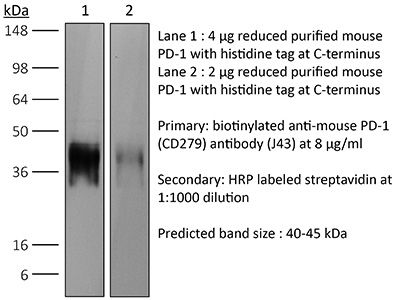
InVivoMAb anti-mouse PD-1 (CD279)
Product Description
Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| Reported Applications |
in vivo blocking of PD-1/PD-L signaling in vitro PD-1 neutralization Western blot |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107747 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of PD-1/PD-L signaling
Li, J., et al (2018). "Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8(+) T Cells" Immunity 48(4): 773-786 e775.
PubMed
The molecular mechanisms whereby CD8(+) T cells become “exhausted” in the tumor microenvironment remain unclear. Programmed death ligand-1 (PD-L1) is upregulated on tumor cells and PD-1-PD-L1 blockade has significant efficacy in human tumors; however, most patients do not respond, suggesting additional mechanisms underlying T cell exhaustion. B7 superfamily member 1 (B7S1), also called B7-H4, B7x, or VTCN1, negatively regulates T cell activation. Here we show increased B7S1 expression on myeloid cells from human hepatocellular carcinoma correlated with CD8(+) T cell dysfunction. B7S1 inhibition suppressed development of murine tumors. Putative B7S1 receptor was co-expressed with PD-1 but not T cell immunoglobulin and mucin-domain containing-3 (Tim-3) at an activated state of early tumor-infiltrating CD8(+) T cells, and B7S1 promoted T cell exhaustion, possibly through Eomes overexpression. Combinatorial blockade of B7S1 and PD-1 synergistically enhanced anti-tumor immune responses. Collectively, B7S1 initiates dysfunction of tumor-infiltrating CD8(+) T cells and may be targeted for cancer immunotherapy.
in vivo blocking of PD-1/PD-L signaling
Imai, Y., et al (2015). "Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis through Inhibition of IL-17A Expression by Innate gammadelta-Low T Cells" J Immunol 195(2): 421-425.
PubMed
Programmed cell death 1 (PD-1) is a key regulatory molecule that has been targeted in human cancers, including melanoma. In clinical testing, Abs against PD-1 have resulted in psoriasiform dermatitis (PsD). To determine whether PD-1 regulates PsD, we compared skin responses of PD-1-deficient (PD-1KO) mice and wild-type (WT) controls in an imiquimod (IMQ)-induced murine model of psoriasis. PD-1KO mice showed severe epidermal hyperplasia, greater neutrophilic infiltration, and higher expression of Th17 cytokines (versus WT mice). IMQ exposure increased PD-1 expression by skin gammadelta-low (GDL) T cells and enhanced expression of PD-L1 by keratinocytes. Three-fold increases in the percentage of IL-17A(+) GDL T cells were observed in skin cell suspensions derived from IMQ-treated PD-1KO mice (versus WT controls), suggesting that the lack of PD-1 has a functional effect not only on alphabeta T cells, but also on GDL T cells, and that PD-1 may play a regulatory role in PsD.
in vivo blocking of PD-1/PD-L signaling
Li, C., et al (2015). "ADAP and SKAP55 deficiency suppresses PD-1 expression in CD8+ cytotoxic T lymphocytes for enhanced anti-tumor immunotherapy" EMBO Mol Med 7(6): 754-769.
PubMed
PD-1 negatively regulates CD8(+) cytotoxic T lymphocytes (CTL) cytotoxicity and anti-tumor immunity. However, it is not fully understood how PD-1 expression on CD8(+) CTL is regulated during anti-tumor immunotherapy. In this study, we have identified that the ADAP-SKAP55 signaling module reduced CD8(+) CTL cytotoxicity and enhanced PD-1 expression in a Fyn-, Ca(2+)-, and NFATc1-dependent manner. In DC vaccine-based tumor prevention and therapeutic models, knockout of SKAP55 or ADAP showed a heightened protection from tumor formation or metastases in mice and reduced PD-1 expression in CD8(+) effector cells. Interestingly, CTLA-4 levels and the percentages of tumor infiltrating CD4(+)Foxp3(+) Tregs remained unchanged. Furthermore, adoptive transfer of SKAP55-deficient or ADAP-deficient CD8(+) CTLs significantly blocked tumor growth and increased anti-tumor immunity. Pretreatment of wild-type CD8(+) CTLs with the NFATc1 inhibitor CsA could also downregulate PD-1 expression and enhance anti-tumor therapeutic efficacy. Together, we propose that targeting the unrecognized ADAP-SKAP55-NFATc1-PD-1 pathway might increase efficacy of anti-tumor immunotherapy.
in vivo blocking of PD-1/PD-L signaling
in vitro PD-1 neutralization
Park, S. J., et al (2014). "Negative role of inducible PD-1 on survival of activated dendritic cells" J Leukoc Biol 95(4): 621-629.
PubMed
PD-1 is a well-established negative regulator of T cell responses by inhibiting proliferation and cytokine production of T cells via interaction with its ligands, B7-H1 (PD-L1) and B7-DC (PD-L2), expressed on non-T cells. Recently, PD-1 was found to be expressed in innate cells, including activated DCs, and plays roles in suppressing production of inflammatory cytokines. In this study, we demonstrate that PD-1 KO DCs exhibited prolonged longevity compared with WT DCs in the dLNs after transfer of DCs into hind footpads. Interestingly, upon LPS stimulation, WT DCs increased the expression of PD-1 and started to undergo apoptosis. DCs, in spleen of LPS-injected PD-1 KO mice, were more resistant to LPS-mediated apoptosis in vivo than WT controls. Moreover, treatment of blocking anti-PD-1 mAb during DC maturation resulted in enhanced DC survival, suggesting that PD-1:PD-L interactions are involved in DC apoptosis. As a result, PD-1-deficient DCs augmented T cell responses in terms of antigen-specific IFN-gamma production and proliferation of CD4 and CD8 T cells to a greater degree than WT DCs. Moreover, PD-1 KO DCs exhibited increased MAPK1 and CD40-CD40L signaling, suggesting a possible mechanism for enhanced DC survival in the absence of PD-1 expression. Taken together, our findings further extend the function of PD-1, which plays an important role in apoptosis of activated DCs and provides important implications for PD-1-mediated immune regulation.
in vivo blocking of PD-1/PD-L signaling
Rabenstein, H., et al (2014). "Differential kinetics of antigen dependency of CD4+ and CD8+ T cells" J Immunol 192(8): 3507-3517.
PubMed
Ag recognition via the TCR is necessary for the expansion of specific T cells that then contribute to adaptive immunity as effector and memory cells. Because CD4+ and CD8+ T cells differ in terms of their priming APCs and MHC ligands we compared their requirements of Ag persistence during their expansion phase side by side. Proliferation and effector differentiation of TCR transgenic and polyclonal mouse T cells were thus analyzed after transient and continuous TCR signals. Following equally strong stimulation, CD4+ T cell proliferation depended on prolonged Ag presence, whereas CD8+ T cells were able to divide and differentiate into effector cells despite discontinued Ag presentation. CD4+ T cell proliferation was neither affected by Th lineage or memory differentiation nor blocked by coinhibitory signals or missing inflammatory stimuli. Continued CD8+ T cell proliferation was truly independent of self-peptide/MHC-derived signals. The subset divergence was also illustrated by surprisingly broad transcriptional differences supporting a stronger propensity of CD8+ T cells to programmed expansion. These T cell data indicate an intrinsic difference between CD4+ and CD8+ T cells regarding the processing of TCR signals for proliferation. We also found that the presentation of a MHC class II-restricted peptide is more efficiently prolonged by dendritic cell activation in vivo than a class I bound one. In summary, our data demonstrate that CD4+ T cells require continuous stimulation for clonal expansion, whereas CD8+ T cells can divide following a much shorter TCR signal.
in vivo blocking of PD-1/PD-L signaling
Sarraj, B., et al (2014). "Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection" Proc Natl Acad Sci U S A 111(33): 12145-12150.
PubMed
Selectin-selectin ligand interactions mediate the initial steps in leukocyte migration, an integral part of immune responses. Fucosyltransferase-VII (FucT-VII), encoded by Fut7, is essential for biosynthesis of selectin ligands. In an established model of cardiac allograft vasculopathy and chronic rejection, Fut7(-/-) recipients exhibited long-term graft survival with minimal vasculopathy compared with WT controls. Graft survival was associated with CD4 T-cell exhaustion in the periphery, characterized by impaired effector cytokine production, defective proliferation, increased expression of inhibitory receptors programmed death-1 (PD-1) and T cell Ig- and mucin-domain-containing molecule-3 (Tim-3), low levels of IL-7Ralpha on CD4 T cells, and reduced migration of polyfunctional CD4 memory T cells to the allograft. Blocking PD-1 triggered rejection only in Fut7(-/-) recipients, whereas depleting regulatory T cells had no effect in either Fut7(-/-) or WT recipients. Adoptive transfer experiments confirmed that this CD4 T cell-exhausted phenotype is seen primarily in Fut7(-/-) CD4 T cells. These data suggest that impaired leukocyte recruitment is a novel mechanism leading to CD4 T-cell exhaustion. Our experimental system serves as an excellent model to study CD4 T-cell exhaustion as a dominant mechanism of transplant tolerance. Further, targeting FucT-VII may serve as a promising strategy to prevent chronic allograft rejection and promote tolerance.
in vivo blocking of PD-1/PD-L signaling
Van der Jeught, K., et al (2014). "Intratumoral administration of mRNA encoding a fusokine consisting of IFN-beta and the ectodomain of the TGF-beta receptor II potentiates antitumor immunity" Oncotarget 5(20): 10100-10113.
PubMed
It is generally accepted that the success of immunotherapy depends on the presence of tumor-specific CD8(+) cytotoxic T cells and the modulation of the tumor environment. In this study, we validated mRNA encoding soluble factors as a tool to modulate the tumor microenvironment to potentiate infiltration of tumor-specific T cells. Intratumoral delivery of mRNA encoding a fusion protein consisting of interferon-beta and the ectodomain of the transforming growth factor-beta receptor II, referred to as Fbeta(2), showed therapeutic potential. The treatment efficacy was dependent on CD8(+) T cells and could be improved through blockade of PD-1/PD-L1 interactions. In vitro studies revealed that administration of Fbeta(2) to tumor cells resulted in a reduced proliferation and increased expression of MHC I but also PD-L1. Importantly, Fbeta(2) enhanced the antigen presenting capacity of dendritic cells, whilst reducing the suppressive activity of myeloid-derived suppressor cells. In conclusion, these data suggest that intratumoral delivery of mRNA encoding soluble proteins, such as Fbeta(2), can modulate the tumor microenvironment, leading to effective antitumor T cell responses, which can be further potentiated through combination therapy.
in vitro PD-1 neutralization
Verhagen, J. and D. C. Wraith (2014). "Blockade of LFA-1 augments in vitro differentiation of antigen-induced Foxp3(+) Treg cells" J Immunol Methods 414: 58-64.
PubMed
Adoptive transfer of antigen-specific, in vitro-induced Foxp3(+) Treg (iTreg) cells protects against autoimmune disease. To generate antigen-specific iTreg cells at high purity, however, remains a challenge. Whereas polyclonal T cell stimulation with anti-CD3 and anti-CD28 antibody yields Foxp3(+) iTreg cells at a purity of 90-95%, antigen-induced iTreg cells typically do not exceed a purity of 65-75%, even in a TCR-transgenic model. In a similar vein to thymic Treg cell selection, iTreg cell differentiation is influenced not only by antigen recognition and the availability of TGF-beta but also by co-factors including costimulation and adhesion molecules. In this study, we demonstrate that blockade of the T cell integrin Leukocyte Function-associated Antigen-1 (LFA-1) during antigen-mediated iTreg cell differentiation augments Foxp3 induction, leading to approximately 90% purity of Foxp3(+) iTreg cells. This increased efficacy not only boosts the yield of Foxp3(+) iTreg cells, it also reduces contamination with activated effector T cells, thus improving the safety of adoptive transfer immunotherapy.
in vitro PD-1 neutralization
Schwager, K., et al (2013). "The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF" J Invest Dermatol 133(3): 751-758.
PubMed
Systemic high-dose IL2 promotes long-term survival in a subset of metastatic melanoma patients, but this treatment is accompanied by severe toxicities. The immunocytokine L19-IL2, in which IL2 is fused to the human L19 antibody capable of selective accumulation on tumor neovasculature, has recently shown encouraging clinical activity in patients with metastatic melanoma. In this study, we have investigated the therapeutic performance of L19-IL2, administered systemically in combination with a murine anti-CTLA-4 antibody or with a second clinical-stage immunocytokine (L19-TNF) in two syngeneic immunocompetent mouse models of cancer. We observed complete tumor eradications when L19-IL2 was used in combination with CTLA-4 blockade. Interestingly, mice cured from F9 tumors developed new lesions when rechallenged with tumor cells after therapy, whereas mice cured from CT26 tumors were resistant to tumor rechallenge. Similarly, L19-IL2 induced complete remissions when administered in a single intratumoral injection in combination with L19-TNF, whereas the two components did not lead to cures when administered as single agents. These findings provide a rationale for combination trials in melanoma, as the individual therapeutic agents have been extensively studied in clinical trials, and the antigen recognized by the L19 antibody has an identical sequence in mouse and man.
in vitro PD-1 neutralization
Noval Rivas, M., et al (2009). "Reviving function in CD4+ T cells adapted to persistent systemic antigen" J Immunol 183(7): 4284-4291.
PubMed
In bone marrow-transplanted patients, chronic graft-versus-host disease is a complication that results from the persistent stimulation of recipient minor histocompatibility Ag (mHA)-specific T cells contained within the graft. In this study, we developed a mouse model where persistent stimulation of donor T cells by recipient’s mHA led to multiorgan T cell infiltration. Exposure to systemic mHA, however, deeply modified T cell function and chronically stimulated T cells developed a long-lasting state of unresponsiveness, or immune adaptation, characterized by their inability to mediate organ immune damages in vivo. However, analysis of the gene expression profile of adapted CD4+ T cells revealed the specific coexpression of genes known to promote differentiation and function of Th1 effector cells as well as genes coding for proteins that control T cell activity, such as cell surface-negative costimulatory molecules and regulatory cytokines. Strikingly, blockade of negative costimulation abolished T cell adaptation and stimulated strong IFN-gamma production and severe multiorgan wasting disease. Negative costimulation was also shown to control lethal LPS-induced toxic shock in mice with adapted T cells, as well as the capacity of adapted T cells to reject skin graft. Our results demonstrate that negative costimulation is the molecular mechanism used by CD4+ T cells to adapt their activity in response to persistent antigenic stimulation. The effector function of CD4+ T cells that have adapted to chronic Ag presentation can be activated by stimuli strong enough to overcome regulatory signals delivered to the T cells by negative costimulation.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
Unraveling Cathepsin S regulation in interleukin-7-mediated anti-tumor immunity reveals its targeting potential against oral cancer.
In J Biomed Sci on 24 July 2025 by Chang, Y. C., Chen, S. J., et al.
PubMed
Immunomodulatory agents benefit a small percentage of patients with oral cancer (OC), a subset of head and neck cancer. Cathepsin S (CTSS), a lysosomal protease, has been frequently associated with tumor immunity. This study aimed to investigate the mechanism by which tumor CTSS affects anti-tumor immunity through the regulation of interleukin-7 (IL-7) to overcome this obstacle.
-
-
-
Cancer Research
ACVR2A attenuation impacts lactate production and hyperglycolytic conditions attracting regulatory T cells in hepatocellular carcinoma.
In Cell Rep Med on 15 April 2025 by Yasukawa, K., Shimada, S., et al.
PubMed
Although ACVR2A mutations are prevalent in non-viral hepatocellular carcinomas (HCCs), the underlying mechanism remains unelucidated. Our molecular investigation reveals that ACVR2A impairment induces hyperglycolysis through the inactivation of the SMAD signaling pathway. Using syngeneic transplantation models and human clinical samples, we clarify that ACVR2A-deficient HCC cells produce and secrete lactate via the upregulation of lactate dehydrogenase A (LDHA) and monocarboxylate transporter 4 (MCT4) expression levels, which promotes regulatory T (Treg) cell accumulation and then acquires resistance to immune checkpoint inhibitors. Remarkably, genetic knockdown and pharmacological inhibition of MCT4 ameliorate the high-lactate milieu in ACVR2A-deficient HCC, resulting in the suppression of intratumoral Treg cell recruitment and the restoration of the sensitivity to PD-1 blockade. These findings furnish compelling evidence that lactate attenuates anti-tumor immunity and that therapeutics targeting this pathway present a promising strategy for mitigating immunotherapy resistance in ACVR2A-deficient HCC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Antigenic cancer persister cells survive direct T cell attack
In bioRxiv on 17 March 2025 by Wang, M. X., Mauch, B. E., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Neoadjuvant anti-4-1BB confers protection against spontaneous metastasis through low-affinity intratumor CD8+T cells in triple-negative breast cancer
In bioRxiv on 2 February 2025 by Lim, B. J. W., Liu, M., et al.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism.
In Nat Immunol on 1 December 2024 by Peralta, R. M., Xie, B., et al.
PubMed
CD8+ T cells are critical mediators of antitumor immunity but differentiate into a dysfunctional state, known as T cell exhaustion, after persistent T cell receptor stimulation in the tumor microenvironment (TME). Exhausted T (Tex) cells are characterized by upregulation of coinhibitory molecules and reduced polyfunctionality. T cells in the TME experience an immunosuppressive metabolic environment via reduced levels of nutrients and oxygen and a buildup of lactic acid. Here we show that terminally Tex cells uniquely upregulate Slc16a11, which encodes monocarboxylate transporter 11 (MCT11). Conditional deletion of MCT11 in T cells reduced lactic acid uptake by Tex cells and improved their effector function. Targeting MCT11 with an antibody reduced lactate uptake specifically in Tex cells, which, when used therapeutically in tumor-bearing mice, resulted in reduced tumor growth. These data support a model in which Tex cells upregulate MCT11, rendering them sensitive to lactic acid present at high levels in the TME.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Single-cell sequencing reveals immune features of treatment response to neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma.
In Nat Commun on 22 October 2024 by Yang, Z., Tian, H., et al.
PubMed
Neoadjuvant immunochemotherapy (nICT) has dramatically changed the treatment landscape of operable esophageal squamous cell carcinoma (ESCC), but factors influencing tumor response to nICT are not well understood. Here, using single-cell RNA sequencing paired with T cell receptor sequencing, we profile tissues from ESCC patients accepting nICT treatment and characterize the tumor microenvironment context. CXCL13+CD8+ Tex cells, a subset of exhausted CD8+ T cells, are revealed to highly infiltrate in pre-treatment tumors and show prominent progenitor exhaustion phenotype in post-treatment samples from responders. We validate CXCL13+CD8+ Tex cells as a predictor of improved response to nICT and reveal CXCL13 to potentiate anti-PD-1 efficacy in vivo. Post-treatment tumors from non-responders are enriched for CXCL13+CD8+ Tex cells with notably remarkable exhaustion phenotype and TNFRSF4+CD4+ Tregs with activated immunosuppressive function and a significant clone expansion. Several critical markers for therapeutic resistance are also identified, including LRRC15+ fibroblasts and SPP1+ macrophages, which may recruit Tregs to form an immunosuppressive landscape. Overall, our findings unravel immune features of distinct therapeutic response to nICT treatment, providing a rationale for optimizing individualized neoadjuvant strategy in ESCC.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Oral reovirus reshapes the gut microbiome and enhances antitumor immunity in colon cancer.
In Nat Commun on 22 October 2024 by Lee, W. S., Lee, S. J., et al.
PubMed
The route of oncolytic virotherapy is pivotal for immunotherapeutic efficacy in advanced cancers. In this preclinical study, an oncolytic reovirus (RC402) is orally administered to induce antitumor immunity. Oral reovirus treatment shows no gross toxicities and effectively suppresses multifocal tumor lesions. Orally administered reovirus interacts with the host immune system in the Peyer's patch of the terminal ileum, increases IgA+ antibody-secreting cells in the lamina propria through MAdCAM-1+ blood vessels, and reshapes the gut microbiome. Oral reovirus promotes antigen presentation, type I/II interferons, and T cell activation within distant tumors, but does not reach or directly infect tumor cells beyond the gastrointestinal tract. In contrast to intratumoral reovirus injection, the presence of the gut microbiome, Batf3+ dendritic cells, type I interferons, and CD8+ T cells are indispensable for orally administered reovirus-induced antitumor immunity. Oral reovirus treatment is most effective when combined with αPD-1(L1) and/or αCTLA-4, leading to complete colon tumor regression and protective immune memory. Collectively, oral reovirus virotherapy is a feasible and effective immunotherapeutic strategy in preclinical studies.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Aberrant cytoplasmic expression of UHRF1 restrains the MHC-I-mediated anti-tumor immune response.
In Nat Commun on 3 October 2024 by Tan, L. M., Yin, T., et al.
PubMed
Immunotherapy successfully complements traditional cancer treatment. However, primary and acquired resistance might limit efficacy. Reduced antigen presentation by MHC-I has been identified as potential resistance factor. Here we show that the epigenetic regulator ubiquitin-like with PHD and ring finger domains 1 (UHRF1), exhibits altered expression and aberrant cytosolic localization in cancerous tissues, where it promotes MHC-I ubiquitination and degradation. Cytoplasmic translocation of UHRF1 is induced by its phosphorylation on a specific serine in response to signals provided by factors present in the tumor microenvironment (TME), such as TGF-β, enabling UHRF1 to bind MHC-I. Downregulation of MHC-I results in suppression of the antigen presentation pathway to establish an immune hostile TME. UHRF1 inactivation by genetic deletion synergizes with immune checkpoint blockade (ICB) treatment and induces an anti-tumour memory response by evoking low-affinity T cells. Our study adds to the understanding of UHRF1 in cancer immune evasion and provides a potential target to synergize with immunotherapy and overcome immunotherapeutic resistance.
-
-
-
Cancer Research
-
Immunology and Microbiology
Systematic investigation of chemo-immunotherapy synergism to shift anti-PD-1 resistance in cancer.
In Nat Commun on 12 April 2024 by Wang, Y., Pattarayan, D., et al.
PubMed
Chemo-immunotherapy combinations have been regarded as one of the most practical ways to improve immunotherapy response in cancer patients. In this study, we integrate the transcriptomics data from anti-PD-1-treated tumors and compound-treated cancer cell lines to systematically screen for chemo-immunotherapy synergisms in silico. Through analyzing anti-PD-1 induced expression changes in patient tumors, we develop a shift ability score to measure if a chemotherapy or a small molecule inhibitor treatment can shift anti-PD-1 resistance in tumor cells. By applying shift ability analysis to 41,321 compounds and 16,853 shRNA treated cancer cell lines transcriptomic data, we characterize the landscape of chemo-immunotherapy synergism and experimentally validated a mitochondrial RNA-dependent mechanism for drug-induced immune activation in tumor. Our study represents an effort to mechanistically characterize chemo-immunotherapy synergism and will facilitate future pre-clinical and clinical studies.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Notch Signaling Regulates Immunosuppressive Tumor-Associated Macrophage Function in Pancreatic Cancer.
In Cancer Immunol Res on 3 January 2024 by Yan, W., Menjivar, R. E., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) continues to have a dismal prognosis. The poor survival of patients with PDA has been attributed to a high rate of early metastasis and low efficacy of current therapies, which partly result from its complex immunosuppressive tumor microenvironment. Previous studies from our group and others have shown that tumor-associated macrophages (TAM) are instrumental in maintaining immunosuppression in PDA. Here, we explored the role of Notch signaling, a key regulator of immune response, within the PDA microenvironment. We identified Notch pathway components in multiple immune cell types within human and mouse pancreatic cancer. TAMs, the most abundant immune cell population in the tumor microenvironment, expressed high levels of Notch receptors, with cognate ligands such as JAG1 expressed on tumor epithelial cells, endothelial cells, and fibroblasts. TAMs with activated Notch signaling expressed higher levels of immunosuppressive mediators, suggesting that Notch signaling plays a role in macrophage polarization within the PDA microenvironment. Genetic inhibition of Notch in myeloid cells led to reduced tumor size and decreased macrophage infiltration in an orthotopic PDA model. Combination of pharmacologic Notch inhibition with PD-1 blockade resulted in increased cytotoxic T-cell infiltration, tumor cell apoptosis, and smaller tumor size. Our work implicates macrophage Notch signaling in the establishment of immunosuppression and indicates that targeting the Notch pathway may improve the efficacy of immune-based therapies in patients with PDA.
-
-
-
Immunology and Microbiology
PD-1 Impairs CD8+ T Cell Granzyme B Production in Aged Mice during Acute Viral Respiratory Infection.
In Immunohorizons on 1 November 2023 by Parks, O. B., Antos, D., et al.
PubMed
CD8+ T cell dysfunction contributes to severe respiratory viral infection outcomes in older adults. CD8+ T cells are the primary cell type responsible for viral clearance. With increasing age, CD8+ T cell function declines in conjunction with an accumulation of cytotoxic tissue-resident memory (TRM) CD8+ T cells. We sought to elucidate the role of PD-1 signaling on aged CD8+ T cell function and accumulation of CD8+ TRM cells during acute viral respiratory tract infection, given the importance of PD-1 regulating CD8+ T cells during acute and chronic infections. PD-1 blockade or genetic ablation in aged mice yielded improved CD8+ T cell granzyme B production comparable to that in young mice during human metapneumovirus and influenza viral infections. Syngeneic transplant and adoptive transfer strategies revealed that improved granzyme B production in aged Pdcd1-/- CD8+ T cells was primarily cell intrinsic because aged wild-type CD8+ T cells did not have increased granzyme B production when transplanted into a young host. PD-1 signaling promoted accumulation of cytotoxic CD8+ TRM cells in aged mice. PD-1 blockade of aged mice during rechallenge infection resulted in improved clinical outcomes that paralleled reduced accumulation of CD8+ TRM cells. These findings suggest that PD-1 signaling impaired CD8+ T cell granzyme B production and contributed to CD8+ TRM cell accumulation in the aged lung. These findings have implications for future research investigating PD-1 checkpoint inhibitors as a potential therapeutic option for elderly patients with severe respiratory viral infections.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Reciprocal transmission of activating and inhibitory signals and cell fate in regenerating T cells.
In Cell Rep on 31 October 2023 by Wang, P. H., Washburn, R. S., et al.
PubMed
The ability of activated progenitor T cells to self-renew while producing differentiated effector cell descendants may underlie immunological memory and persistent responses to ongoing infection. The nature of stem-like T cells responding to cancer and during treatment with immunotherapy is not clear. The subcellular organization of dividing progenitor CD8+ T cells from mice challenged with syngeneic tumors is examined here. Three-dimensional microscopy reveals an activating hub composed of polarized CD3, CD28, and phosphatidylinositol 3-kinase (PI3K) activity at the putative immunological synapse with an inhibitory hub composed of polarized PD-1 and CD73 at the opposite pole of mitotic blasts. Progenitor T cells from untreated and inhibitory checkpoint blockade-treated mice yield a differentiated TCF1- daughter cell, which inherits the PI3K activation hub, alongside a discordantly fated, self-renewing TCF1+ sister cell. Dynamic organization of opposite activating and inhibitory signaling poles in mitotic lymphocytes may account for the enigmatic durability of specific immunity.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Synergism Between IL21 and Anti-PD-1 Combination Therapy is Underpinned by the Coordinated Reprogramming of the Immune Cellular Network in the Tumor Microenvironment.
In Cancer Res Commun on 1 August 2023 by Wu, S., Huang, H., et al.
PubMed
T cell-stimulating cytokines and immune checkpoint inhibitors (ICI) are an ideal combination for increasing response rates of cancer immunotherapy. However, the results of clinical trials have not been satisfying. It is important to understand the mechanism of synergy between these two therapeutic modalities. Here, through integrated analysis of multiple single-cell RNA sequencing (scRNA-seq) datasets of human tumor-infiltrating immune cells, we demonstrate that IL21 is produced by tumor-associated T follicular helper cells and hyperactivated/exhausted CXCL13+CD4+ T cells in the human tumor microenvironment (TME). In the mouse model, the hyperactivated/exhausted CD4+ T cell-derived IL21 enhances the helper function of CD4+ T cells that boost CD8+ T cell-mediated immune responses during PD-1 blockade immunotherapy. In addition, we demonstrated that IL21's antitumor activity did not require T-cell trafficking. Using scRNA-seq analysis of the whole tumor-infiltrating immune cells, we demonstrated that IL21 treatment in combination with anti-PD-1 blockade synergistically drives tumor antigen-specific CD8+ T cells to undergo clonal expansion and differentiate toward the hyperactive/exhausted functional state in the TME. In addition, IL21 treatment and anti-PD-1 blockade synergistically promote dendritic cell (DC) activation and maturation to mature DC as well as monocyte to type 1 macrophage (M1) differentiation in the TME. Furthermore, the combined treatment reprograms the immune cellular network by reshaping cell-cell communication in the TME. Our study establishes unique mechanisms of synergy between IL21 and PD-1-based ICI in the TME through the coordinated promotion of type 1 immune responses.
-
-
-
Immunology and Microbiology
-
Cancer Research
-
Mus musculus (Mouse)
The GPCR-Gαs-PKA signaling axis promotes T cell dysfunction and cancer immunotherapy failure.
In Nat Immunol on 1 August 2023 by Wu, V. H., Yung, B. S., et al.
PubMed
Immune checkpoint blockade (ICB) targeting PD-1 and CTLA-4 has revolutionized cancer treatment. However, many cancers do not respond to ICB, prompting the search for additional strategies to achieve durable responses. G-protein-coupled receptors (GPCRs) are the most intensively studied drug targets but are underexplored in immuno-oncology. Here, we cross-integrated large singe-cell RNA-sequencing datasets from CD8+ T cells covering 19 distinct cancer types and identified an enrichment of Gαs-coupled GPCRs on exhausted CD8+ T cells. These include EP2, EP4, A2AR, β1AR and β2AR, all of which promote T cell dysfunction. We also developed transgenic mice expressing a chemogenetic CD8-restricted Gαs-DREADD to activate CD8-restricted Gαs signaling and show that a Gαs-PKA signaling axis promotes CD8+ T cell dysfunction and immunotherapy failure. These data indicate that Gαs-GPCRs are druggable immune checkpoints that might be targeted to enhance the response to ICB immunotherapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Immunohistochemistry
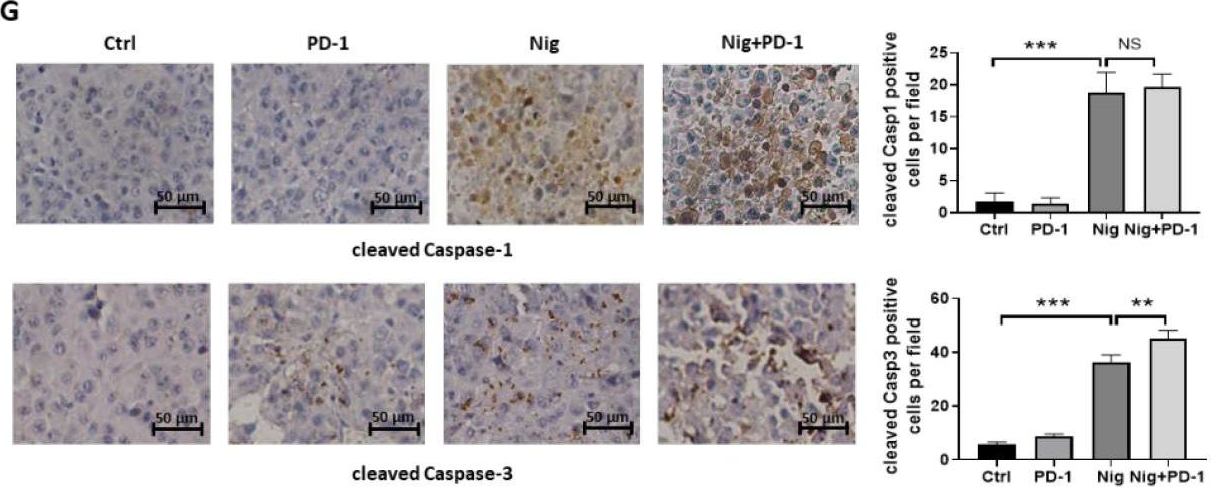
Nigericin Boosts Anti-Tumor Immune Response via Inducing Pyroptosis in Triple-Negative Breast Cancer.
In Cancers (Basel) on 16 June 2023 by Wu, L., Bai, S., et al.
PubMed
Although immune checkpoint inhibitors improved the clinical outcomes of advanced triple negative breast cancer (TBNC) patients, the response rate remains relatively low. Nigericin is an antibiotic derived from Streptomyces hydrophobicus. We found that nigericin caused cell death in TNBC cell lines MDA-MB-231 and 4T1 by inducing concurrent pyroptosis and apoptosis. As nigericin facilitated cellular potassium efflux, we discovered that it caused mitochondrial dysfunction, leading to mitochondrial ROS production, as well as activation of Caspase-1/GSDMD-mediated pyroptosis and Caspase-3-mediated apoptosis in TNBC cells. Notably, nigericin-induced pyroptosis could amplify the anti-tumor immune response by enhancing the infiltration and anti-tumor effect of CD4+ and CD8+ T cells. Moreover, nigericin showed a synergistic therapeutic effect when combined with anti-PD-1 antibody in TNBC treatment. Our study reveals that nigericin may be a promising anti-tumor agent, especially in combination with immune checkpoint inhibitors for advanced TNBC treatment.
-
-
-
Mus musculus (Mouse)
Human Metapneumovirus Reinfection in Aged Mice Recapitulates Increased Disease Severity in Elderly Humans Infected with Human Metapneumovirus.
In Immunohorizons on 1 June 2023 by Parks, O. B., Eddens, T., et al.
PubMed
Human metapneumovirus (HMPV) is a leading cause of respiratory infection in adults >65 y. Nearly all children worldwide are seropositive for HMPV by age 5 y, but reinfections occur throughout life, and there is no licensed vaccine. Recurrent HMPV infection is mild and self-resolving in immunocompetent individuals. However, elderly individuals develop severe respiratory disease on HMPV reinfection that leads to a high risk for morbidity and mortality. In this study, we developed a mouse model to mirror HMPV reinfection in elderly humans. C57BL/6J mice were infected with HMPV at 6-7 wk old, aged in-house, and rechallenged with high-dose virus at 70 wk. Aged rechallenged mice had profound weight loss similar to primary infected mice, increased lung histopathology, and accumulated cytotoxic CD8+CD44+CD62L-CD69+CD103+ memory cells despite having undetectable lung virus titer. When aged mice 14 mo postinfection (p.i.) or young mice 5 wk p.i. were restimulated with HMPV cognate Ag to mimic epitope vaccination, aged mice had an impaired CD8+ memory response. Convalescent serum transfer from young naive or 5 wk p.i. mice into aged mice on day of infection did not protect. Aged mice vaccinated with UV-inactivated HMPV also exhibited diminished protection and poor CD8+ memory response compared with young mice. These results suggest aged individuals with HMPV reinfection have a dysregulated CD8+ memory T cell response that fails to protect and exacerbates disease. Moreover, aged mice exhibited a poor memory response to either epitope peptide or UV-inactivated vaccination, suggesting that aged CD8+ T cell dysfunction presents a barrier to effective vaccination strategies.
-
-
-
Flow cytometry/Cell sorting
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Endosialin-positive tumor-derived pericytes promote tumor progression through impeding the infiltration of CD8+ T cells in clear cell renal cell carcinoma.
In Cancer Immunol Immunother on 1 June 2023 by Lu, T., Zhang, J., et al.
PubMed
Immune checkpoint blockade (ICB) therapy can be effective against clear cell renal cell carcinoma (ccRCC), but many patients show no benefit. Tumor-derived pericytes (TDPs) may promote tumor progression by influencing T cells and are an immunotherapy target; however, they may comprise functionally distinct subtypes. We aimed to identify markers of tumor-promoting TDPs and develop TDP-targeting strategies to enhance ICB therapy effectiveness against ccRCC.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
In vivo experiments
-
Mus musculus (Mouse)
Arginase 1 is a key driver of immune suppression in pancreatic cancer.
In Elife on 2 February 2023 by Menjivar, R. E., Nwosu, Z. C., et al.
PubMed
An extensive fibroinflammatory stroma rich in macrophages is a hallmark of pancreatic cancer. In this disease, it is well appreciated that macrophages are immunosuppressive and contribute to the poor response to immunotherapy; however, the mechanisms of immune suppression are complex and not fully understood. Immunosuppressive macrophages are classically defined by the expression of the enzyme Arginase 1 (ARG1), which we demonstrated is potently expressed in pancreatic tumor-associated macrophages from both human patients and mouse models. While routinely used as a polarization marker, ARG1 also catabolizes arginine, an amino acid required for T cell activation and proliferation. To investigate this metabolic function, we used a genetic and a pharmacologic approach to target Arg1 in pancreatic cancer. Genetic inactivation of Arg1 in macrophages, using a dual recombinase genetically engineered mouse model of pancreatic cancer, delayed formation of invasive disease, while increasing CD8+ T cell infiltration. Additionally, Arg1 deletion induced compensatory mechanisms, including Arg1 overexpression in epithelial cells, namely Tuft cells, and Arg2 overexpression in a subset of macrophages. To overcome these compensatory mechanisms, we used a pharmacological approach to inhibit arginase. Treatment of established tumors with the arginase inhibitor CB-1158 exhibited further increased CD8+ T cell infiltration, beyond that seen with the macrophage-specific knockout, and sensitized the tumors to anti-PD1 immune checkpoint blockade. Our data demonstrate that Arg1 drives immune suppression in pancreatic cancer by depleting arginine and inhibiting T cell activation.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Notch signaling regulates immunosuppressive tumor-associated macrophage function in pancreatic cancer
In bioRxiv on 13 January 2023 by Yan, W., Steele, N. G., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Host stimulator of interferon genes is essential for the efficacy of anti-programmed cell death protein 1 inhibitors in non-small cell lung cancer.
In Immunology on 1 December 2022 by Zhou, L., Xu, Q., et al.
PubMed
The stimulator of interferon genes (STING) pathway is important for anticancer immune responses. However, the relative contributions of host and tumour STING in anti-programmed cell death protein 1 (anti-PD-1) inhibitor responses in non-small cell lung cancer (NSCLC) are unknown. STING expression in tumour and blood was associated with anti-PD-1 therapy in NSCLC patients; Moreover, loss of PD-1 inhibitor therapeutic potency was demonstrated in STING KO (knock out) splenocytes and STING KO mice. STING knock-down in tumour cells had no effect. STING on CD8+ T cells and host cells, not tumour cells, correlated with clinical effect of anti-PD-1 therapy in NSCLC patients. Finally, adoptive transfer of CD8+ T cells restored PD-1 inhibitor anticancer effects. STING in host cells but not in tumour cells mediates anti-PD-1 inhibitor responses in cancer immunotherapy and could be used to select advantageous NSCLC patients from immunotherapy.
-