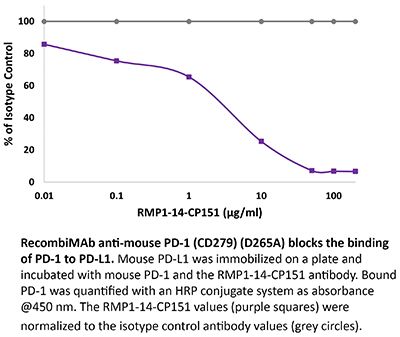
RecombiMAb anti-mouse PD-1 (CD279) (D265A)
(switched from rat IgG2a)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb mouse IgG2a (D265A) isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Mutations | D265A |
| Immunogen | Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| Reported Applications |
in vivo blocking of PD-1/PD-L signaling* *Reported for the original rat IgG2a RMP1-14 antibody |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from CHO cell supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2927525 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of PD-1/PD-L signaling
Maldonado MDM, Gracia-Hernandez M, Le LH, Iida M, Gulley JL, Donahue RN, Palena C, Schlom J, Hamilton DH (2025). "Combination of a therapeutic cancer vaccine targeting the endogenous retroviral envelope protein ERVMER34-1 with immune-oncology agents
PubMed
Background: Endogenous retroviruses (ERVs) are remnants of retrovirus germline infections that occurred over the course of evolution and constitute between 5% and 8% of the human genome. While ERVs tend to be epigenetically silenced in normal adult human tissues, they are often overexpressed in carcinomas and may represent novel immunotherapeutic targets. This study characterizes the ERV envelope protein ERVMER34-1 as a target for a therapeutic cancer vaccine. Methods: The expression of ERVMER34-1 in multiple healthy adult and cancer tissues was assessed, as was its immunogenicity, to ascertain whether specific T cells could lyse human carcinoma cell lines expressing ERVMER34-1. Furthermore, the ability of a rationally designed ERVMER34-1-targeted therapeutic vaccine to induce tumor clearance in two murine carcinoma models expressing ERVMER34-1 was examined either as a monotherapy or in combination with anti-programmed cell death protein-1/programmed death-ligand 1 monoclonal antibody (mAb) or the interleukin-15 superagonist N-803. Results: The ERVMER34-1 protein was shown to be overexpressed in 232/376 of human carcinomas analyzed while being absent in most healthy adult tissues. High levels of ERVMER34-1 RNA expression associate with decreased survival in uveal melanoma, adenoid cystic, and head and neck carcinomas. ERVMER34-1-specific T cells were detected in peripheral blood mononuclear cells (PBMCs) of patients with cancer but not healthy donors following an overnight stimulation. However, reactive T cells are readily expanded from both healthy donor and patient with cancer PBMCs following a 7- day in vitro stimulation. Furthermore, ERVMER34-1-specific T cells selectively kill human carcinoma cell lines expressing ERVMER34-1. A novel, rationally designed, therapeutic cancer vaccine targeting ERVMER34-1 mediated tumor control in established syngeneic murine tumors expressing the full-length ERVMER34-1 protein. When combined with checkpoint blockade, the vaccine promoted expansion of neoepitope-reactive T cells whose function was further enhanced when combined with N-803. This expansion of neoepitope-reactive T cells was associated with tumor control. Conclusions: This study reveals the potential of a vaccine that targets the retroviral envelope protein ERVMER34-1 and supports its continued development toward clinical testing as a new class of therapeutic cancer vaccine.
in vivo blocking of PD-1/PD-L signaling
Lepland A, Peranzoni E, Haljasorg U, Asciutto EK, Crespí-Amer M, Modesti L, Kilk K, Lombardia M, Acosta G, Royo M, Peterson P, Marigo I, Teesalu T, Scodeller P (2025). "Peptide-Drug Conjugate for Therapeutic Reprogramming of Tumor-Associated Macropha
PubMed
In triple-negative breast cancer (TNBC), pro-tumoral macrophages promote metastasis and suppress the immune response. To target these cells, a previously identified CD206 (mannose receptor)-binding peptide, mUNO was engineered to enhance its affinity and proteolytic stability. The new rationally designed peptide, MACTIDE, includes a trypsin inhibitor loop, from the Sunflower Trypsin Inhibitor-I. Binding studies to recombinant CD206 revealed a 15-fold lower KD for MACTIDE compared to parental mUNO. Mass spectrometry further demonstrated a 5-fold increase in MACTIDE's half-life in tumor lysates compared to mUNO. Homing studies in TNBC-bearing mice shows that fluorescein (FAM)-MACTIDE precisely targeted CD206+ tumor-associated macrophages (TAM) upon intravenous, intraperitoneal, and even oral administration, with minimal liver accumulation. MACTIDE was conjugated to Verteporfin, an FDA-approved photosensitizer and YAP/TAZ pathway inhibitor to create the conjugate MACTIDE-V. In the orthotopic 4T1 TNBC mouse model, non-irradiated MACTIDE-V-treated mice exhibited anti-tumoral effects comparable to those treated with irradiated MACTIDE-V, with fewer signs of toxicity, prompting further investigation into the laser-independent activity of the conjugate. In vitro studies using bone marrow-derived mouse macrophages showed that MACTIDE-V excluded YAP from the nucleus, increased phagocytic activity, and upregulated several genes associated with cytotoxic anti-tumoral macrophages. In mouse models of TNBC, MACTIDE-V slowed primary tumor growth, suppressed lung metastases, and increased markers of phagocytosis and antigen presentation in TAM and monocytes, increasing the tumor infiltration of several lymphocyte subsets. MACTIDE-V is proposed as a promising peptide-drug conjugate for modulating macrophage function in breast cancer immunotherapy.
in vivo blocking of PD-1/PD-L signaling
Yuan X, Hao X, Chan HL, Zhao N, Pedroza DA, Liu F, Le K, Smith AJ, Calderon SJ, Lieu N, Soth MJ, Jones P, Zhang XH, Rosen JM (2024). "CREB-binding protein/P300 bromodomain inhibition reduces neutrophil accumulation and activates antitumor immunity in
PubMed
Tumor-associated neutrophils (TANs) have been shown to promote immunosuppression and tumor progression, and a high TAN frequency predicts poor prognosis in triple-negative breast cancer (TNBC). Dysregulation of CREB-binding protein (CBP)/P300 function has been observed with multiple cancer types. The bromodomain (BRD) of CBP/P300 has been shown to regulate its activity. In this study, we found that IACS-70654, a selective CBP/P300 BRD inhibitor, reduced TANs and inhibited the growth of neutrophil-enriched TNBC models. In the bone marrow, CBP/P300 BRD inhibition reduced the tumor-driven abnormal differentiation and proliferation of neutrophil progenitors. Inhibition of CBP/P300 BRD also stimulated the immune response by inducing an IFN response and MHCI expression in tumor cells and increasing tumor-infiltrated cytotoxic T cells. Moreover, IACS-70654 improved the response of a neutrophil-enriched TNBC model to docetaxel and immune checkpoint blockade. This provides a rationale for combining a CBP/P300 BRD inhibitor with standard-of-care therapies in future clinical trials for neutrophil-enriched TNBC.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
ACTM-838, a novel systemically delivered bacterial immunotherapy that enriches in solid tumors and delivers IL-15/IL-15Rα and STING payloads to engage innate and adaptive immunity in the TME and enable a durable anti-tumor immune response.
In Oncotarget on 6 October 2025 by Cron, K. R., Fang, P., et al.
PubMed
STACT is a modular, genetically engineered live attenuated S. Typhimurium bacterial platform that enables tissue-specific localization and cell-targeted delivery of large, multiplexed payloads via systemic administration. It has been engineered to minimize systemic toxicity and to enrich in the tumor microenvironment (TME) via metabolic dependency and showed a decreased systemic inflammatory cytokine profile compared to its parent strain VNP20009. ACTM-838 utilizes the STACT platform to deliver IL-15/IL15Rα and a constitutively active STING to tumor-resident phagocytic antigen-presenting cells. Upon intravenous (IV) dosing to tumor-bearing mice, ACTM-838 distributed and enriched in the TME, exhibited specific uptake in tumor-resident phagocytic cells and led to expression of human IL-15/IL15Rα and murine IFNα in the tumor. ACTM-838 induced comprehensive TME changes to an immune permissive anti-tumor phenotype with a decrease in exhausted T-cells and Tregs and an increase in cytolytic T-cells and MHCII-high proliferating myeloid cells. ACTM-838-treated tumors exhibited upregulated anti-tumor innate and adaptive immunity expression profiles, T-, NK- and B-cell infiltration and downregulated cell cycle, DNA damage and TGFβ responses. Single-cell RNAseq and flow cytometry data confirmed activation and infiltration of both innate and adaptive immune cells. ACTM-838 showed durable anti-tumor efficacy in multiple murine tumor models and synergized with anti-PD1 therapy in combination.
-
-
-
Immunology and Microbiology
-
Cancer Research
Immature monocytic cells within tumors differentiate into immunosuppressive cells in resistant tumors to immunotherapy.
In iScience on 15 August 2025 by Levin, S., Benguigui, M., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have improved outcomes in advanced cancers, yet resistance remains a major obstacle. Here, we investigated the role of myeloid cells in shaping the immunosuppressive tumor microenvironment that contributes to ICI resistance. Using mutagenized ICI-sensitive and resistant 4T1 breast cancer clones, we performed single-cell RNA sequencing to characterize immune cell populations post-ICI therapy. We identified monocytic dendritic progenitors (MDPs) and common monocytic progenitors (cMOPs) enriched in sensitive tumors, which may differentiate into immunosuppressive cells in resistant tumors. Analysis of public datasets confirmed the presence of MDP-cMOPs in tumors and blood of patients with breast, lung, and colorectal cancer. We found high expression of CXCR4 and IL6R in MDP-cMOPs, and inhibiting these pathways blocked their recruitment and differentiation. Combined targeting of CXCR4 and IL6 pathway with ICI improved responses in resistant tumors, highlighting MDP-cMOPs as contributors to immunotherapy resistance and potential therapeutic targets.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Combination of a therapeutic cancer vaccine targeting the endogenous retroviral envelope protein ERVMER34-1 with immune-oncology agents facilitates expansion of neoepitope-specific T cells and promotes tumor control.
In J Immunother Cancer on 13 May 2025 by Maldonado, M. D. M., Gracia-Hernandez, M., et al.
PubMed
Endogenous retroviruses (ERVs) are remnants of retrovirus germline infections that occurred over the course of evolution and constitute between 5% and 8% of the human genome. While ERVs tend to be epigenetically silenced in normal adult human tissues, they are often overexpressed in carcinomas and may represent novel immunotherapeutic targets. This study characterizes the ERV envelope protein ERVMER34-1 as a target for a therapeutic cancer vaccine.
-
-
-
Cancer Research
Peptide-Drug Conjugate for Therapeutic Reprogramming of Tumor-Associated Macrophages in Breast Cancer.
In Adv Sci (Weinh) on 1 March 2025 by Lepland, A., Peranzoni, E., et al.
PubMed
In triple-negative breast cancer (TNBC), pro-tumoral macrophages promote metastasis and suppress the immune response. To target these cells, a previously identified CD206 (mannose receptor)-binding peptide, mUNO was engineered to enhance its affinity and proteolytic stability. The new rationally designed peptide, MACTIDE, includes a trypsin inhibitor loop, from the Sunflower Trypsin Inhibitor-I. Binding studies to recombinant CD206 revealed a 15-fold lower KD for MACTIDE compared to parental mUNO. Mass spectrometry further demonstrated a 5-fold increase in MACTIDE's half-life in tumor lysates compared to mUNO. Homing studies in TNBC-bearing mice shows that fluorescein (FAM)-MACTIDE precisely targeted CD206+ tumor-associated macrophages (TAM) upon intravenous, intraperitoneal, and even oral administration, with minimal liver accumulation. MACTIDE was conjugated to Verteporfin, an FDA-approved photosensitizer and YAP/TAZ pathway inhibitor to create the conjugate MACTIDE-V. In the orthotopic 4T1 TNBC mouse model, non-irradiated MACTIDE-V-treated mice exhibited anti-tumoral effects comparable to those treated with irradiated MACTIDE-V, with fewer signs of toxicity, prompting further investigation into the laser-independent activity of the conjugate. In vitro studies using bone marrow-derived mouse macrophages showed that MACTIDE-V excluded YAP from the nucleus, increased phagocytic activity, and upregulated several genes associated with cytotoxic anti-tumoral macrophages. In mouse models of TNBC, MACTIDE-V slowed primary tumor growth, suppressed lung metastases, and increased markers of phagocytosis and antigen presentation in TAM and monocytes, increasing the tumor infiltration of several lymphocyte subsets. MACTIDE-V is proposed as a promising peptide-drug conjugate for modulating macrophage function in breast cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
CREB-binding protein/P300 bromodomain inhibition reduces neutrophil accumulation and activates antitumor immunity in triple-negative breast cancer.
In JCI Insight on 17 September 2024 by Yuan, X., Hao, X., et al.
PubMed
Tumor-associated neutrophils (TANs) have been shown to promote immunosuppression and tumor progression, and a high TAN frequency predicts poor prognosis in triple-negative breast cancer (TNBC). Dysregulation of CREB-binding protein (CBP)/P300 function has been observed with multiple cancer types. The bromodomain (BRD) of CBP/P300 has been shown to regulate its activity. In this study, we found that IACS-70654, a selective CBP/P300 BRD inhibitor, reduced TANs and inhibited the growth of neutrophil-enriched TNBC models. In the bone marrow, CBP/P300 BRD inhibition reduced the tumor-driven abnormal differentiation and proliferation of neutrophil progenitors. Inhibition of CBP/P300 BRD also stimulated the immune response by inducing an IFN response and MHCI expression in tumor cells and increasing tumor-infiltrated cytotoxic T cells. Moreover, IACS-70654 improved the response of a neutrophil-enriched TNBC model to docetaxel and immune checkpoint blockade. This provides a rationale for combining a CBP/P300 BRD inhibitor with standard-of-care therapies in future clinical trials for neutrophil-enriched TNBC.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Therapeutic Tumor Macrophage Reprogramming in Breast Cancer Through a Peptide-Drug Conjugate
In bioRxiv on 12 August 2024 by Lepland, A., Peranzoni, E., et al.
-