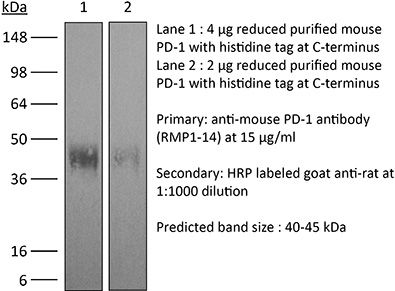
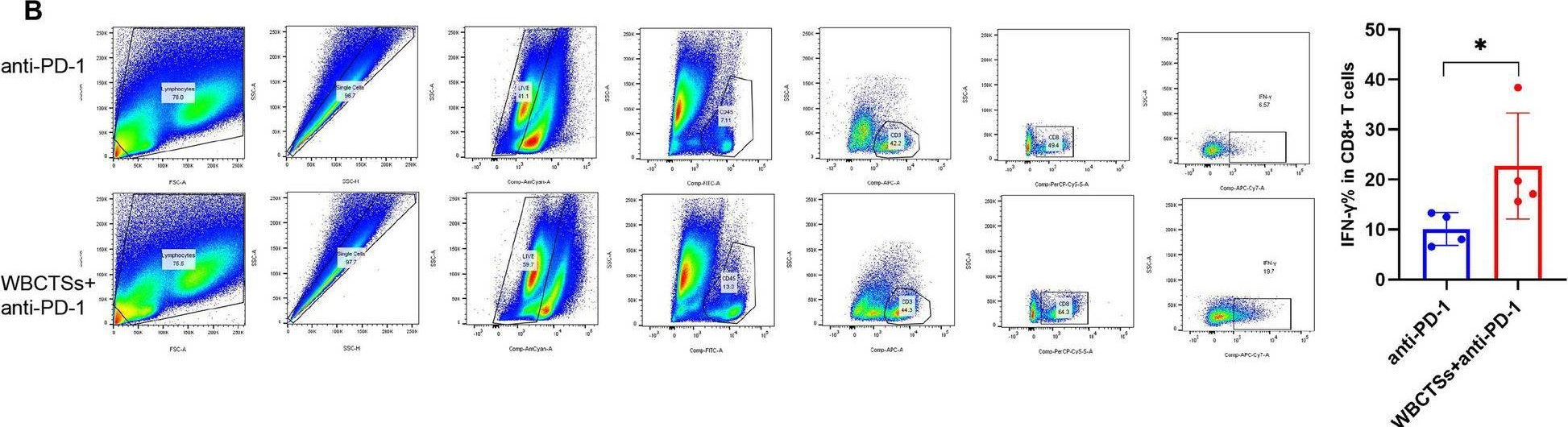
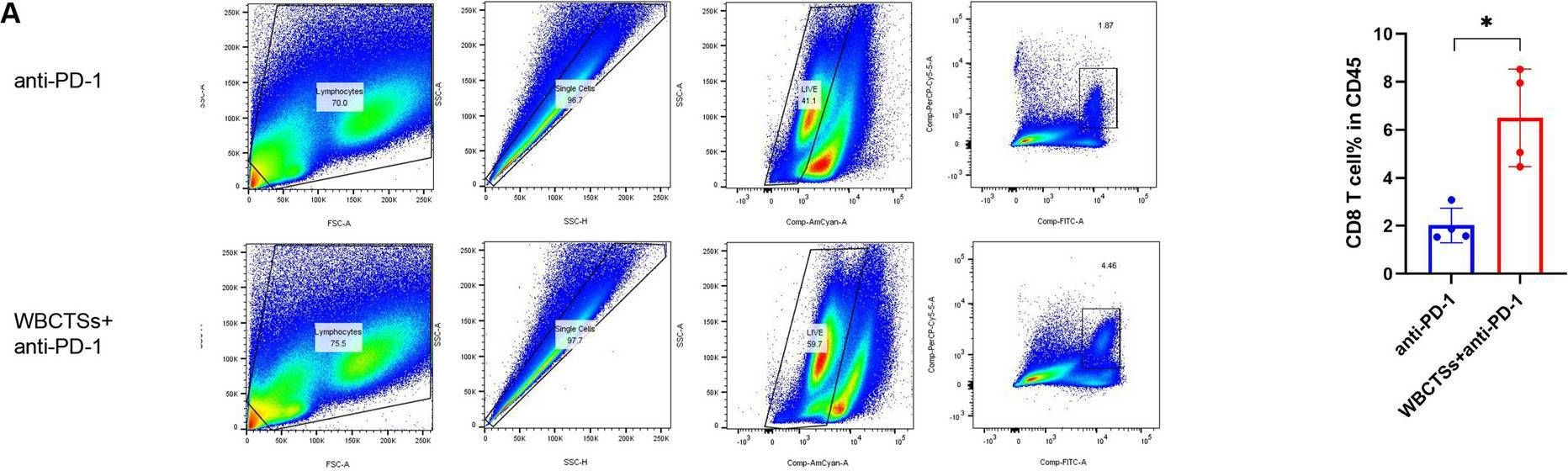
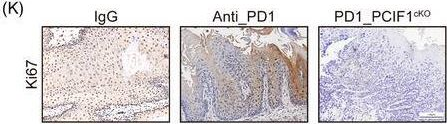
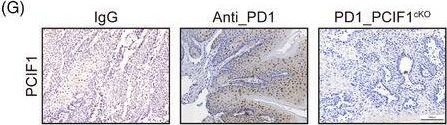
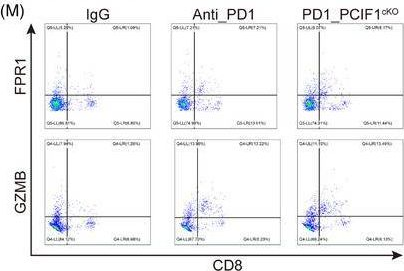
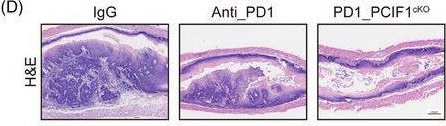
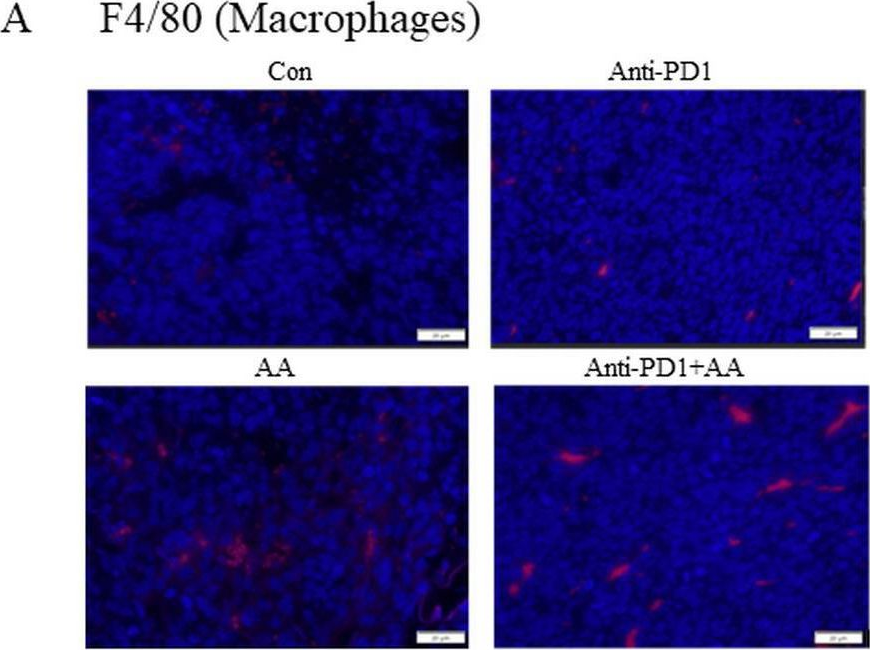
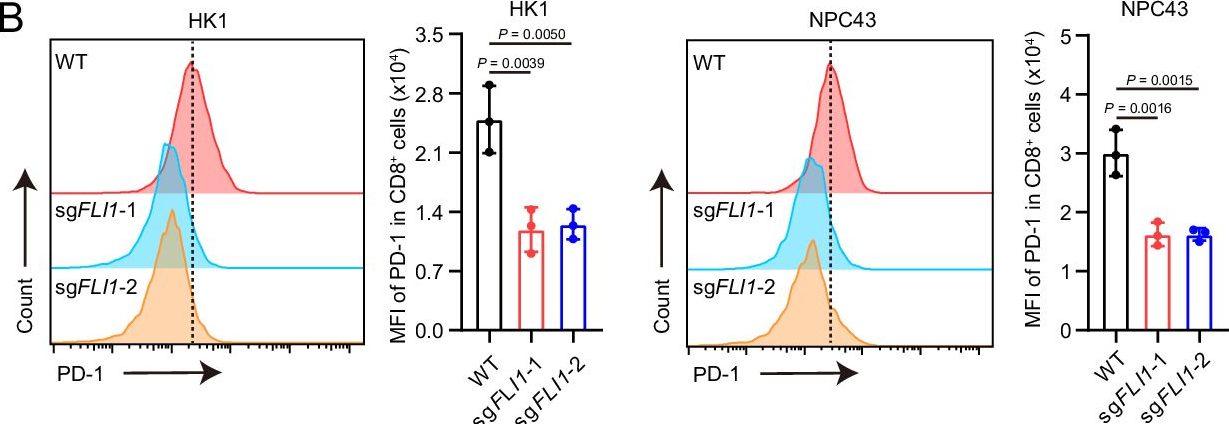
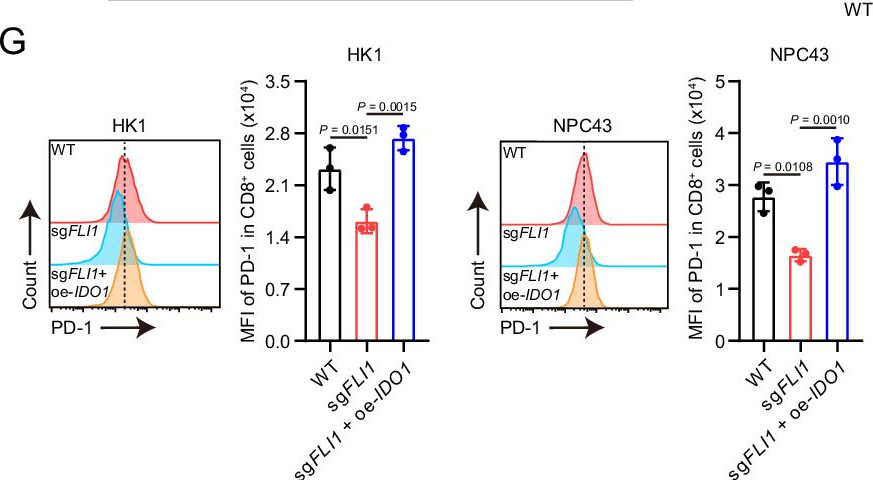
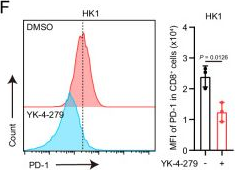
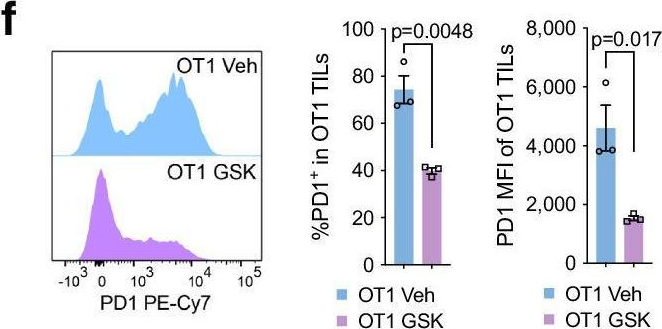
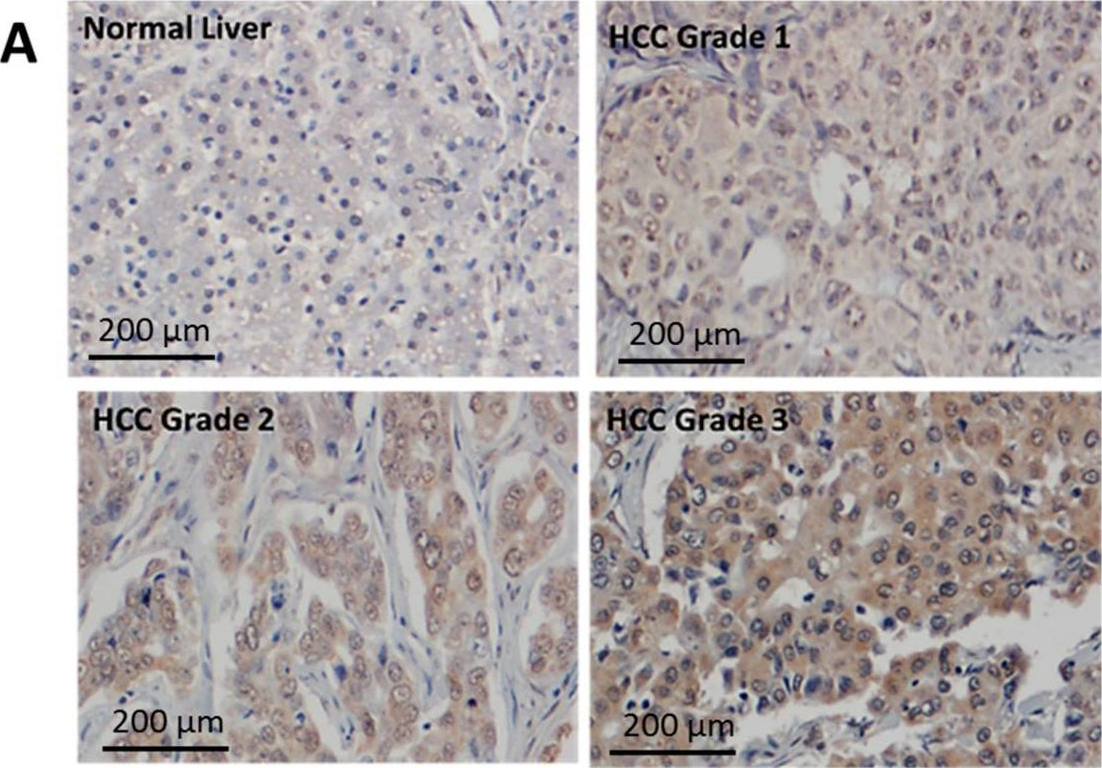
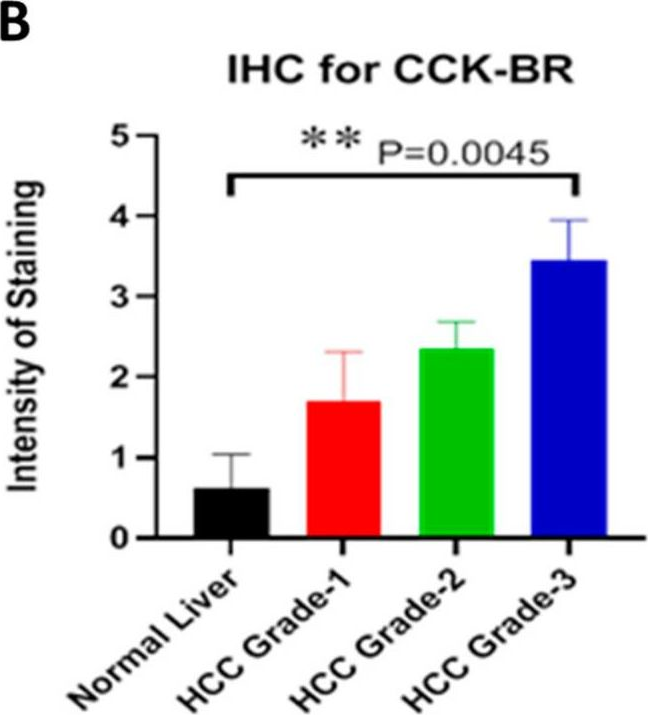
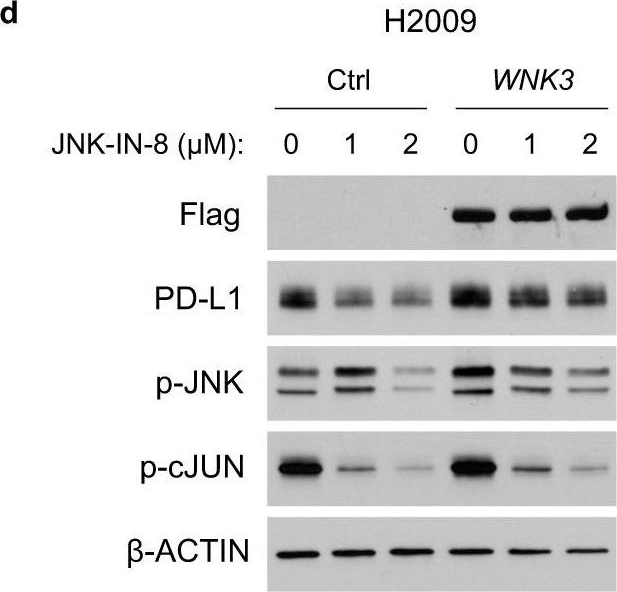
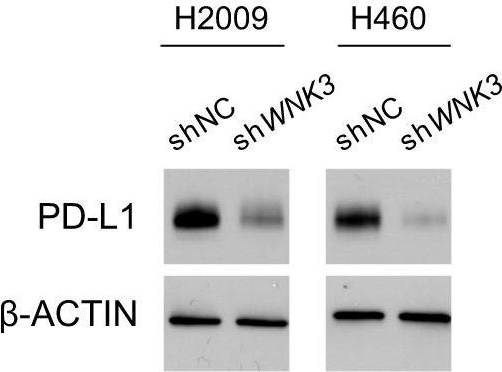
InVivoMAb anti-mouse PD-1 (CD279)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| Reported Applications | in vivo blocking of PD-1/PD-L signaling |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949053 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of PD-1/PD-L signaling
Grasselly, C., et al. (2018). "The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent" Front Immunol 9: 2100.
PubMed
In spite of impressive response rates in multiple cancer types, immune checkpoint inhibitors (ICIs) are active in only a minority of patients. Alternative strategies currently aim to combine immunotherapies with conventional agents such as cytotoxic chemotherapies. Here, we performed a study of PD-1 or PDL-1 blockade in combination with reference chemotherapies in four fully immunocompetent mouse models of cancer. We analyzed both the in vivo antitumor response, and the tumor immune infiltrate 4 days after the first treatment. in vivo tumor growth experiments revealed variable responsiveness to ICIs between models. We observed enhanced antitumor effects of the combination of immunotherapy with chemotherapy in the MC38 colon and MB49 bladder models, a lack of response in the 4T1 breast model, and an inhibition of ICIs activity in the MBT-2 bladder model. Flow cytometry analysis of tumor samples showed significant differences in all models between untreated and treated mice. At baseline, all the tumor models studied were predominantly infiltrated with cells harboring an immunosuppressive phenotype. Early alterations of the tumor immune infiltrate after treatment were found to be highly variable. We found that the balance between effector cells and immunosuppressive cells in the tumor microenvironment could be altered with some treatment combinations, but this effect was not always correlated with an impact on in vivo tumor growth. These results show that the combination of cytotoxic chemotherapy with ICIs may result in enhanced, similar or reduced antitumor activity, in a model- and regimen-dependent fashion. The present investigations should help to select appropriate combination regimens for ICIs.
in vivo blocking of PD-1/PD-L signaling
Triplett, T. A., et al. (2018). "Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme" Nat Biotechnol 36(8): 758-764.
PubMed
Increased tryptophan (Trp) catabolism in the tumor microenvironment (TME) can mediate immune suppression by upregulation of interferon (IFN)-gamma-inducible indoleamine 2,3-dioxygenase (IDO1) and/or ectopic expression of the predominantly liver-restricted enzyme tryptophan 2,3-dioxygenase (TDO). Whether these effects are due to Trp depletion in the TME or mediated by the accumulation of the IDO1 and/or TDO (hereafter referred to as IDO1/TDO) product kynurenine (Kyn) remains controversial. Here we show that administration of a pharmacologically optimized enzyme (PEGylated kynureninase; hereafter referred to as PEG-KYNase) that degrades Kyn into immunologically inert, nontoxic and readily cleared metabolites inhibits tumor growth. Enzyme treatment was associated with a marked increase in the tumor infiltration and proliferation of polyfunctional CD8(+) lymphocytes. We show that PEG-KYNase administration had substantial therapeutic effects when combined with approved checkpoint inhibitors or with a cancer vaccine for the treatment of large B16-F10 melanoma, 4T1 breast carcinoma or CT26 colon carcinoma tumors. PEG-KYNase mediated prolonged depletion of Kyn in the TME and reversed the modulatory effects of IDO1/TDO upregulation in the TME.
in vivo blocking of PD-1/PD-L signaling
Moynihan, K. D., et al. (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo blocking of PD-1/PD-L signaling
Vanpouille-Box, C., et al. (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo blocking of PD-1/PD-L signaling
Twyman-Saint Victor, C., et al. (2015). "Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer" Nature 520(7547): 373-377.
PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
in vivo blocking of PD-1/PD-L signaling
Zander, R. A., et al. (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo blocking of PD-1/PD-L signaling
Zelenay, S., et al. (2015). "Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity" Cell 162(6): 1257-1270.
PubMed
The mechanisms by which melanoma and other cancer cells evade anti-tumor immunity remain incompletely understood. Here, we show that the growth of tumors formed by mutant Braf(V600E) mouse melanoma cells in an immunocompetent host requires their production of prostaglandin E2, which suppresses immunity and fuels tumor-promoting inflammation. Genetic ablation of cyclooxygenases (COX) or prostaglandin E synthases in Braf(V600E) mouse melanoma cells, as well as in Nras(G12D) melanoma or in breast or colorectal cancer cells, renders them susceptible to immune control and provokes a shift in the tumor inflammatory profile toward classic anti-cancer immune pathways. This mouse COX-dependent inflammatory signature is remarkably conserved in human cutaneous melanoma biopsies, arguing for COX activity as a driver of immune suppression across species. Pre-clinical data demonstrate that inhibition of COX synergizes with anti-PD-1 blockade in inducing eradication of tumors, implying that COX inhibitors could be useful adjuvants for immune-based therapies in cancer patients.
in vivo blocking of PD-1/PD-L signaling
Evans, E. E., et al. (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo blocking of PD-1/PD-L signaling
Ngiow, S. F., et al. (2015). "A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1" Cancer Res 75(18): 3800-3811.
PubMed
Despite successes, thus far, a significant proportion of the patients treated with anti-PD1 antibodies have failed to respond. We use mouse tumor models of anti-PD1 sensitivity and resistance and flow cytometry to assess tumor-infiltrating immune cells immediately after therapy. We demonstrate that the expression levels of T-cell PD1 (PD1(lo)), myeloid, and T-cell PDL1 (PDL1(hi)) in the tumor microenvironment inversely correlate and dictate the efficacy of anti-PD1 mAb and function of intratumor CD8(+) T cells. In sensitive tumors, we reveal a threshold for PD1 downregulation on tumor-infiltrating CD8(+) T cells below which the release of adaptive immune resistance is achieved. In contrast, PD1(hi) T cells in resistant tumors fail to be rescued by anti-PD1 therapy and remain dysfunctional unless intratumor PDL1(lo) immune cells are targeted. Intratumor Tregs are partly responsible for the development of anti-PD1-resistant tumors and PD1(hi) CD8(+) T cells. Our analyses provide a framework to interrogate intratumor CD8(+) T-cell PD1 and immune PDL1 levels and response in human cancer. Cancer Res; 75(18); 3800-11. (c)2015 AACR.
in vivo blocking of PD-1/PD-L signaling
McGray, A. J., et al. (2014). "Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor" Mol Ther 22(1): 206-218.
PubMed
Despite clear evidence of immunogenicity, cancer vaccines only provide a modest clinical benefit. To evaluate the mechanisms that limit tumor regression following vaccination, we have investigated the weak efficacy of a highly immunogenic experimental vaccine using a murine melanoma model. We discovered that the tumor adapts rapidly to the immune attack instigated by tumor-specific CD8+ T cells in the first few days following vaccination, resulting in the upregulation of a complex set of biological networks, including multiple immunosuppressive processes. This rapid adaptation acts to prevent sustained local immune attack, despite continued infiltration by increasing numbers of tumor-specific T cells. Combining vaccination with adoptive transfer of tumor-specific T cells produced complete regression of the treated tumors but did not prevent the adaptive immunosuppression. In fact, the adaptive immunosuppressive pathways were more highly induced in regressing tumors, commensurate with the enhanced level of immune attack. Examination of tumor infiltrating T-cell functionality revealed that the adaptive immunosuppression leads to a progressive loss in T-cell function, even in tumors that are regressing. These novel observations that T cells produced by therapeutic intervention can instigate a rapid adaptive immunosuppressive response within the tumor have important implications for clinical implementation of immunotherapies.
in vivo blocking of PD-1/PD-L signaling
Mittal, D., et al. (2014). "Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor" Cancer Res 74(14): 3652-3658.
PubMed
Adenosine targeting is an attractive new approach to cancer treatment, but no clinical study has yet examined adenosine inhibition in oncology despite the safe clinical profile of adenosine A2A receptor inhibitors (A2ARi) in Parkinson disease. Metastasis is the main cause of cancer-related deaths worldwide, and therefore we have studied experimental and spontaneous mouse models of melanoma and breast cancer metastasis to demonstrate the efficacy and mechanism of a combination of A2ARi in combination with anti-PD-1 monoclonal antibody (mAb). This combination significantly reduces metastatic burden and prolongs the life of mice compared with either monotherapy alone. Importantly, the combination was only effective when the tumor expressed high levels of CD73, suggesting a tumor biomarker that at a minimum could be used to stratify patients that might receive this combination. The mechanism of the combination therapy was critically dependent on NK cells and IFNgamma, and to a lesser extent, CD8(+) T cells and the effector molecule, perforin. Overall, these results provide a strong rationale to use A2ARi with anti-PD-1 mAb for the treatment of minimal residual and metastatic disease.
in vivo blocking of PD-1/PD-L signaling
van der Werf, N., et al. (2013). "Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection" PLoS Pathog 9(3): e1003215.
PubMed
The suppression of protective Type 2 immunity is a principal factor driving the chronicity of helminth infections, and has been attributed to a range of Th2 cell-extrinsic immune-regulators. However, the intrinsic fate of parasite-specific Th2 cells within a chronic immune down-regulatory environment, and the resultant impact such fate changes may have on host resistance is unknown. We used IL-4gfp reporter mice to demonstrate that during chronic helminth infection with the filarial nematode Litomosoides sigmodontis, CD4(+) Th2 cells are conditioned towards an intrinsically hypo-responsive phenotype, characterised by a loss of functional ability to proliferate and produce the cytokines IL-4, IL-5 and IL-2. Th2 cell hypo-responsiveness was a key element determining susceptibility to L. sigmodontis infection, and could be reversed in vivo by blockade of PD-1 resulting in long-term recovery of Th2 cell functional quality and enhanced resistance. Contrasting with T cell dysfunction in Type 1 settings, the control of Th2 cell hypo-responsiveness by PD-1 was mediated through PD-L2, and not PD-L1. Thus, intrinsic changes in Th2 cell quality leading to a functionally hypo-responsive phenotype play a key role in determining susceptibility to filarial infection, and the therapeutic manipulation of Th2 cell-intrinsic quality provides a potential avenue for promoting resistance to helminths.
in vivo blocking of PD-1/PD-L signaling
Holmgaard, R. B., et al. (2013). "Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4" J Exp Med 210(7): 1389-1402.
PubMed
The cytotoxic T lymphocyte antigen-4 (CTLA-4)-blocking antibody ipilimumab results in durable responses in metastatic melanoma, though therapeutic benefit has been limited to a fraction of patients. This calls for identification of resistance mechanisms and development of combinatorial strategies. Here, we examine the inhibitory role of indoleamine 2,3-dioxygenase (IDO) on the antitumor efficacy of CTLA-4 blockade. In IDO knockout mice treated with anti-CTLA-4 antibody, we demonstrate a striking delay in B16 melanoma tumor growth and increased overall survival when compared with wild-type mice. This was also observed with antibodies targeting PD-1-PD-L1 and GITR. To highlight the therapeutic relevance of these findings, we show that CTLA-4 blockade strongly synergizes with IDO inhibitors to mediate rejection of both IDO-expressing and nonexpressing poorly immunogenic tumors, emphasizing the importance of the inhibitory role of both tumor- and host-derived IDO. This effect was T cell dependent, leading to enhanced infiltration of tumor-specific effector T cells and a marked increase in the effector-to-regulatory T cell ratios in the tumors. Overall, these data demonstrate the immunosuppressive role of IDO in the context of immunotherapies targeting immune checkpoints and provide a strong incentive to clinically explore combination therapies using IDO inhibitors irrespective of IDO expression by the tumor cells.
in vivo blocking of PD-1/PD-L signaling
John, L. B., et al. (2013). "Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells" Clin Cancer Res 19(20): 5636-5646.
PubMed
PURPOSE: To determine the antitumor efficacy and toxicity of a novel combination approach involving adoptive T-cell immunotherapy using chimeric antigen receptor (CAR) T cells with an immunomodulatory reagent for blocking immunosuppression. EXPERIMENTAL DESIGN: We examined whether administration of a PD-1 blocking antibody could increase the therapeutic activity of CAR T cells against two different Her-2(+) tumors. The use of a self-antigen mouse model enabled investigation into the efficacy, mechanism, and toxicity of this combination approach. RESULTS: In this study, we first showed a significant increase in the level of PD-1 expressed on transduced anti-Her-2 CD8(+) T cells following antigen-specific stimulation with PD-L1(+) tumor cells and that markers of activation and proliferation were increased in anti-Her-2 T cells in the presence of anti-PD-1 antibody. In adoptive transfer studies in Her-2 transgenic recipient mice, we showed a significant improvement in growth inhibition of two different Her-2(+) tumors treated with anti-Her-2 T cells in combination with anti-PD-1 antibody. The therapeutic effects observed correlated with increased function of anti-Her-2 T cells following PD-1 blockade. Strikingly, a significant decrease in the percentage of Gr1(+) CD11b(+) myeloid-derived suppressor cells (MDSC) was observed in the tumor microenvironment of mice treated with the combination therapy. Importantly, increased antitumor effects were not associated with any autoimmune pathology in normal tissue expressing Her-2 antigen. CONCLUSION: This study shows that specifically blocking PD-1 immunosuppression can potently enhance CAR T-cell therapy that has significant implications for potentially improving therapeutic outcomes of this approach in patients with cancer.
in vivo blocking of PD-1/PD-L signaling
Curran, M. A., et al. (2010). "PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors" Proc Natl Acad Sci U S A 107(9): 4275-4280.
PubMed
Vaccination with irradiated B16 melanoma cells expressing either GM-CSF (Gvax) or Flt3-ligand (Fvax) combined with antibody blockade of the negative T-cell costimulatory receptor cytotoxic T-lymphocyte antigen-4 (CTLA-4) promotes rejection of preimplanted tumors. Despite CTLA-4 blockade, T-cell proliferation and cytokine production can be inhibited by the interaction of programmed death-1 (PD-1) with its ligands PD-L1 and PD-L2 or by the interaction of PD-L1 with B7-1. Here, we show that the combination of CTLA-4 and PD-1 blockade is more than twice as effective as either alone in promoting the rejection of B16 melanomas in conjunction with Fvax. Adding alphaPD-L1 to this regimen results in rejection of 65% of preimplanted tumors vs. 10% with CTLA-4 blockade alone. Combination PD-1 and CTLA-4 blockade increases effector T-cell (Teff) infiltration, resulting in highly advantageous Teff-to-regulatory T-cell ratios with the tumor. The fraction of tumor-infiltrating Teffs expressing CTLA-4 and PD-1 increases, reflecting the proliferation and accumulation of cells that would otherwise be anergized. Combination blockade also synergistically increases Teff-to-myeloid-derived suppressor cell ratios within B16 melanomas. IFN-gamma production increases in both the tumor and vaccine draining lymph nodes, as does the frequency of IFN-gamma/TNF-alpha double-producing CD8(+) T cells within the tumor. These results suggest that combination blockade of the PD-1/PD-L1- and CTLA-4-negative costimulatory pathways allows tumor-specific T cells that would otherwise be inactivated to continue to expand and carry out effector functions, thereby shifting the tumor microenvironment from suppressive to inflammatory.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
Unraveling the role of HDAC3 as an immunotherapy prognostic biomarker and therapeutic target in advanced non-small cell lung cancer.
In Respir Res on 12 June 2025 by Dai, L., Huang, L., et al.
PubMed
This study investigates HDAC3 as a potential immunotherapy biomarker in advanced non-small cell lung cancer (aNSCLC), focusing on its association with treatment response to immune checkpoint inhibitors (ICIs).
-
-
-
Cancer Research
-
Immunology and Microbiology
Loss of LAPTM4A inhibits M2 polarization of tumor-associated macrophages in glioblastoma, promoting immune activation and enhancing anti-PD1 therapy.
In Commun Biol on 11 June 2025 by Geng, J., Liang, B., et al.
PubMed
Glioma is a highly aggressive central nervous system tumor with limited treatment options, presenting a significant challenge for effective therapy. Despite advancements, the role of tumor-associated macrophages (TAMs) in glioma remains poorly understood, especially regarding their polarization and its impact on the immune response. This study investigates the effects of Lysosomal-associated protein transmembrane 4 A (LAPTM4A) deficiency on the polarization of TAMs and its role in modulating anti-tumor immunity. Using C57BL/6 male mice, we established an orthotopic glioma model and employed single-cell RNA sequencing, flow cytometry, in vitro co-culture systems, and in vivo anti-PD-1 therapy experiments to explore the functional role of LAPTM4A. We found that LAPTM4A promotes M2 polarization of TAMs, contributing to glioma progression by enhancing cell proliferation and invasion. In contrast, LAPTM4A-deficient glioma models show a shift towards M1 macrophage phenotypes, leading to stronger immune activation and increased sensitivity to anti-PD-1 therapy. These results suggest that targeting LAPTM4A may provide a novel strategy to improve glioma treatment by modulating TAM polarization and enhancing immune responses. This research lays the groundwork for future therapies aimed at reprogramming the tumor microenvironment to combat glioblastoma.
-
-
-
Western Blotting
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Targeting PERP promotes anti-tumor immunity in HNSCC by regulating tumor immune microenvironment and metabolic homeostasis.
In Mol Cancer on 7 June 2025 by Wang, X., Tian, Y., et al.
PubMed
PERP may have the potential to function as an oncogene. However, the precise function, prognostic value, and predictive significance remain shrouded in ambiguity.
-
-
-
Cancer Research
-
Immunology and Microbiology
Glutamate transporter SLC1A6 promotes resistance to immunotherapy in cancer.
In Cancer Immunol Immunother on 7 June 2025 by Li, C., Lin, Y., et al.
PubMed
Resistance to immune checkpoint inhibitors remains a significant challenge in the treatment of cancer. Emerging evidence suggests that metabolic reprogramming plays a crucial role in tumor metabolism and progression. Our study strived to investigate the role and underlying mechanisms of the glutamate transporter SLC1A6 in resistance to immunotherapy of cancer.
-
-
-
Immunology and Microbiology
Characterisation of an autochthonous mouse ccRCC model of immune checkpoint inhibitor therapy resistance.
In Sci Rep on 5 June 2025 by Peighambari, A., Huang, H., et al.
PubMed
Many metastatic clear cell renal cell carcinomas (ccRCC) are resistant to immune checkpoint inhibitor therapies, however the mechanisms underlying sensitivity or resistance remain incompletely characterised. We demonstrate that ccRCCs in the Vhl/Trp53/Rb1 mutant mouse model are resistant to combined anti-PD-1/anti-CTLA-4 therapy alone and in combination with additional therapeutic agents that reflect current ccRCC clinical trials. However, in some animals in vivo checkpoint therapy allowed isolated splenic T cells to recognise cultured ccRCC cells from the same animal, implicating the tumour microenvironment in suppression of T cell activation. We identified putative immunosuppressive myeloid cell populations with features similar to myeloid cells in the microenvironment of human ccRCC. The expression patterns of immune checkpoint ligands in both the mouse model and in human ccRCC suggests that several checkpoint systems other than PD-1 and CTLA-4 are likely to represent the dominant T cell suppressive forces in ccRCC. Our findings characterise an autochthonous mouse ccRCC model of immune checkpoint inhibitor therapy resistance and pave the way for a systematic functional dissection of the identified potential molecular barriers to effective immune therapy of ccRCC.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Multi-omics unveils BCAA metabolism markers L-leucine and HMGCS1 as prognostic marker for immunotherapy efficacy in non-small cell lung cancer.
In Respir Res on 2 June 2025 by Dai, L., Wang, X., et al.
PubMed
This study aims to identify branched-chain amino acid (BCAA) plasma metabolites and gene signatures that enhance prognostic assessments in non-small cell lung cancer (NSCLC) patients receiving immunotherapy.
-
-
-
Cancer Research
-
Cell Biology
Gut microbial metabolite 4-hydroxybenzeneacetic acid drives colorectal cancer progression via accumulation of immunosuppressive PMN-MDSCs.
In J Clin Invest on 2 June 2025 by Liao, Q., Zhou, X., et al.
PubMed
Colorectal cancer (CRC) is characterized by an immune-suppressive microenvironment that contributes to tumor progression and immunotherapy resistance. The gut microbiome produces diverse metabolites that feature unique mechanisms of interaction with host targets, yet the role of many metabolites in CRC remains poorly understood. In this study, the microbial metabolite 4-hydroxybenzeneacetic acid (4-HPA) promoted the infiltration of PMN myeloid-derived suppressor cells (PMN-MDSCs) in the tumor microenvironment, consequently inhibiting the antitumor response of CD8+ T cells and promoting CRC progression in vivo. Mechanistically, 4-HPA activates the JAK2/STAT3 pathway, which upregulates CXCL3 transcription, thereby recruiting PMN-MDSCs to the CRC microenvironment. Selective knockdown of CXCL3 resensitized tumors to anti-PD-1 immunotherapy in vivo. Chlorogenic acid reduces the production of 4-HPA by microbiota, likewise abolishing 4-HPA-mediated immunosuppression. The 4-HPA content in CRC tissues was notably increased in patients with advanced CRC. Overall, the gut microbiome uses 4-HPA as a messenger to control chemokine-dependent accumulation of PMN-MDSC cells and regulate antitumor immunity in CRC. Our findings provide a scientific basis for establishing clinical intervention strategies to reverse the tumor immune microenvironment and improve the efficacy of immunotherapy by reducing the interaction among intestinal microbiota, tumor cells, and tumor immune cells.
-
-
-
Cancer Research
-
Cell Biology
Age-dependent differences in breast tumor microenvironment: challenges and opportunities for efficacy studies in preclinical models.
In Cell Death Differ on 1 June 2025 by Falvo, P., Gruener, S., et al.
PubMed
Immunity suffers a function deficit during aging, and the incidence of cancer is increased in the elderly. However, most cancer models employ young mice, which are poorly representative of adult cancer patients. We have previously reported that Triple-Therapy (TT), involving antigen-presenting-cell activation by vinorelbine and generation of TCF1+-stem-cell-like T cells (scTs) by cyclophosphamide significantly improved anti-PD-1 efficacy in anti-PD1-resistant models like Triple-Negative Breast Cancer (TNBC) and Non-Hodgkin's Lymphoma (NHL), due to T-cell-mediated tumor killing. Here, we describe the effect of TT on TNBC growth and on tumor-microenvironment (TME) of young (6-8w, representative of human puberty) versus adult (12 m, representative of 40y-humans) mice. TT-efficacy was similar in young and adults, as CD8+ scTs were only marginally reduced in adults. However, single-cell analyses revealed major differences in the TME: adults had fewer CD4+ scTs, B-naïve and NK-cells, and more memory-B-cells. Cancer-associated-fibroblasts (CAF) with an Extracellular Matrix (ECM) deposition-signature (Matrix-CAFs) were more common in young mice, while pro-inflammatory stromal populations and myofibroblasts were more represented in adults. Matrix-CAFs in adult mice displayed decreased ECM-remodeling abilities, reduced collagen deposition, and a different pattern of interactions with the other cells of the TME. Taken together, our results suggest that age-dependent differences in the TME should be considered when designing preclinical studies.
-
-
-
Cancer Research
Cancer therapy via neoepitope-specific monoclonal antibody cocktails.
In Cancer Immunol Immunother on 31 May 2025 by Hartman, C. J., Mohamed, A. O., et al.
PubMed
Cellular heterogeneity presents a significant challenge to cancer treatment. Antibody therapies targeting individual tumor-associated antigens can be extremely effective but are not suited for all patients and often fail against tumors with heterogeneous expression as tumor cells with low or no antigen expression escape targeting and develop resistance. Simultaneously targeting multiple tumor-specific proteins with multiple antibodies has the potential to overcome this barrier and improve efficacy, but relatively few widely expressed cancer-specific antigens are known. In contrast, neoepitopes, which arise from mutations unique to tumor cells, are considerably more abundant. However, since neoepitopes are not commonly shared between individuals, a patient-customized approach is necessary and motivates efforts to develop an efficient means to identify suitable target mutations and isolate neoepitope-specific monoclonal antibodies. Here, focusing on the latter goal, we use directed evolution in yeast and phage display systems to engineer antibodies from nonimmune, human antibody fragment libraries that are specific for neoepitopes previously reported in the B16F10 melanoma model. We demonstrate proof-of-concept for a pipeline that supports rapid isolation and functional enhancement of multiple neoepitope peptide-targeted monoclonal antibodies and demonstrate their robust binding to B16F10 cells and potent effector functions in vitro. These antibodies were combined and evaluated in vivo for anticancer activity in tumor-bearing mice, where they suppressed B16F10 tumor growth and prolonged survival. These findings emphasize the potential for clinical application of patient-customized antibody cocktails in the treatment of the many cancers poorly addressed by current therapies.
-
-
-
Cancer Research
-
Stem Cells and Developmental Biology
Cleavage of CAD by caspase-3 determines the cancer cell fate during chemotherapy.
In Nat Commun on 30 May 2025 by Ma, J., Zhao, J., et al.
PubMed
Metabolic heterogeneity resulting from the intra-tumoral heterogeneity mediates massive adverse outcomes of tumor therapy, including chemotherapeutic resistance, but the mechanisms inside remain largely unknown. Here, we find that the de novo pyrimidine synthesis pathway determines the chemosensitivity. Chemotherapeutic drugs promote the degradation of cytosolic Carbamoyl-phosphate synthetase II, Aspartate transcarbamylase, and Dihydroorotase (CAD), an enzyme that is rate-limiting for pyrimidine synthesis, leading to apoptosis. We also find that CAD needs to be cleaved by caspase-3 on its Asp1371 residue, before its degradation. Overexpressing CAD or mutating Asp1371 to block caspase-3 cleavage confers chemoresistance in xenograft and Cldn18-ATK gastric cancer models. Importantly, mutations related to Asp1371 of CAD are found in tumor samples that failed neoadjuvant chemotherapy and pharmacological targeting of CAD-Asp1371 mutations using RMY-186 ameliorates chemotherapy efficacy. Our work reveals the vulnerability of de novo pyrimidine synthesis during chemotherapy, highlighting CAD as a promising therapeutic target and biomarker.
-
-
-
Cancer Research
-
Immunology and Microbiology
VSV-CHIKV activates antitumor immunity by inducing pyroptosis in a melanoma model.
In Discov Oncol on 29 May 2025 by Wu, F., Zhan, Y., et al.
PubMed
Melanoma is the most dangerous skin cancer due to its difficulty in treatment, high recurrence rate and metastatic ability. As a vector for oncolytic viruses (OVs), vesicular stomatitis virus (VSV) has been shown to be effective against malignant melanoma. However, the glycoprotein G protein of VSV has potential neurotoxicity. It has been shown that replacing glycoprotein G with E3-E2-6K-E1 of chikungunya virus (CHIKV) reduces its neurotoxicity and targets gliomas. Therefore, the aim of this study was to investigate the oncolytic effect of recombinant VSV-CHIKV on melanoma and the underlying mechanism. In this study, we found that recombinant VSV-CHIKV triggered GSDMD-mediated melanoma cell pyroptosis. Importantly, the NLRP3/Caspase-1/GSDMD axis was activated after VSV-CHIKV infection in melanoma cell lines and in a xenograft mouse model. Inhibition of GSDMD blocked cell pyroptosis, antitumor immunity and the tumor response in response to VSV-CHIKV treatment, suggesting that VSV-CHIKV act through the GSDMD pathway. VSV-CHIKV-triggered GSDMD-mediated tumor pyroptosis recruited cytotoxic T lymphocytes (CTLs) into the tumor microenvironment, which was accompanied by the release of inflammatory mediators. This remodeled the tumor microenvironment and turned immunologically "cold" tumors into "hot" tumors, thereby sensitized these tumors to checkpoint blockade. Finally, the combination therapy of VSV-CHIKV and an immune checkpoint inhibitor (anti-PD-1) prolonged the survival of mice. In conclusion, the VSV-CHIKV strategy is an attractive biologic therapy against melanoma.
-
-
-
Immunology and Microbiology
A lyophilizable LNP vaccine enables STING-reinforced postoperational adjuvant immunotherapy.
In J Nanobiotechnology on 26 May 2025 by Yang, Y., Guo, J., et al.
PubMed
Immune checkpoint blockade therapy (iCBT) has revolutionized cancer treatment, however, there is a low response rate, especially in treating postsurgical reoccurring tumors. Vaccine based immunotherapy can sensitize iCBT, but its development was largely hindered by inefficient delivery and high requirements of storage. In this study, the vaccine loaded with immunostimulant was employed to improve iCBT-based adjuvant postsurgical therapy. A lyophilized, antigen E7 peptide and manganese ion (Mn2+) co-delivered tumor vaccine was developed based on lipid nanoparticles (EM@LNP). The vaccination efficacy was examined in both prophylactic and therapeutic schemes in murine subcutaneous models, the synergetic effect of vaccination combined with anti-PD-1 therapy was further investigated in post-operative tumor model. EM@LNP vaccination elicited effective CD8+T cell response through modulating tumor immunosuppressive microenvironment and conferring immune memory, demonstrating potent immunization in both preventive and therapeutic schemes. What's more, EM@LNP vaccination orchestrated with iCBT, efficiently repressing tumor recurrence. Further mechanism studies using inhibitor for cells invitro and the investigation using STING-/- mice confirmed that the cGAS-STING signaling pathway activated by Mn2+ is indispensable for LNP vaccination and the coordination with iCBT-based adjuvant immunotherapy. In summary, this study shows a lyophilized LNP vaccine could significantly amplify iCBT efficiency, providing a translational strategy of adjuvant immunotherapy for treating postsurgical tumor recurrence.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
IL-1β blockade prevents cardiotoxicity and improves the efficacy of immune checkpoint blockers and chemotherapy against pancreatic cancer in mice with obesity.
In J Immunother Cancer on 24 May 2025 by Talele, N. P., Kumra, H., et al.
PubMed
Immune checkpoint blockers (ICBs) have revolutionized cancer therapy, yet they remain largely ineffective in treating pancreatic ductal adenocarcinoma (PDAC). Moreover, ICBs can cause severe immune-related adverse events (irAEs), including fatal cardiac toxicity. Finally, obesity is a risk factor in PDAC that may differentially modulate ICB efficacy in a malignancy-dependent manner.
-
-
-
Cancer Research
Oral ENPP1 inhibitor designed using generative AI as next generation STING modulator for solid tumors.
In Nat Commun on 23 May 2025 by Pu, C., Cui, H., et al.
PubMed
Despite the STING-type-I interferon pathway playing a key role in effective anti-tumor immunity, the therapeutic benefit of direct STING agonists appears limited. In this study, we use several artificial intelligence techniques and patient-based multi-omics data to show that Ectonucleotide Pyrophosphatase/Phosphodiesterase 1 (ENPP1), which hydrolyzes STING-activating cyclic GMP-AMP (cGAMP), is a safer and more effective STING-modulating target than direct STING agonism in multiple solid tumors. We then leverage our generative chemistry artificial intelligence-based drug design platform to facilitate the design of ISM5939, an orally bioavailable ENPP1-selective inhibitor capable of stabilizing extracellular cGAMP and activating bystander antigen-presenting cells without inducing either toxic inflammatory cytokine release or tumor-infiltrating T-cell death. In murine syngeneic models across cancer types, ISM5939 synergizes with targeting the PD-1/PD-L1 axis and chemotherapy in suppressing tumor growth with good tolerance. Our findings provide evidence supporting ENPP1 as an innate immune checkpoint across solid tumors and reports an AI design-aided ENPP1 inhibitor, ISM5939, as a cutting-edge STING modulator for cancer therapy, paving a path for immunotherapy advancements.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
HDAC6 facilitates LUAD progression by inducing EMT and enhancing macrophage polarization towards the M2 phenotype.
In NPJ Precis Oncol on 22 May 2025 by Jiang, Y., Zhang, J., et al.
PubMed
Histone deacetylase 6 (HDAC6) plays a critical role in lung adenocarcinoma (LUAD) prognosis and the tumor immune microenvironment (TIME). This study, utilizing public datasets and experimental validation, revealed that HDAC6 is upregulated in LUAD, correlating with poor survival outcomes and an immunosuppressive TIME characterized by increased Tregs, CAFs, M2 macrophages, and MDSCs. HDAC6-high patients showed reduced immunotherapy response. HDAC6 knockout inhibited tumor growth, suppressed PI3K/AKT/mTOR signaling and EMT, and enhanced apoptosis and M1 macrophage recruitment. HDAC6 inhibition synergized with anti-PD-1 therapy, suggesting a potential combinatorial strategy for LUAD treatment. HDAC6 serves as a key prognostic marker and therapeutic target in LUAD.
-
-
-
Cancer Research
Glioma-neuronal circuit remodeling induces regional immunosuppression.
In Nat Commun on 22 May 2025 by Nejo, T., Krishna, S., et al.
PubMed
Neuronal activity-driven mechanisms influence glioblastoma cell proliferation and invasion, while glioblastoma remodels neuronal circuits. Although a subpopulation of malignant cells enhances neuronal connectivity, their impact on the immune system remains unclear. Here, we show that glioblastoma regions with enhanced neuronal connectivity exhibit regional immunosuppression, characterized by distinct immune cell compositions and the enrichment of anti-inflammatory tumor-associated macrophages (TAMs). In preclinical models, knockout of Thrombospondin-1 (TSP1/Thbs1) in glioblastoma cells suppresses synaptogenesis and glutamatergic neuronal hyperexcitability. Furthermore, TSP1 knockout restores antigen presentation-related genes, promotes the infiltration of pro-inflammatory TAMs and CD8 + T-cells in the tumor, and alleviates TAM-mediated T-cell suppression. Pharmacological inhibition of glutamatergic signaling also shifts TAMs toward a less immunosuppressive state, prolongs survival in mice, and shows the potential to enhance the efficacy of immune cell-based therapy. These findings confirm that glioma-neuronal circuit remodeling is strongly linked with regional immunosuppression and suggest that targeting glioma-neuron-immune crosstalk could provide avenues for immunotherapy.
-
-
-
Cancer Research
NECTIN4 regulates the cell surface expression of CD155 in non-small cell lung cancer cells and induces tumor resistance to PD-1 inhibitors.
In Cancer Immunol Immunother on 20 May 2025 by Mizusaki, S., Yoneshima, Y., et al.
PubMed
The development of immune checkpoint inhibitors has changed treatment strategies for some patients with non-small cell lung cancer (NSCLC). However, resistance remains a major problem, requiring the elucidation of resistance mechanisms, which might aid the development of novel therapeutic strategies. The upregulation of CD155, a primary ligand of the immune checkpoint receptor TIGIT, has been implicated in a mechanism of resistance to PD-1/PD-L1 inhibitors, and it is therefore important to characterize the mechanisms underlying the regulation of CD155 expression in tumor cells. The aim of this study was to identify a Nectin that might regulate CD155 expression in NSCLC and affect anti-tumor immune activity. In this study, we demonstrated that NECTIN4 regulated the cell surface expression and stabilization of CD155 by interacting and co-localizing with CD155 on the cell surface. In a syngeneic mouse model, NECTIN4-overexpressing cells exhibited increased cell surface CD155 and resistance to anti-PD-1 antibodies. Of note, combination therapy with anti-PD-1 and anti-TIGIT antibodies significantly suppressed tumor growth. These findings provide new insights into the mechanisms of resistance to anti-PD-1 antibodies and suggest that NECTIN4 could serve as a valuable marker in therapeutic strategies targeting TIGIT.
-
-
-
Cancer Research
-
Immunology and Microbiology
USP2 inhibition unleashes CD47-restrained phagocytosis and enhances anti-tumor immunity.
In Nat Commun on 16 May 2025 by Dai, P., Sun, Y., et al.
PubMed
The CD47/SIRPα axis conveys a 'don't eat me' signal, thereby thwarting the phagocytic clearance of tumor cells. Although blocking antibodies targeting CD47 have demonstrated promising anti-tumor effects in preclinical models, clinical trials involving human cancer patients have not yielded ideal results. Exploring the regulatory mechanisms of CD47 is imperative for devising more efficacious combinational therapies. Here, we report that inhibiting USP2 prompts CD47 degradation and reshapes the tumor microenvironment (TME), thereby enhancing anti-PD-1 immunotherapy. Mechanistically, USP2 interacts with CD47, stabilizing it through deubiquitination. USP2 inhibition destabilizes CD47, thereby boosting macrophage phagocytosis. Single-cell RNA sequencing shows USP2 inhibition reprograms TME, evidenced by increasing M1 macrophages and CD8+ T cells while reducing M2 macrophages. Combining ML364 with anti-PD-1 reduces tumor burden in mouse models. Clinically, low USP2 expression predicts a better response to anti-PD-1 treatment. Our findings uncover the regulatory mechanism of CD47 by USP2 and targeting this axis boosts anti-tumor immunity.
-
-
-
Cancer Research
-
Immunology and Microbiology
Sensitivity to immune checkpoint inhibitors in BRAF/MEK inhibitor refractory melanoma.
In J Immunother Cancer on 15 May 2025 by Patel, R. P., Lim, L. R. J., et al.
PubMed
Resistance to BRAF and MEK inhibitors (BRAFi/MEKi) in metastatic melanoma frequently results in cross-resistance to immune checkpoint inhibitors (ICI), limiting effective treatment options. However, a subset of BRAFi/MEKi-resistant patients remains responsive to second-line ICI, suggesting heterogeneous underlying resistance mechanisms. This study aimed to explore the tumor immune microenvironment in BRAFi/MEKi-resistant melanoma to uncover factors influencing sensitivity to second-line ICI therapy.
-
-
-
Immunology and Microbiology
Targeting legumain-mediated cell-cell interaction sensitizes glioblastoma to immunotherapy in preclinical models.
In J Clin Invest on 15 May 2025 by Pang, L., Guo, S., et al.
PubMed
Tumor-associated macrophages (TAMs) are the most prominent immune cell population in the glioblastoma (GBM) tumor microenvironment and play critical roles in promoting tumor progression and immunosuppression. Here we identified that TAM-derived legumain (LGMN) exhibited a dual role in regulating the biology of TAMs and GBM cells. LGMN promoted macrophage infiltration in a cell-autonomous manner by activating the GSK3β/STAT3 pathway. Moreover, TAM-derived LGMN activated integrin αv/AKT/p65 signaling to drive GBM cell proliferation and survival. Targeting of LGMN-directed macrophage (inhibiting GSK3β and STAT3) and GBM cell (inhibiting integrin αv) mechanisms resulted in an antitumor effect in immunocompetent GBM mouse models that was further enhanced by combination with anti-PD-1 therapy. Our study reveals a paracrine and autocrine mechanism of TAM-derived LGMN that promotes GBM progression and immunosuppression, providing effective therapeutic targets to improve immunotherapy in GBM.
-