InVivoPlus rat IgG1 isotype control, anti-trinitrophenol
Product Description
Specifications
| Isotype | Rat IgG1, λ |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687813 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Groves, H. T., et al (2020). "Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence" mBio 11(1).
PubMed
Respiratory viral infections are extremely common, but their impacts on the composition and function of the gut microbiota are poorly understood. We previously observed a significant change in the gut microbiota after viral lung infection. Here, we show that weight loss during respiratory syncytial virus (RSV) or influenza virus infection was due to decreased food consumption, and that the fasting of mice altered gut microbiota composition independently of infection. While the acute phase tumor necrosis factor alpha (TNF-α) response drove early weight loss and inappetence during RSV infection, this was not sufficient to induce changes in the gut microbiota. However, the depletion of CD8(+) cells increased food intake and prevented weight loss, resulting in a reversal of the gut microbiota changes normally observed during RSV infection. Viral infection also led to changes in the fecal gut metabolome, with a significant shift in lipid metabolism. Sphingolipids, polyunsaturated fatty acids (PUFAs), and the short-chain fatty acid (SCFA) valerate were all increased in abundance in the fecal metabolome following RSV infection. Whether this and the impact of infection-induced anorexia on the gut microbiota are part of a protective anti-inflammatory response during respiratory viral infections remains to be determined. IMPORTANCE The gut microbiota has an important role in health and disease: gut bacteria can generate metabolites that alter the function of immune cells systemically. Understanding the factors that can lead to changes in the gut microbiome may help to inform therapeutic interventions. This is the first study to systematically dissect the pathway of events from viral lung infection to changes in gut microbiota. We show that the cellular immune response to viral lung infection induces inappetence, which in turn alters the gut microbiome and metabolome. Strikingly, there was an increase in lipids that have been associated with the resolution of disease. This opens up new paths of investigation: first, what is the (presumably secreted) factor made by the T cells that can induce inappetence? Second, is inappetence an adaptation that accelerates recovery from infection, and if so, does the microbiome play a role in this?
Al Sayed, M. F., et al (2019). "T-cell-Secreted TNFα Induces Emergency Myelopoiesis and Myeloid-Derived Suppressor Cell Differentiation in Cancer" Cancer Res 79(2): 346-359.
PubMed
Hematopoiesis in patients with cancer is characterized by reduced production of red blood cells and an increase in myelopoiesis, which contributes to the immunosuppressive environment in cancer. Some tumors produce growth factors that directly stimulate myelopoiesis such as G-CSF or GM-CSF. However, for a majority of tumors that do not directly secrete hematopoietic growth factors, the mechanisms involved in the activation of myelopoiesis are poorly characterized. In this study, we document in different murine tumor models activated hematopoiesis with increased proliferation of long-term and short-term hematopoietic stem cells and myeloid progenitor cells. As a consequence, the frequency of myeloid-derived suppressor cells and its ratio to CD8(+) T cells increased in tumor-bearing mice. Activation of hematopoiesis and myeloid differentiation in tumor-bearing mice was induced by TNFα, which was mainly secreted by activated CD4(+) T cells. Therefore, the activated adaptive immune system in cancer induces emergency myelopoiesis and immunosuppression. SIGNIFICANCE: These findings characterize a regulatory circuit linking activated T cells to suppression of tumor-specific immune responses, providing a conceptual advance in the understanding of emergency-hematopoiesis in cancer and opening new targets for therapeutic approaches.
Claser, C., et al (2019). "Lung endothelial cell antigen cross-presentation to CD8(+)T cells drives malaria-associated lung injury" Nat Commun 10(1): 4241.
PubMed
Malaria-associated acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are life-threatening manifestations of severe malaria infections. The pathogenic mechanisms that lead to respiratory complications, such as vascular leakage, remain unclear. Here, we confirm that depleting CD8(+)T cells with anti-CD8β antibodies in C57BL/6 mice infected with P. berghei ANKA (PbA) prevent pulmonary vascular leakage. When we transfer activated parasite-specific CD8(+)T cells into PbA-infected TCRβ(-/-) mice (devoid of all T-cell populations), pulmonary vascular leakage recapitulates. Additionally, we demonstrate that PbA-infected erythrocyte accumulation leads to lung endothelial cell cross-presentation of parasite antigen to CD8(+)T cells in an IFNγ-dependent manner. In conclusion, pulmonary vascular damage in ALI is a consequence of IFNγ-activated lung endothelial cells capturing, processing, and cross-presenting malaria parasite antigen to specific CD8(+)T cells induced during infection. The mechanistic understanding of the immunopathogenesis in malaria-associated ARDS and ALI provide the basis for development of adjunct treatments.
Bauche, D., et al (2018). "LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis" Immunity 49(2): 342-352 e345.
PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3(+) regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1beta production from intestinal-resident CX3CR1(+) macrophages but not CD103(+) dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1(+) macrophage production of IL-23 and IL-1beta. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1(+) tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function.
Kugel, C. H., 3rd, et al (2018). "Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations" Clin Cancer Res 24(21): 5347-5356.
PubMed
Purpose: We have shown that the aged microenvironment increases melanoma metastasis, and decreases response to targeted therapy, and here we queried response to anti-PD1.Experimental Design: We analyzed the relationship between age, response to anti-PD1, and prior therapy in 538 patients. We used mouse models of melanoma, to analyze the intratumoral immune microenvironment in young versus aged mice and confirmed our findings in human melanoma biopsies.Results: Patients over the age of 60 responded more efficiently to anti-PD-1, and likelihood of response to anti-PD-1 increased with age, even when we controlled for prior MAPKi therapy. Placing genetically identical tumors in aged mice (52 weeks) significantly increased their response to anti-PD1 as compared with the same tumors in young mice (8 weeks). These data suggest that this increased response in aged patients occurs even in the absence of a more complex mutational landscape. Next, we found that young mice had a significantly higher population of regulatory T cells (Tregs), skewing the CD8(+):Treg ratio. FOXP3 staining of human melanoma biopsies revealed similar increases in Tregs in young patients. Depletion of Tregs using anti-CD25 increased the response to anti-PD1 in young mice.Conclusions: While there are obvious limitations to our study, including our inability to conduct a meta-analysis due to a lack of available data, and our inability to control for mutational burden, there is a remarkable consistency in these data from over 500 patients across 8 different institutes worldwide. These results stress the importance of considering age as a factor for immunotherapy response. Clin Cancer Res; 24(21); 5347-56. ©2018 AACR See related commentary by Pawelec, p. 5193.
Kuranda, K., et al (2018). "Exposure to wild-type AAV drives distinct capsid immunity profiles in humans" J Clin Invest 128(12): 5267-5279.
PubMed
Recombinant adeno-associated virus (AAV) vectors have been broadly adopted as a gene delivery tool in clinical trials, owing to their high efficiency of transduction of several host tissues and their low immunogenicity. However, a considerable proportion of the population is naturally exposed to the WT virus from which AAV vectors are derived, which leads to the acquisition of immunological memory that can directly determine the outcome of gene transfer. Here, we show that prior exposure to AAV drives distinct capsid immunity profiles in healthy subjects. In peripheral blood mononuclear cells (PBMCs) isolated from AAV-seropositive donors, recombinant AAV triggered TNF-α secretion in memory CD8+ T cells, B cell differentiation into antibody-secreting cells, and anti-capsid antibody production. Conversely, PBMCs isolated from AAV-seronegative individuals appeared to carry a population of NK cells reactive to AAV. Further, we demonstrated that the AAV capsid activates IL-1β and IL-6 cytokine secretion in monocyte-related dendritic cells (moDCs). IL-1β and IL-6 blockade inhibited the anti-capsid humoral response in vitro and in vivo. These results provide insights into immune responses to AAV in humans, define a possible role for moDCs and NK cells in capsid immunity, and open new avenues for the modulation of vector immunogenicity.
Zhang, Y., et al (2018). "Macrophage-Associated PGK1 Phosphorylation Promotes Aerobic Glycolysis and Tumorigenesis" Mol Cell 71(2): 201-215.e207.
PubMed
Macrophages are a dominant leukocyte population in the tumor microenvironment and actively promote cancer progression. However, the molecular mechanism underlying the role of macrophages remains poorly understood. Here we show that polarized M2 macrophages enhance 3-phosphoinositide-dependent protein kinase 1 (PDPK1)-mediated phosphoglycerate kinase 1 (PGK1) threonine (T) 243 phosphorylation in tumor cells by secreting interleukin-6 (IL-6). This phosphorylation facilitates a PGK1-catalyzed reaction toward glycolysis by altering substrate affinity. Inhibition of PGK1 T243 phosphorylation or PDPK1 in tumor cells or neutralization of macrophage-derived IL-6 abrogates macrophage-promoted glycolysis, proliferation, and tumorigenesis. In addition, PGK1 T243 phosphorylation correlates with PDPK1 activation, IL-6 expression, and macrophage infiltration in human glioblastoma multiforme (GBM). Moreover, PGK1 T243 phosphorylation also correlates with malignance and prognosis of human GBM. Our findings demonstrate a novel mechanism of macrophage-promoted tumor growth by regulating tumor cell metabolism, implicating the therapeutic potential to disrupt the connection between macrophages and tumor cells by inhibiting PGK1 phosphorylation.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
TIM3+ breast cancer cells license immune evasion during micrometastasis outbreak.
In Cancer Cell on 11 August 2025 by Rozalén, C., Sangrador, I., et al.
PubMed
In metastasis, the dynamics of tumor-immune interactions during micrometastasis remain unclear. Identifying the vulnerabilities of micrometastases before outbreaking into macrometastases can reveal therapeutic opportunities for metastasis. Here, we report a function of T cell immunoglobulin and mucin domain 3 (TIM3) in tumor cells during micrometastasis using breast cancer (BC) metastasis mouse models. TIM3 is highly upregulated in micrometastases, promoting survival, stemness, and immune escape. TIM3+ tumor cells are specifically selected during early seeding of micrometastasis. Mechanistically, TIM3 increases β-catenin/interleukin-1β (IL-1β) signaling, leading to stemness and immune-evasion by inducing immunosuppressive γδ T cells and reducing CD8 T cells during micrometastasis. Clinical data confirm increased TIM3+ tumor cells in BC metastasis and TIM3+ tumor cells as a biomarker of poor outcome in BC patients. (Neo)adjuvant TIM3 blockade reduces the metastatic seeding and incidence in preclinical models. These findings unveil a specific mechanism of micrometastasis immune-evasion and the potential use of TIM3 blockade for subclinical metastasis.
-
-
-
Genetics
Microbiota regulates the TET1-mediated DNA hydroxymethylation program in innate lymphoid cell differentiation.
In Nat Commun on 5 June 2024 by Zhang, X., Gao, X., et al.
PubMed
Innate lymphoid cell precursors (ILCPs) develop into distinct subsets of innate lymphoid cells (ILCs) with specific functions. The epigenetic program underlying the differentiation of ILCPs into ILC subsets remains poorly understood. Here, we reveal the genome-wide distribution and dynamics of the DNA methylation and hydroxymethylation in ILC subsets and their respective precursors. Additionally, we find that the DNA hydroxymethyltransferase TET1 suppresses ILC1 but not ILC2 or ILC3 differentiation. TET1 deficiency promotes ILC1 differentiation by inhibiting TGF-β signaling. Throughout ILCP differentiation at postnatal stage, gut microbiota contributes to the downregulation of TET1 level. Microbiota decreases the level of cholic acid in the gut, impairs TET1 expression and suppresses DNA hydroxymethylation, ultimately resulting in an expansion of ILC1s. In adult mice, TET1 suppresses the hyperactivation of ILC1s to maintain intestinal homeostasis. Our findings provide insights into the microbiota-mediated epigenetic programming of ILCs, which links microbiota-DNA methylation crosstalk to ILC differentiation.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Tumour extracellular vesicles and particles induce liver metabolic dysfunction.
In Nature on 1 June 2023 by Wang, G., Li, J., et al.
PubMed
Cancer alters the function of multiple organs beyond those targeted by metastasis1,2. Here we show that inflammation, fatty liver and dysregulated metabolism are hallmarks of systemically affected livers in mouse models and in patients with extrahepatic metastasis. We identified tumour-derived extracellular vesicles and particles (EVPs) as crucial mediators of cancer-induced hepatic reprogramming, which could be reversed by reducing tumour EVP secretion via depletion of Rab27a. All EVP subpopulations, exosomes and principally exomeres, could dysregulate hepatic function. The fatty acid cargo of tumour EVPs-particularly palmitic acid-induced secretion of tumour necrosis factor (TNF) by Kupffer cells, generating a pro-inflammatory microenvironment, suppressing fatty acid metabolism and oxidative phosphorylation, and promoting fatty liver formation. Notably, Kupffer cell ablation or TNF blockade markedly decreased tumour-induced fatty liver generation. Tumour implantation or pre-treatment with tumour EVPs diminished cytochrome P450 gene expression and attenuated drug metabolism in a TNF-dependent manner. We also observed fatty liver and decreased cytochrome P450 expression at diagnosis in tumour-free livers of patients with pancreatic cancer who later developed extrahepatic metastasis, highlighting the clinical relevance of our findings. Notably, tumour EVP education enhanced side effects of chemotherapy, including bone marrow suppression and cardiotoxicity, suggesting that metabolic reprogramming of the liver by tumour-derived EVPs may limit chemotherapy tolerance in patients with cancer. Our results reveal how tumour-derived EVPs dysregulate hepatic function and their targetable potential, alongside TNF inhibition, for preventing fatty liver formation and enhancing the efficacy of chemotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Kindlin-1 regulates IL-6 secretion and modulates the immune environment in breast cancer models.
In Elife on 8 March 2023 by Webb, E. R., Dodd, G. L., et al.
PubMed
The adhesion protein Kindlin-1 is over-expressed in breast cancer where it is associated with metastasis-free survival; however, the mechanisms involved are poorly understood. Here, we report that Kindlin-1 promotes anti-tumor immune evasion in mouse models of breast cancer. Deletion of Kindlin-1 in Met-1 mammary tumor cells led to tumor regression following injection into immunocompetent hosts. This was associated with a reduction in tumor infiltrating Tregs. Similar changes in T cell populations were seen following depletion of Kindlin-1 in the polyomavirus middle T antigen (PyV MT)-driven mouse model of spontaneous mammary tumorigenesis. There was a significant increase in IL-6 secretion from Met-1 cells when Kindlin-1 was depleted and conditioned media from Kindlin-1-depleted cells led to a decrease in the ability of Tregs to suppress the proliferation of CD8+ T cells, which was dependent on IL-6. In addition, deletion of tumor-derived IL-6 in the Kindlin-1-depleted tumors reversed the reduction of tumor-infiltrating Tregs. Overall, these data identify a novel function for Kindlin-1 in regulation of anti-tumor immunity, and that Kindlin-1 dependent cytokine secretion can impact the tumor immune environment.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Neuroscience
Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion.
In Sci Adv on 3 February 2023 by Sun, C., Ye, Y., et al.
PubMed
One of the major obstacles to treating pancreatic ductal adenocarcinoma (PDAC) is its immunoresistant microenvironment. The functional importance and molecular mechanisms of Schwann cells in PDAC remains largely elusive. We characterized the gene signature of tumor-associated nonmyelinating Schwann cells (TASc) in PDAC and indicated that the abundance of TASc was correlated with immune suppressive tumor microenvironment and the unfavorable outcome of patients with PDAC. Depletion of pancreatic-specific TASc promoted the tumorigenesis of PDAC tumors. TASc-expressed long noncoding RNA (lncRNA) plasmacytoma variant translocation 1 (PVT1) was triggered by the tumor cell-produced interleukin-6. Mechanistically, PVT1 modulated RAF proto-oncogene serine/threonine protein kinase-mediated phosphorylation of tryptophan 2,3-dioxygenase in TASc, facilitating its enzymatic activities in catalysis of tryptophan to kynurenine. Depletion of TASc-expressed PVT1 suppressed PDAC tumor growth. Furthermore, depletion of TASc using a small-molecule inhibitor effectively sensitized PDAC to immunotherapy, signifying the important roles of TASc in PDAC immune resistance.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
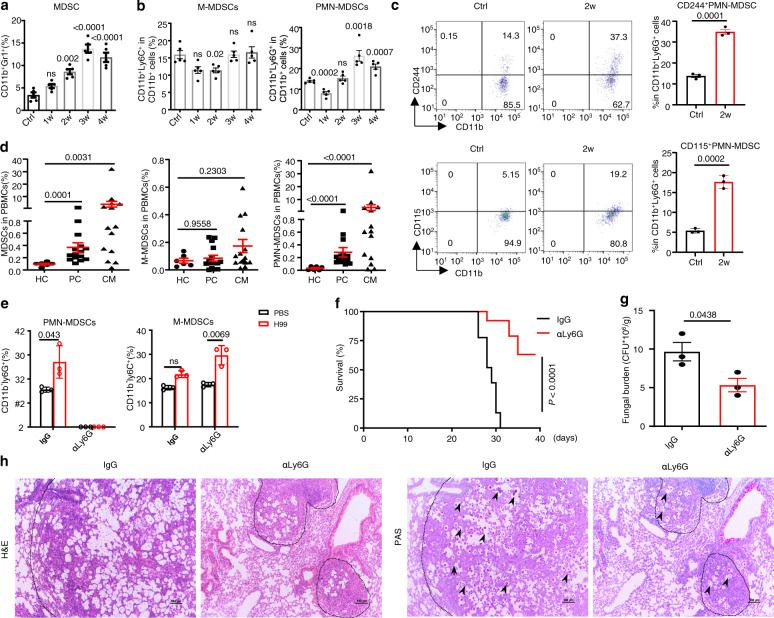
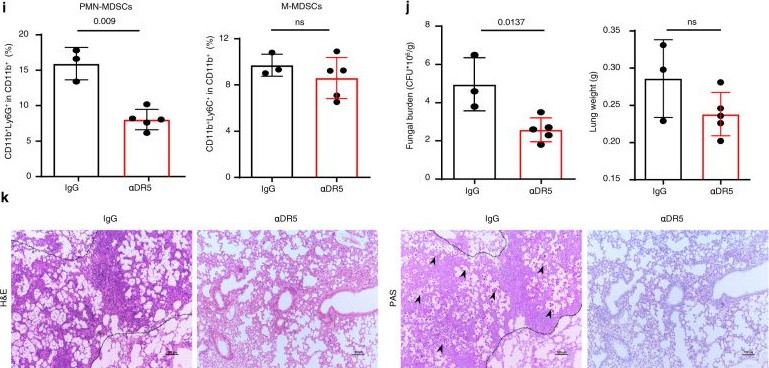
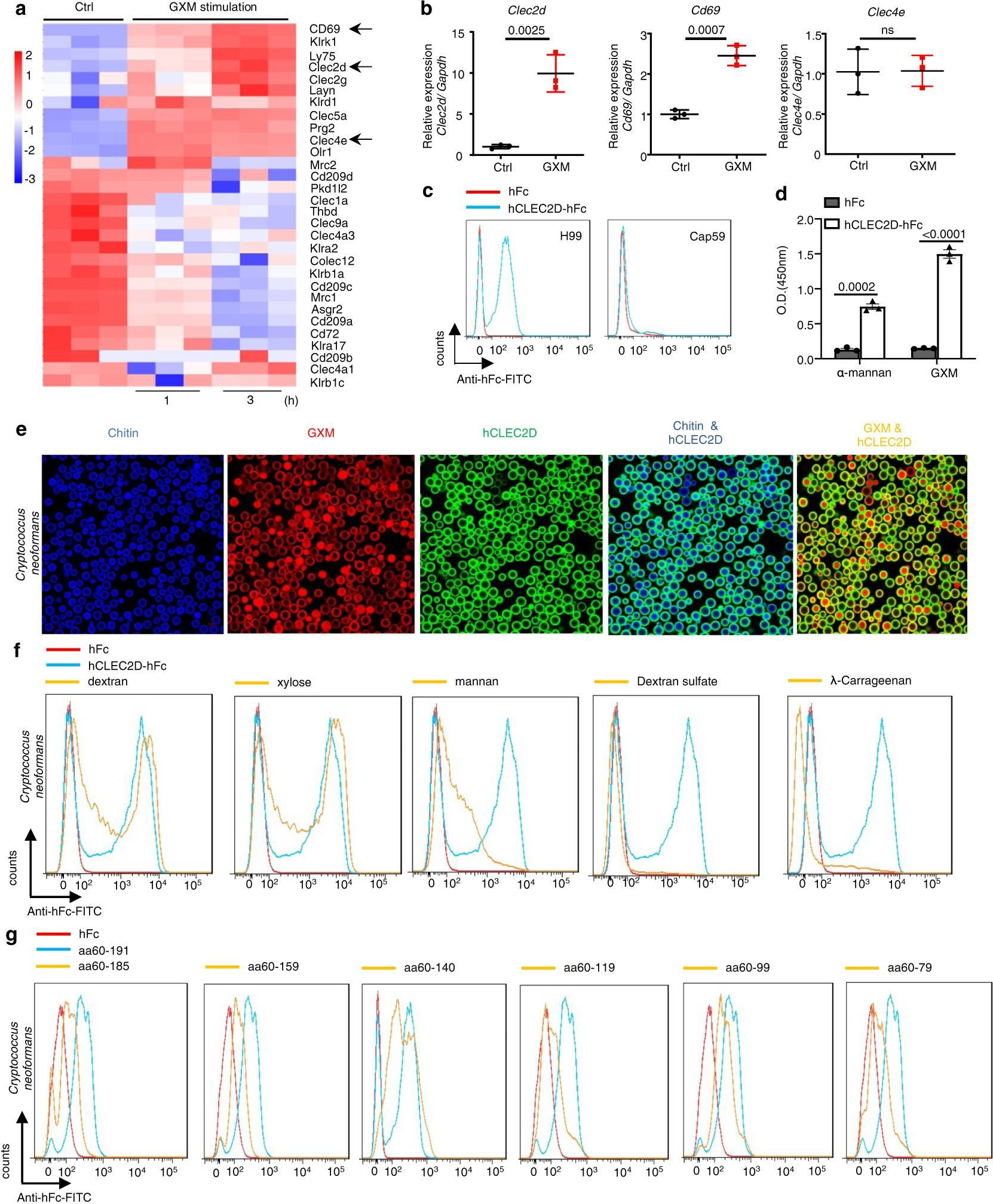
Inhibition of myeloid-derived suppressor cell arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection.
In Nat Commun on 14 July 2022 by Li, Y. N., Wang, Z. W., et al.
PubMed
Cryptococcosis is a potentially lethal disease that is primarily caused by the fungus Cryptococcus neoformans, treatment options for cryptococcosis are limited. Here, we show glucuronoxylomannan, the major polysaccharide component of C. neoformans, induces the recruitment of neutrophilic myeloid-derived suppressor cells in mice and patients with cryptococcosis. Depletion of neutrophilic myeloid-derived suppressor cells enhances host defense against C. neoformans infection. We identify C-type lectin receptor-2d recognizes glucuronoxylomannan to potentiate the immunosuppressive activity of neutrophilic myeloid-derived suppressor cells by initiating p38-mediated production of the enzyme arginase-1, which inhibits T-cell mediated antifungal responses. Notably, pharmacological inhibition of arginase-1 expression by a specific inhibitor of p38, SB202190, or an orally available receptor tyrosine kinase inhibitor, vandetanib, significantly enhances T-cell mediated antifungal responses against cryptococcosis. These data reveal a crucial suppressive role of neutrophilic myeloid-derived suppressor cells during cryptococcosis and highlight a promising immunotherapeutic application by inhibiting arginase-1 production to combat infectious diseases.
-
-
-
Cancer Research
Loss of the adhesion protein Kindlin-1 stimulates tumor clearance via modulation of Tregs
In bioRxiv on 5 March 2022 by Webb, E. R., Dodd, G., et al.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
Anti-Ly6G binding and trafficking mediate positive neutrophil selection to unleash the anti-tumor efficacy of radiation therapy.
In Oncoimmunology on 8 February 2021 by Boivin, G., Ancey, P. B., et al.
PubMed
The anti-Ly6G antibody is used to deplete Ly6Gpos neutrophils and study their role in diverse pathologies. However, depletion is never absolute, as Ly6Glow neutrophils resistant to depletion rapidly emerge. Studying the functionality of these residual neutrophils is necessary to interpret anti-Ly6G-based experimental designs. In vitro, we found anti-Ly6G binding induced Ly6G internalization, surface Ly6G paucity, and primed the oxidative burst of neutrophils upon TNF α co-stimulation. In vivo, we found neutrophils resistant to anti-Ly6G depletion exhibited anti-neutrophil-cytoplasmic-antibodies. In the pre-clinical KrasLox-STOP-Lox-G12D/WT; Trp53Flox/Flox mouse lung tumor model, abnormal neutrophil accumulation and aging was accompanied with an N2-like SiglecFpos polarization and ly6g downregulation. Consequently, SiglecFpos neutrophils exposed to anti-Ly6G reverted to Ly6Glow and were resistant to depletion. Noting that anti-Ly6G mediated neutrophil depletion alone had no anti-tumor effect, we found a long-lasting rate of tumor regression (50%) by combining anti-Ly6G with radiation-therapy, in this model reputed to be refractory to standard anticancer therapies. Mechanistically, anti-Ly6G regulated neutrophil aging while radiation-therapy enhanced the homing of anti-Ly6G-boundSiglecFneg neutrophils to tumors. This anti-tumor effect was recapitulated by G-CSF administration prior to RT and abrogated with an anti-TNFα antibody co-administration. In summary, we report that incomplete depletion of neutrophils using targeted antibodies can intrinsically promote their oxidative activity. This effect depends on antigen/antibody trafficking and can be harnessed locally using select delivery of radiation-therapy to impair tumor progression. This underutilized aspect of immune physiology may be adapted to expand the scope of neutrophil-related research.
-
-
-
Immunocytochemistry-immunofluorescence
-
Mus musculus (Mouse)
Durable and controlled depletion of neutrophils in mice.
In Nat Commun on 2 June 2020 by Boivin, G., Faget, J., et al.
PubMed
Neutrophils are an essential part of the innate immune system. To study their importance, experimental studies often aim to deplete these cells, generally by injecting anti-Ly6G or anti-Gr1 antibodies. However, these approaches are only partially effective, transient or lack specificity. Here we report that neutrophils remaining after anti-Ly6G treatment are newly derived from the bone marrow, instead of depletion escapees. Mechanistically, newly generated, circulating neutrophils have lower Ly6G membrane expression, and consequently reduced targets for anti-Ly6G-mediated depletion. To overcome this limitation, we develop a double antibody-based depletion strategy that enhances neutrophil elimination by anti-Ly6G treatment. This approach achieves specific, durable and controlled reduction of neutrophils in vivo, and may be suitable for studying neutrophil function in experimental models.
-
-
-
Control
-
Control
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations.
In Clin Cancer Res on 1 November 2018 by Kugel, C. H., Douglass, S. M., et al.
PubMed
Purpose: We have shown that the aged microenvironment increases melanoma metastasis, and decreases response to targeted therapy, and here we queried response to anti-PD1.Experimental Design: We analyzed the relationship between age, response to anti-PD1, and prior therapy in 538 patients. We used mouse models of melanoma, to analyze the intratumoral immune microenvironment in young versus aged mice and confirmed our findings in human melanoma biopsies.Results: Patients over the age of 60 responded more efficiently to anti-PD-1, and likelihood of response to anti-PD-1 increased with age, even when we controlled for prior MAPKi therapy. Placing genetically identical tumors in aged mice (52 weeks) significantly increased their response to anti-PD1 as compared with the same tumors in young mice (8 weeks). These data suggest that this increased response in aged patients occurs even in the absence of a more complex mutational landscape. Next, we found that young mice had a significantly higher population of regulatory T cells (Tregs), skewing the CD8+:Treg ratio. FOXP3 staining of human melanoma biopsies revealed similar increases in Tregs in young patients. Depletion of Tregs using anti-CD25 increased the response to anti-PD1 in young mice.Conclusions: While there are obvious limitations to our study, including our inability to conduct a meta-analysis due to a lack of available data, and our inability to control for mutational burden, there is a remarkable consistency in these data from over 500 patients across 8 different institutes worldwide. These results stress the importance of considering age as a factor for immunotherapy response. Clin Cancer Res; 24(21); 5347-56. ©2018 AACRSee related commentary by Pawelec, p. 5193.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Immune checkpoint blockade combined with IL-6 and TGF-β inhibition improves the therapeutic outcome of mRNA-based immunotherapy.
In Int J Cancer on 1 August 2018 by Bialkowski, L., Van der Jeught, K., et al.
PubMed
Improved understanding of cancer immunology has provided insight into the phenomenon of frequent tumor recurrence after initially successful immunotherapy. A delicate balance exists between the capacity of the immune system to control tumor growth and various resistance mechanisms that arise to avoid or even counteract the host's immune system. These resistance mechanisms include but are not limited to (i) adaptive expression of inhibitory checkpoint molecules in response to the proinflammatory environment and (ii) amplification of cancer stem cells, a small fraction of tumor cells possessing the capacity for self-renewal and mediating treatment resistance and formation of metastases after long periods of clinical remission. Several individual therapeutic agents have so far been developed to revert T-cell exhaustion or disrupt the cross-talk between cancer stem cells and the tumor-promoting microenvironment. Here, we demonstrate that a three-arm combination therapy-consisting of an mRNA-based vaccine to induce antigen-specific T-cell responses, monoclonal antibodies blocking inhibitory checkpoint molecules (PD-1, TIM-3, LAG-3), and antibodies targeting IL-6 and TGF-β-improves the therapeutic outcome in subcutaneous TC-1 tumors and significantly prolongs survival of treated mice. Our findings point to a need for a rational development of multidimensional anticancer therapies, aiming at the induction of tumor-specific immunity and simultaneously targeting multiple resistance mechanisms.
-
-
-
Immunology and Microbiology
Spleen-derived IFN-γ induces generation of PD-L1+-suppressive neutrophils during endotoxemia.
In J Leukoc Biol on 1 December 2017 by Langereis, J. D., Pickkers, P., et al.
PubMed
The immune inhibitory checkpoint molecule programmed death ligand (PD-L)-1 is increasingly recognized as an important player in the immune suppression observed in patients with sepsis, but its role has mainly been studied in monocytes. In an earlier study, we demonstrated that experimental human endotoxemia results in mobilization of a subset of PD-L1-expressing neutrophils displaying an IFN-γ-induced transcriptome profile. Herein, we identify the source of IFN-γ production during murine endotoxemia and its role in the generation of PD-L1+-suppressive neutrophils. We demonstrate that, similar to what we found in humans, murine endotoxemia results in the influx of a subset of PD-L1+ neutrophils in the circulation, and incubation of mouse neutrophils with recombinant IFN-γ profoundly increases PD-L1 expression. Furthermore, administration of anti-IFN-γ abrogated the generation of PD-L1+ neutrophils in endotoxemic mice. The critical involvement of the spleen is illustrated by the fact that splenectomy nullified circulating IFN-γ levels and substantially reduced the abundance of PD-L1+ neutrophils, whereas cotreatment with recombinant IFN-γ resulted in complete restoration of generation of PD-L1+ neutrophils in splenectomized mice. Finally, the functional importance of spleen-derived PD-L1+ neutrophils is exemplified by the finding that the profound decrease in T-lymphocyte proliferation observed in cells from endotoxemic mice was attenuated in cells from splenectomized animals. We demonstrated that spleen-derived IFN-γ induces generation of PD-L1+-suppressive neutrophils, implying that the spleen is critically involved in immune suppression during inflammatory diseases such as sepsis. Furthermore, our data suggest that IFN-γ plays a dual role by enhancing innate immunity and at the same time suppressing adaptive immune responses.
-