients with lung cancer. T cells from these patients responded vigorously to autologous tumour antigens after culture with allogeneic-IgG-loaded DCs, recapitulating our findings in mice. These results reveal that tumour-binding allogeneic IgG can induce powerful antitumour immunity that can be exploited for cancer immunotherapy.
Nakatsukasa, H., et al (2015). "The DNA-binding inhibitor Id3 regulates IL-9 production in CD4(+) T cells" Nat Immunol 16(10): 1077-1084.
PubMed
The molecular mechanisms by which signaling via transforming growth factor-beta (TGF-beta) and interleukin 4 (IL-4) control the differentiation of CD4(+) IL-9-producing helper T cells (TH9 cells) remain incompletely understood. We found here that the DNA-binding inhibitor Id3 regulated TH9 differentiation, as deletion of Id3 increased IL-9 production from CD4(+) T cells. Mechanistically, TGF-beta1 and IL-4 downregulated Id3 expression, and this process required the kinase TAK1. A reduction in Id3 expression enhanced binding of the transcription factors E2A and GATA-3 to the Il9 promoter region, which promoted Il9 transcription. Notably, Id3-mediated control of TH9 differentiation regulated anti-tumor immunity in an experimental melanoma-bearing model in vivo and also in human CD4(+) T cells in vitro. Thus, our study reveals a previously unrecognized TAK1-Id3-E2A-GATA-3 pathway that regulates TH9 differentiation.
Bulliard, Y., et al (2013). "Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies" J Exp Med 210(9): 1685-1693.
PubMed
Fc gamma receptor (FcgammaR) coengagement can facilitate antibody-mediated receptor activation in target cells. In particular, agonistic antibodies that target tumor necrosis factor receptor (TNFR) family members have shown dependence on expression of the inhibitory FcgammaR, FcgammaRIIB. It remains unclear if engagement of FcgammaRIIB also extends to the activities of antibodies targeting immunoregulatory TNFRs expressed by T cells. We have explored the requirement for activating and inhibitory FcgammaRs for the antitumor effects of antibodies targeting the TNFR glucocorticoid-induced TNFR-related protein (GITR; TNFRSF18; CD357) expressed on activated and regulatory T cells (T reg cells). We found that although FcgammaRIIB was dispensable for the in vivo efficacy of anti-GITR antibodies, in contrast, activating FcgammaRs were essential. Surprisingly, the dependence on activating FcgammaRs extended to an antibody targeting the non-TNFR receptor CTLA-4 (CD152) that acts as a negative regulator of T cell immunity. We define a common mechanism that correlated with tumor efficacy, whereby antibodies that coengaged activating FcgammaRs expressed by tumor-associated leukocytes facilitated the selective elimination of intratumoral T cell populations, particularly T reg cells. These findings may have broad implications for antibody engineering efforts aimed at enhancing the therapeutic activity of immunomodulatory antibodies.
Kerzerho, J., et al (2013). "Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity" J Allergy Clin Immunol 131(4): 1048-1057, 1057 e1041-1042.
PubMed
BACKGROUND: Asthma is defined as a chronic inflammatory disease of the airways; however, the underlying physiologic and immunologic processes are not fully understood. OBJECTIVE: The aim of this study was to determine whether TH9 cells develop in vivo in a model of chronic airway hyperreactivity (AHR) and what factors control this development. METHOD: We have developed a novel chronic allergen exposure model using the clinically relevant antigen Aspergillus fumigatus to determine the time kinetics of TH9 development in vivo. RESULTS: TH9 cells were detectable in the lungs after chronic allergen exposure. The number of TH9 cells directly correlated with the severity of AHR, and anti-IL-9 treatment decreased airway inflammation. Moreover, we have identified programmed cell death ligand (PD-L) 2 as a negative regulator of TH9 cell differentiation. Lack of PD-L2 was associated with significantly increased TGF-beta and IL-1alpha levels in the lungs, enhanced pulmonary TH9 differentiation, and higher morbidity in the sensitized mice. CONCLUSION: Our findings suggest that PD-L2 plays a pivotal role in the regulation of TH9 cell development in chronic AHR, providing novel strategies for modulating adaptive immunity during chronic allergic responses.
Licona-Limon, P., et al (2013). "Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection" Immunity 39(4): 744-757.
PubMed
Type 2 inflammatory cytokines, including interleukin-4 (IL-4), IL-5, IL-9, and IL-13, drive the characteristic features of immunity against parasitic worms and allergens. Whether IL-9 serves an essential role in the initiation of host-protective responses is controversial, and the importance of IL-9- versus IL-4-producing CD4(+) effector T cells in type 2 immunity is incompletely defined. Herein, we generated IL-9-deficient and IL-9-fluorescent reporter mice that demonstrated an essential role for this cytokine in the early type 2 immunity against Nippostrongylus brasiliensis. Whereas T helper 9 (Th9) cells and type 2 innate lymphoid cells (ILC2s) were major sources of infection-induced IL-9 production, the adoptive transfer of Th9 cells, but not Th2 cells, caused rapid worm expulsion, marked basophilia, and increased mast cell numbers in Rag2-deficient hosts. Taken together, our data show a critical and nonredundant role for Th9 cells and IL-9 in host-protective type 2 immunity against parasitic worm infection.
Rayamajhi, M., et al (2012). "Lung B cells promote early pathogen dissemination and hasten death from inhalation anthrax" Mucosal Immunol 5(4): 444-454.
PubMed
Sampling of mucosal antigens regulates immune responses but may also promote dissemination of mucosal pathogens. Lung dendritic cells (LDCs) capture antigens and traffic them to lung-draining lymph nodes (LDLNs) dependent on the chemokine receptor CCR7 (chemokine (C-C motif) receptor 7). LDCs also capture lung pathogens such as Bacillus anthracis (BA). However, we show here that the initial traffic of BA spores from lungs to LDLNs is largely independent of LDCs and CCR7, occurring instead in association with B cells. BA spores rapidly bound B cells in lungs and cultured mouse and human B cells. Binding was independent of the B-cell receptor (BCR). B cells instilled in the lungs trafficked to LDLNs and BA spore traffic to LDLNs was impaired by B-cell deficiency. Depletion of B cells also delayed death of mice receiving a lethal BA infection. These results suggest that mucosal B cells traffic BA, and possibly other antigens, from lungs to LDLNs.
Schafer, H., et al (2012). "Myofibroblast-induced tumorigenicity of pancreatic ductal epithelial cells is L1CAM dependent" Carcinogenesis 33(1): 84-93.
PubMed
Pancreatic ductal adenocarcinoma (PDAC) and chronic pancreatitis, representing one risk factor for PDAC, are characterized by a marked desmoplasia enriched of pancreatic myofibroblasts (PMFs). Thus, PMFs are thought to essentially promote pancreatic tumorigenesis. We recently demonstrated that the adhesion molecule L1CAM is involved in epithelial-mesenchymal transition of PMF-cocultured H6c7 human ductal epithelial cells and that L1CAM is expressed already in ductal structures of chronic pancreatitis with even higher elevation in primary tumors and metastases of PDAC patients. This study aimed at investigating whether PMFs and L1CAM drive malignant transformation of pancreatic ductal epithelial cells by enhancing their tumorigenic potential. Cell culture experiments demonstrated that in the presence of PMFs, H6c7 cells exhibit a profound resistance against death ligand-induced apoptosis. This apoptosis protection was similarly observed in H6c7 cells stably overexpressing L1CAM. Intrapancreatic inoculation of H6c7 cells together with PMFs (H6c7co) resulted in tumor formation in 7/8 and liver metastases in 6/8 severe combined immunodeficiency (SCID) mice, whereas no tumors and metastases were detectable after inoculation of H6c7 cells alone. Likewise, tumor outgrowth and metastases resulted from inoculation of L1CAM-overexpressing H6c7 cells in 5/7 and 3/7 SCID mice, respectively, but not from inoculation of mock-transfected H6c7 cells. Treatment of H6c7co tumor-bearing mice with the L1CAM antibody L1-9.3/2a inhibited tumor formation and liver metastasis in 100 and 50%, respectively, of the treated animals. Overall, these data provide new insights into the mechanisms of how PMFs and L1CAM contribute to malignant transformation of pancreatic ductal epithelial cells in early stages of pancreatic tumorigenesis.
Lamere, M. W., et al (2011). "Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance" J Virol 85(10): 5027-5035.
PubMed
Seasonal influenza epidemics recur due to antigenic drift of envelope glycoprotein antigens and immune evasion of circulating viruses. Additionally, antigenic shift can lead to influenza pandemics. Thus, a universal vaccine that protects against multiple influenza virus strains could alleviate the continuing impact of this virus on human health. In mice, accelerated clearance of a new viral strain (cross-protection) can be elicited by prior infection (heterosubtypic immunity) or by immunization with the highly conserved internal nucleoprotein (NP). Both heterosubtypic immunity and NP-immune protection require antibody production. Here, we show that systemic immunization with NP readily accelerated clearance of a 2009 pandemic H1N1 influenza virus isolate in an antibody-dependent manner. However, human immunization with trivalent inactivated influenza virus vaccine (TIV) only rarely and modestly boosted existing levels of anti-NP IgG. Similar results were observed in mice, although the reaction could be enhanced with adjuvants, by adjusting the stoichiometry among NP and other vaccine components, and by increasing the interval between TIV prime and boost. Importantly, mouse heterosubtypic immunity that had waned over several months could be enhanced by injecting purified anti-NP IgG or by boosting with NP protein, correlating with a long-lived increase in anti-NP antibody titers. Thus, current immunization strategies poorly induce NP-immune antibody that is nonetheless capable of contributing to long-lived cross-protection. The high conservation of NP antigen and the known longevity of antibody responses suggest that the antiviral activity of anti-NP IgG may provide a critically needed component of a universal influenza vaccine.
Libbey, J. E., et al (2011). "Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection" J Virol 85(14): 6913-6922.
PubMed
Cells that can participate in an innate immune response within the central nervous system (CNS) include infiltrating cells (polymorphonuclear leukocytes , macrophages, and natural killer cells) and resident cells (microglia and sometimes astrocytes). The proinflammatory cytokine interleukin-6 (IL-6) is produced by all of these cells and has been implicated in the development of behavioral seizures in the Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model. The assessment, via PCR arrays, of the mRNA expression levels of a large number of chemokines (ligands and receptors) in TMEV-infected and mock-infected C57BL/6 mice both with and without seizures did not clearly demonstrate the involvement of PMNs, monocytes/macrophages, or NK cells in the development of seizures, possibly due to overlapping function of the chemokines. Additionally, C57BL/6 mice unable to recruit or depleted of infiltrating PMNs and NK cells had seizure rates comparable to those of controls following TMEV infection, and therefore PMNs and NK cells do not significantly contribute to seizure development. In contrast, C57BL/6 mice treated with minocycline, which affects monocytes/macrophages, microglial cells, and PMNs, had significantly fewer seizures than controls following TMEV infection, indicating monocytes/macrophages and resident microglial cells are important in seizure development. Irradiated bone marrow chimeric mice that were either IL-6-deficient mice reconstituted with wild-type bone marrow cells or wild-type mice reconstituted with IL-6-deficient bone marrow cells developed significantly fewer behavioral seizures following TMEV infection. Therefore, both resident CNS cells and infiltrating cells are necessary for seizure development.
Product Citations
-
-
Cell Biology
Macrophages eat more after disruption of cis interactions between CD47 and the checkpoint receptor SIRPα.
In Journal of Cell Science on 1 January 2020 by Hayes, B. H., Tsai, R. K., et al.
PubMed
The macrophage checkpoint receptor SIRPα signals against phagocytosis by binding CD47 expressed on all cells - including macrophages. Here, inhibiting cis interactions between SIRPα and CD47 on the same macrophage increases eating approximately the same as inhibiting trans interactions. Antibody blockade of CD47, as pursued in clinical trials against cancer, is applied separately to human-derived macrophages and to red blood cell (RBC) targets for phagocytosis, and both scenarios produce surprisingly similar increases in RBC engulfment. Blockade of both macrophages and targets results in hyper-phagocytosis, and knockdown of macrophage-CD47 likewise increases eating of 'foreign' cells and particles, decreases SIRPα's baseline inhibitory signaling, and linearly increases binding of soluble-CD47 in trans, consistent with cis-trans competition. Many cell types express both SIRPα and CD47, including mouse melanoma B16 cells, and CRISPR-mediated deletions modulate B16 phagocytosis consistent with cis-trans competition. Additionally, soluble SIRPα binding to human-CD47 displayed on Chinese hamster ovary (CHO) cells is suppressed by SIRPα co-display, and atomistic computations confirm SIRPα bends and binds CD47 in cis. Safety and efficacy profiles for CD47-SIRPα blockade might therefore reflect a disruption of both cis and trans interactions. © 2020. Published by The Company of Biologists Ltd.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Triggering the TCR Developmental Checkpoint Activates a Therapeutically Targetable Tumor Suppressive Pathway in T-cell Leukemia.
In Cancer Discovery on 1 September 2016 by Trinquand, A., dos Santos, N. R., et al.
PubMed
Cancer onset and progression involves the accumulation of multiple oncogenic hits, which are thought to dominate or bypass the physiologic regulatory mechanisms in tissue development and homeostasis. We demonstrate in T-cell acute lymphoblastic leukemia (T-ALL) that, irrespective of the complex oncogenic abnormalities underlying tumor progression, experimentally induced, persistent T-cell receptor (TCR) signaling has antileukemic properties and enforces a molecular program resembling thymic negative selection, a major developmental event in normal T-cell development. Using mouse models of T-ALL, we show that induction of TCR signaling by high-affinity self-peptide/MHC or treatment with monoclonal antibodies to the CD3ε chain (anti-CD3) causes massive leukemic cell death. Importantly, anti-CD3 treatment hampered leukemogenesis in mice transplanted with either mouse- or patient-derived T-ALLs. These data provide a strong rationale for targeted therapy based on anti-CD3 treatment of patients with TCR-expressing T-ALL and demonstrate that endogenous developmental checkpoint pathways are amenable to therapeutic intervention in cancer cells. T-ALLs are aggressive malignant lymphoid proliferations of T-cell precursors characterized by high relapse rates and poor prognosis, calling for the search for novel therapeutic options. Here, we report that the lineage-specific TCR/CD3 developmental checkpoint controlling cell death in normal T-cell progenitors remains switchable to induce massive tumor cell apoptosis in T-ALL and is amenable to preclinical therapeutic intervention. Cancer Discov; 6(9); 972-85. ©2016 AACR.See related commentary by Lemonnier and Mak, p. 946This article is highlighted in the In This Issue feature, p. 932. ©2016 American Association for Cancer Research.
-
-
-
Cancer Research
Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects.
In Gut Microbes on 1 December 2025 by Liang, Y., Du, M., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy with limited effective treatment options. Emerging evidence links enriched environment (EE)-induced eustress to PDAC inhibition. However, the underlying mechanisms remain unclear. In this study, we explored the role of gut microbiota in PDAC-suppressive effects of EE. We demonstrated that depletion of gut microbiota with antibiotics abolished EE-induced tumor suppression, while fecal microbiota transplantation (FMT) from EE mice significantly inhibited tumor growth in both subcutaneous and orthotopic PDAC models housed in standard environment. 16S rRNA sequencing revealed that EE enhanced gut microbiota diversity and selectively enriched probiotic Lactobacillus, particularly L. reuteri. Treatment with L. reuteri significantly suppressed PDAC tumor growth and increased natural killer (NK) cell infiltration into the tumor microenvironment. Depletion of NK cells alleviated the anti-tumor effects of L. reuteri, underscoring the essential role of NK cell-mediated immunity in anti-tumor response. Clinical analysis of PDAC patients showed that higher fecal Lactobacillus abundance correlated with improved progression-free and overall survival, further supporting the therapeutic potential of L. reuteri in PDAC. Overall, this study identifies gut microbiota as a systemic regulator of PDAC under psychological stress. Supplementation of psychobiotic Lactobacillus may offer a novel therapeutic strategy for PDAC.
-
-
-
Cancer Research
An immunometabolic prodrug strategy overcomes DHODH inhibitor resistance in refractory melanoma.
In J Exp Clin Cancer Res on 14 November 2025 by Hai, Y., Wang, W., et al.
PubMed
Metabolic reprogramming, particularly upregulated de novo pyrimidine biosynthesis, drives cancer progression and immune evasion. Dihydroorotate dehydrogenase (DHODH), a key enzyme in this pathway, is a promising therapeutic target, but its inhibitors often face resistance in immune-refractory melanoma, linked to low basal stimulator of interferon genes (STING) expression.
-
-
-
Cancer Research
-
Genetics
Oncostatin M induces epigenetic reprogramming in renal cell carcinoma-associated endothelial cells.
In Commun Biol on 6 November 2025 by Nguyen-Tran, H. H., Nguyen, T. N., et al.
PubMed
The molecular and functional changes in endothelial cells during disease progression such as cancer have been noted but the mechanism of their activation is still under-studied. Previously we discovered that tumor-derived Oncostatin M induced tumor-associated vascular phenotypes, and the activated endothelial cells in turn promoted tumor progression and metastasis of clear-cell renal cell carcinoma (ccRCC). However, the mechanism of Oncostatin M action remains unknown. Here, we reveal that Oncostatin M signaling triggers specific epigenetic reprogramming of endothelial cells through upregulation of lysine acetyltransferase 6B, leading to increased histone 3 lysine 14 acetylation (H3K14ac) in vitro and in vivo. H3K14ac-modified chromatins upregulate specific gene sets associated with hypoxic response, hyper-angiogenesis, inflammation, and mesenchymal transition. Targeting H3K14ac in endothelial cells by interfering with acetyltransferase 6B function or neutralizing Oncostatin M ameliorates the premalignant hyperplastic phenotypes in the autochthonous ccRCC mouse model and diminishes tumor growth and metastasis in the ccRCC xenograft model.
-
-
-
Immunology and Microbiology
Functional CFTR may be required for Prevotella melaninogenica regulation of epithelial cell defense against Staphylococcus aureus.
In J Cyst Fibros on 5 November 2025 by Goryachok, M., Fairbanks-Mahnke, A., et al.
PubMed
Prevotella melaninogenica is enriched in the lungs of people with cystic fibrosis (pwCF), yet its functional impact on respiratory tract homeostasis remains incompletely understood. Prior studies identified immune modulatory effects following lung exposure to Prevotella, but the relevance of these findings for CF infections is unknown.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Immunology and Microbiology
Blocking CCR1+ macrophages overcomes resistance to immune checkpoint inhibitors in melanoma.
In Cell Commun Signal on 3 November 2025 by Su, X., Huang, R., et al.
PubMed
Chemokines and their receptors play a pivotal role in shaping the tumor microenvironment (TME) and modulating immune responses by orchestrating immune cell recruitment, spatial positioning, and facilitating cell-cell interactions. However, the exact mechanisms underlying chemokine signaling across different cell populations within the TME remain poorly understood. In this study, we utilized multiple-omics approaches to explore the relationship between CCR1+ macrophages, CD8+ exhausted T (Tex) cells, and immune checkpoint blockade (ICB) therapy response, as well as the role of chemokine signaling in the formation of CCR1+ macrophage and CD8+ Tex cell niches. We found that CCR1+ macrophages were closely associated with ICB outcomes in melanoma. Additionally, combination therapy with a CCR1 antagonist and anti-PD-1 monoclonal antibody significantly reduced tumor burden in melanoma mouse models, which was attributed to the substantial depletion of CD8+ Tex cells. Further, CCR1+ macrophages were found to co-localize with CD8+ Tex cells in human melanoma tissue, and the CCR1+ macrophage-CD8+ Tex cell niche was correlated with ICB treatment response in mice. Importantly, the CCR1-CCL3 axis was identified as a critical mediator in the formation of this niche. Overall, our study underscores the spatial relationship between CCR1+ macrophages and CD8+ Tex cells in ICB therapy, providing a promising strategy to overcome ICB resistance in melanoma.
-
-
Evaluating the Therapeutic Efficacy of an Anti-BAFF Receptor Antibody Using a Rheumatoid Arthritis Mouse Model.
In Antibodies (Basel) on 20 October 2025 by Aharon, A., Birnboim-Perach, R., et al.
PubMed
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint inflammation that leads to tissue damage and disability. RA affects approximately 0.5-1% of the global population and is driven by a complex interplay of genetic susceptibility, environmental factors, and immune dysregulation. While biologic and targeted synthetic DMARDs improved RA treatment, they have limitations in efficacy, safety, and accessibility. B-cell-targeting therapies, such as anti-CD20, have shown effectiveness, but only with broad immunosuppression, which can increase infection risk and compromise humoral immunity. Therefore, there is an unmet need for more selective therapeutic strategies that modulate pathogenic immune pathways while preserving protective immune functions. It has been suggested that targeting the BAFF pathway may offer a more favorable therapeutic approach compared to targeting CD20.
-
-
Immunology and Microbiology
-
Cancer Research
Macrophage repolarization by immune checkpoint blockade drives T cell engagement in the tumor microenvironment.
In iScience on 17 October 2025 by Kwok, T., Silva-Junior, I. A., et al.
PubMed
Immunotherapy combinations can improve patient outcomes, yet the interactions within the tumor microenvironment (TME) that drive therapeutic synergy are poorly understood. Tumor establishment drives monocyte recruitment and differentiation into tumor-associated macrophages (TAMs), which have essential roles in coordinating immune responses and are thus attractive targets for therapeutic modulation. In a murine model of combination anti-programmed cell death protein 1 (PD-1) and its ligand (anti-PD-L1) checkpoint blockade, tumor control was associated with increased infiltration of CD8+ T cells and M1-like repolarization of TAMs. Live-cell imaging of the tumor microenvironment revealed close contacts between tumor-infiltrating CD8+ T cells and TAMs, in which the extent of the contact interfaces increased with combination immunotherapy. Treatment with anti-PD-L1 was able to increase macrophage expression of pro-inflammatory factors and phagocytic activity, suggesting a role for TAMs in reactivating CD8+ T cells in the TME. However, co-treatment with anti-PD-1 was ultimately necessary for tumor control, indicating the need for combination targeting of the TME.
-
-
-
Immunology and Microbiology
Non-neutralizing antibodies to influenza A matrix-protein-2-ectodomain are broadly effective therapeutics and resistant to viral escape mutations.
In Sci Adv on 12 September 2025 by Kim, T., Bimler, L., et al.
PubMed
Influenza A viruses remain a global health threat, yet no universal antibody therapy exists. Clinical programs have centered on neutralizing mAbs, only to be thwarted by strain specificity and rapid viral escape. We instead engineered three non-neutralizing IgG2a mAbs that target distinct, overlapping epitopes within the conserved N terminus of the M2 ectodomain (M2e). Combined at low dose, this "triple M2e-mAb" confers robust prophylactic and therapeutic protection in mice challenged with diverse human and zoonotic IAV strains, including highly pathogenic variants. Therapeutic efficacy depends on Fc-mediated effector activity via FcγRI, FcγRIII, and FcγRIV, rather than in vitro neutralization. Serial passaging in triple M2e-mAb-treated immunocompetent and immunodeficient hosts failed to generate viral escape mutants. Our findings redefine the influenza-specific antibody therapeutic design and support Fc-optimized, non-neutralizing M2e-mAbs as a broadly effective, mutation-resistant, off-the-shelve therapy with direct relevance to human pandemic preparedness.
-
-
-
Cancer Research
Intrinsic Properties of the Lymph Node Render It Immunologically Susceptible to Metastasis.
In Cancer Discov on 4 September 2025 by Kahn, B., Ng, R. W. S., et al.
PubMed
Lymph nodes (LN) are the staging grounds for antitumor immunity; therefore, their high susceptibility to metastatic colonization is a paradox. Previous studies have suggested that extrinsic tumor-derived factors precondition the draining LN to enable tumor cell survival by promoting a state of immune suppression. In this study, we investigate whether properties of the LN itself may impede its ability to clear metastasizing tumor cells. Using multiple immunocompetent transplant models, we show that LNs possess intrinsic features, independent of preconditioning, which make them an advantageous site for tumor cells to evade T-cell control. Tumor growth in the LN is facilitated by regulatory T cells, which locally suppress the cytolytic capacity of tumor-specific CD8+ T cells by restricting IL2. These findings identify an intrinsic mechanism that contributes to the high rate of LN metastasis in solid tumors.
-
-
-
Cancer Research
-
Immunology and Microbiology
Arginine depletion potentiates standard-of-care chemo-immunotherapy in preclinical models of high-risk neuroblastoma.
In J Exp Clin Cancer Res on 14 August 2025 by Hanssen, K. M., Murray, J., et al.
PubMed
Dysregulated amino acid metabolism creates cancer-specific vulnerabilities. Neuroblastoma tumors have dysregulated arginine metabolism that renders them sensitive to systemic arginine deprivation. Arginase therapy has been proposed as a therapeutic approach for neuroblastoma treatment and has a favorable safety profile in pediatric cancer patients, however optimal therapeutic combinations remain unexplored.
-
-
-
Cancer Research
a-TIGIT mAb belrestotug in combination with anti-PD1 induces an immunocompetent tumor microenvironment (TME)
In medRxiv on 25 July 2025 by Cuende, J., Rosewick, N., et al.
-
-
-
Cancer Research
Neutralization of acyl coenzyme A binding protein for the experimental prevention and treatment of hepatocellular carcinoma.
In Cell Rep Med on 15 July 2025 by Li, S., Motiño, O., et al.
PubMed
Acyl coenzyme A binding protein (ACBP encoded by diazepam binding inhibitor DBI) is involved in non-malignant liver diseases. Here, we show that DBI mRNA and circulating ACBP/DBI levels are increased in patients with hepatocellular carcinoma (HCC). We investigated its role in hepatocarcinogenesis in mice, inhibiting ACBP/DBI by three methods: (1) inducible whole-body or liver-specific knockout of DBI, (2) a point mutation of the ACBP/DBI receptor (GABRG2), and (3) induction of autoantibodies neutralizing ACBP/DBI. ACBP/DBI plays a major pro-carcinogenic role in HCC induced by intrahepatic transplantation of HCC cell lines, transgenic co-expression of the two oncogenes Myc and Ctnnb1, and chronic challenge with a Western-style diet together with either carbon tetrachloride (CCl4) or diethylnitrosamine. ACBP/DBI inhibition normalizes HCC-associated gene expression, reducing oncogenic alterations in cell cycle-, immunomodulatory-, and ferroptosis-regulatory genes. ACBP/DBI inhibition increases HCC responses to PD-1 blockade and sensitizes HCC to the therapeutic induction of ferroptosis. Hence, ACBP/DBI constitutes an actionable target involved in HCC pathogenesis.
-
-
-
Immunology and Microbiology
-
Cancer Research
IL-2 immunotherapy rescues irradiation-induced T cell exhaustion in mouse colon cancer.
In iScience on 20 June 2025 by Yong, C. S. M., Telarovic, I., et al.
PubMed
Radiotherapy (RT) can stimulate anti-cancer T cell responses, and cytokines, notably interleukin-2 (IL-2), are necessary for optimal T cell function and memory. However, timing and IL-2 receptor (IL-2R) bias of IL-2 signals are ill-defined. Using image-guided RT in a mouse colon cancer model, we observed single high-dose (20 Gy) RT transiently upregulated IL-2Rα (CD25) on effector CD8+ T cells, facilitating the use of CD25-biased IL-2 immunotherapy. Timed administration of CD25-biased IL-2 treatment after RT favored intratumoral expansion of CD8+ T cells over regulatory T cells, which resulted in comparable anti-tumor effects as with RT plus IL-2Rβ (CD122)-biased IL-2 immunotherapy. Moreover, intratumoral CD8+ T cells of animals receiving combined IL-2R-biased IL-2 and RT showed reduced markers of exhaustion. These combination treatments affected both primary irradiated and distant non-irradiated tumors and achieved durable responses. We demonstrate that timed IL-2R subunit-biased IL-2 immunotherapy synergizes with single high-dose RT to achieve potent anti-cancer immunity.
-
-
-
Cancer Research
Intrinsic immunosuppressive features of monocytes suppress CAR-T19 through IL-1 pathway modulation in mantle cell lymphoma.
In Mol Ther Oncol on 18 June 2025 by Yun, K., Sakemura, R. L., et al.
PubMed
CD19-targeted chimeric antigen receptor T cells (CAR-T19) have shown remarkable success in B cell malignancies, but most patients relapse within 1-2 years. Here, we identified interleukin-1 (IL-1) receptor antagonist (IL-1ra) as a mediator of M2-like macrophage-derived inhibition of CAR-T19 in mantle cell lymphoma (MCL), as well as a potential target to enhance CAR-T19 efficacy. In preclinical models that recapitulated interactions between tumor, macrophages, and T cells, we demonstrated that M2-derived IL-1ra impairs IL-1 signaling and functions of CAR-T19. These findings were validated using clinical samples from the ZUMA-2 trial that led to the FDA approval of CAR-T19 in MCL. Single-cell RNA sequencing of CAR-T19 products and baseline myeloid cells indicated downregulated IL-1β production, enriched immunosuppressive phenotypes, and IL-1ra upregulation in the non-responder monocytes, as well as impaired IL-1β signaling and T cell functions in the non-responder CAR-T19 products. Furthermore, our preclinical studies of IL-1β showed enhanced CAR-T antitumor activities. Collectively, these data present a potential role for IL-1 signaling and IL-1ra in CAR-T19 failure.
-
-
Interleukin-27 is antiviral at the maternal-fetal interface.
In Research Square on 5 June 2025 by Jurado, K. A., Merlino, M., et al.
-
-
Cancer Research
-
Stem Cells and Developmental Biology
Cleavage of CAD by caspase-3 determines the cancer cell fate during chemotherapy.
In Nat Commun on 30 May 2025 by Ma, J., Zhao, J., et al.
PubMed
Metabolic heterogeneity resulting from the intra-tumoral heterogeneity mediates massive adverse outcomes of tumor therapy, including chemotherapeutic resistance, but the mechanisms inside remain largely unknown. Here, we find that the de novo pyrimidine synthesis pathway determines the chemosensitivity. Chemotherapeutic drugs promote the degradation of cytosolic Carbamoyl-phosphate synthetase II, Aspartate transcarbamylase, and Dihydroorotase (CAD), an enzyme that is rate-limiting for pyrimidine synthesis, leading to apoptosis. We also find that CAD needs to be cleaved by caspase-3 on its Asp1371 residue, before its degradation. Overexpressing CAD or mutating Asp1371 to block caspase-3 cleavage confers chemoresistance in xenograft and Cldn18-ATK gastric cancer models. Importantly, mutations related to Asp1371 of CAD are found in tumor samples that failed neoadjuvant chemotherapy and pharmacological targeting of CAD-Asp1371 mutations using RMY-186 ameliorates chemotherapy efficacy. Our work reveals the vulnerability of de novo pyrimidine synthesis during chemotherapy, highlighting CAD as a promising therapeutic target and biomarker.
-
-
Interferon-γ and IL-27 positively regulate type 1 regulatory T cell development during adaptive tolerance.
In iScience on 16 May 2025 by Lecky, D. A. J., Sheriff, L., et al.
PubMed
Strong T cell receptor (TCR) and interleukin (IL)-27 signaling influence type 1 regulatory (Tr1) T cell development, but whether other signals determine their differentiation is unclear. Utilizing Tg4 TCR transgenic mice, we established a model for rapid Tr1 cell induction. A single high dose of [4Y]-MBP peptide drove the differentiation of Il10+ T cells with Tr1 cell mRNA and protein signatures. Kinetic transcriptional and phenotypic analyses revealed that the Tr1 cell module was transient and preceded by Ifng transcription in other CD4+ T cells. Changes in Tr1 cell frequency correlated with altered macrophage activation, while neutralization of interferon (IFN)γ reduced Tr1 cell frequency and the TCR signal strength markers Nur77, inducible T cell costimulator (ICOS), and OX40. Antibody depletion experiments inferred that the relevant source of IFNγ was not natural killer (NK) cell derived. Additionally, blocking IL-27 in combination with IFNγ neutralization additively reduced Tr1 cell frequency in vivo. These findings reveal that IFNγ has a non-redundant role in augmenting Tr1 cell differentiation in vivo.
-
Interleukin-27 is antiviral at the maternal-fetal interface
In bioRxiv on 28 April 2025 by Merlino, M. S., Barksdale, B., et al.