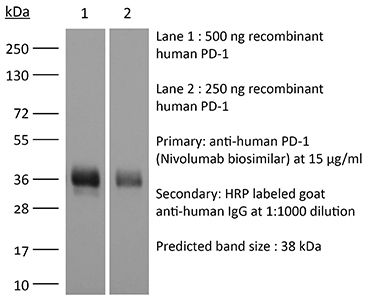
InVivoSIM anti-human PD-1 (Nivolumab Biosimilar)
Product Description
Specifications
| Isotype | Human IgG4 |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG4 (S228P) isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Mutations | S228P |
| Immunogen | Human PD-1 |
| Reported Applications |
in vitro PD-1 neutralization in vivo blocking of PD-1/PD-L signaling in vitro functional assay in vivo functional assay Flow Cytometry Immunohistochemistry Western Blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894724 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo functional assay
Ruggiu M, Guérin MV, Corre B, Bardou M, Alonso R, Russo E, Garcia Z, Feldmann L, Lemaître F, Dusseaux M, Grandjean CL, Bousso P (2024). "Anti-PD-1 therapy triggers Tfh cell-dependent IL-4 release to boost CD8 T cell responses in tumor-draining lymph
PubMed
Anti-PD-1 therapy targets intratumoral CD8+ T cells to promote clinical responses in cancer patients. Recent evidence suggests an additional activity in the periphery, but the underlying mechanism is unclear. Here, we show that anti-PD-1 mAb enhances CD8+ T cell responses in tumor-draining lymph nodes by stimulating cytokine production in follicular helper T cells (Tfh). In two different models, anti-PD-1 mAb increased the activation and proliferation of tumor-specific T cells in lymph nodes. Surprisingly, anti-PD-1 mAb did not primarily target CD8+ T cells but instead stimulated IL-4 production by Tfh cells, the major population bound by anti-PD-1 mAb. Blocking IL-4 or inhibiting the Tfh master transcription factor BCL6 abrogated anti-PD-1 mAb activity in lymph nodes while injection of IL-4 complexes was sufficient to recapitulate anti-PD-1 mAb activity. A similar mechanism was observed in a vaccine model. Finally, nivolumab also boosted human Tfh cells in humanized mice. We propose that Tfh cells and IL-4 play a key role in the peripheral activity of anti-PD-1 mAb.
in vivo blocking of PD-1/PD-L signaling
Western Blot
Deng R, Tian R, Li X, Xu Y, Li Y, Wang X, Li H, Wang L, Xu B, Yang D, Tang S, Xue B, Zuo C, Zhu H (2024). "ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis" iScience 27(4):10953
PubMed
Hepatitis B virus (HBV) infection generally elicits weak type-I interferon (IFN) immune response in hepatocytes, covering the regulatory effect of IFN-stimulated genes. In this study, low level of IFN-stimulated gene 12a (ISG12a) predicted malignant transformation and poor prognosis of HBV-associated hepatocellular carcinoma (HCC), whereas high level of ISG12a indicated active NK cell phenotypes. ISG12a interacts with TRIM21 to inhibit the phosphorylation activation of protein kinase B (PKB, also known as AKT) and β-catenin, suppressing PD-L1 expression to block PD-1/PD-L1 signaling, thereby enhancing the anticancer effect of NK cells. The suppression of PD-1-deficient NK-92 cells on HBV-associated tumors was independent of ISG12a expression, whereas the anticancer effect of PD-1-expressed NK-92 cells on HBV-associated tumors was enhanced by ISG12a and treatments of atezolizumab and nivolumab. Thus, tumor intrinsic ISG12a promotes the anticancer effect of NK cells by regulating PD-1/PD-L1 signaling, presenting the significant role of innate immunity in defending against HBV-associated HCC.
in vivo blocking of PD-1/PD-L signaling
Das S, Valton J, Duchateau P, Poirot L (2023). "Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy" Front Immunol .
PubMed
Adoptive cell therapy based on chimeric antigen receptor (CAR)-engineered T-cells has proven to be lifesaving for many cancer patients. However, its therapeutic efficacy has so far been restricted to only a few malignancies, with solid tumors proving to be especially recalcitrant to efficient therapy. Poor intra-tumor infiltration by T cells and T cell dysfunction due to a desmoplastic, immunosuppressive microenvironment are key barriers for CAR T-cell success against solid tumors. Cancer-associated fibroblasts (CAFs) are critical components of the tumor stroma, evolving specifically within the tumor microenvironment (TME) in response to tumor cell cues. The CAF secretome is a significant contributor to the extracellular matrix and a plethora of cytokines and growth factors that induce immune suppression. Together they form a physical and chemical barrier which induces a T cell-excluding 'cold' TME. CAF depletion in stroma rich solid tumors can thus provide an opportunity to convert immune evasive tumors susceptible to tumor-antigen CAR T-cell cytotoxicity. Using our TALEN-based gene editing platform we engineered non-alloreactive, immune evasive CAR T-cells (termed UCAR T-cells) targeting the unique CAF marker Fibroblast Activation Protein, alpha (FAP). In an orthotopic mouse model of triple-negative breast cancer (TNBC) composed of patient derived-CAFs and tumor cells, we demonstrate the efficacy of our engineered FAP UCAR T-cells in CAF depletion, reduction of desmoplasia and successful tumor infiltration. Furthermore, while previously resistant, pre-treatment with FAP UCAR T-cells now sensitized these tumors to Mesothelin (Meso) UCAR T-cell infiltration and anti-tumor cytotoxicity. Combination therapy of FAP UCAR, Meso UCAR T cells and the checkpoint inhibitor anti-PD-1 significantly reduced tumor burden and prolonged mice survival. Our study thus proposes a novel treatment paradigm for successful CAR T-cell immunotherapy against stroma-rich solid tumors.
in vitro functional assay
Lam W, Hu R, Liu SH, Cheng P, Cheng YC (2023). "YIV-906 enhances nuclear factor of activated T-cells (NFAT) activity of T cells and promotes immune checkpoint blockade antibody action and CAR T-cell activity" Front Pharmacol .
PubMed
YIV-906 is a systems biology botanical cancer drug, inspired by a traditional Chinese herbal formulation. Results from eight Phase I/II to II clinical studies demonstrated the potential of YIV-906 to prolong survival and improve the quality of life of cancer patients. As an immunomodulator in the tumor microenvironment, YIV-906 can turn cold tumors hot and potentiate anti-tumor activity for different classes of anticancer agents; and as a cytoprotector in the GI, YIV-906 can reduce non-hematological side effects and speed up damaged tissue recovery. YIV-906 enhanced anti-PD1 action against hepatoma in mice by stimulating both innate and adaptive immunity. In a Jurkat cell-staphylococcal superantigen E (SEE)-Raji cell culture model, YIV-906 promoted T cell activation with upregulation of CD69 by enhancing NFAT activity, with or without PD1-PD-L1 interaction. YIV-906 could trigger the phosphorylation of TCR downstream signaling cascades without the involvement of TCR. YIV-906 could inhibit SHP1 and SHP2 activities, which dephosphorylates TCR downstream proteins due to the PD1-PD-L1 interaction. Therefore, YIV-906 could enhance anti-PD1 action to rescue the depressed NFAT activity of Jurkat cells due to the PD1-PD-L1 interaction. In addition, YIV-906 enhanced the NFAT activity and killing capability of Jurkat cells expressing chimeric antigen receptor (CAR-CD19-CD3z) toward CD19 expressing cells, such as Raji cells, with or without PD1-PD-L1 overexpression. Ingredient herb S (Scutellaria baicalensis Georgi) of YIV-906 and some S compounds were found to play key roles in these activities. In conclusion, YIV-906 modulates adaptive immunity by activating T effector cells mainly through its action on SHP1/2. YIV-906 could also facilitate immune checkpoint blockade therapy or CAR-T cell therapy for cancer treatment.
in vivo blocking of PD-1/PD-L signaling
Qiu H, Zmina PM, Huang AY, Askew D, Bedogni B (2018). "Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties" Cancer Lett .
PubMed
We have previously shown that Notch1 plays a critical role in modulating melanoma tumor cell growth and survival. Here we show that Notch1 also contributes to an immune-suppressive tumor microenvironment (TME). Notch1 inhibition reduces immune suppressive cells (i.e. MDSCs and Tregs) while allowing the recruitment of functional CD8(+) T cells, leading to a decrease in the Tregs/CD8(+) ratio, a key parameter in assessing positive responses to immune-checkpoint inhibitors. Inhibition of Notch1 improves the antitumor activity of nivolumab and ipilimumab, particularly when given in combination. Mechanistically, tumor-associated Notch1 regulates the expression of several chemokines involved in MDSCs and Tregs recruitment. Among them, CCL5, IL6 and IL8, or MIP2 in mouse, were consistently reduced by Notch1 depletion in several human and mouse melanoma cell lines. Notch1 controls the transcription of IL8 and IL6; and the secretion of CCL5 likely by inhibiting the expression of SNAP23, a member of the SNAREs family of proteins involved in cell exocytosis. Inhibition of SNAP23 decreases CCL5 secretion similarly to Notch1 inhibition. Hence, targeting Notch1 would affect both melanoma intrinsic growth/survival properties, and provide an immune-responsive TME, thus improving immune therapy efficacy.
Product Citations
-
-
Immunology and Microbiology
Immune checkpoint landscape of human atherosclerosis and influence of cardiometabolic factors.
In Nat Cardiovasc Res on 1 December 2024 by Barcia Durán, J. G., Das, D., et al.
PubMed
Immune checkpoint inhibitor (ICI) therapies can increase the risk of cardiovascular events in survivors of cancer by worsening atherosclerosis. Here we map the expression of immune checkpoints (ICs) within human carotid and coronary atherosclerotic plaques, revealing a network of immune cell interactions that ICI treatments can unintentionally target in arteries. We identify a population of mature, regulatory CCR7+FSCN1+ dendritic cells, similar to those described in tumors, as a hub of IC-mediated signaling within plaques. Additionally, we show that type 2 diabetes and lipid-lowering therapies alter immune cell interactions through PD-1, CTLA4, LAG3 and other IC targets in clinical development, impacting plaque inflammation. This comprehensive map of the IC interactome in healthy and cardiometabolic disease states provides a framework for understanding the potential adverse and beneficial impacts of approved and investigational ICIs on atherosclerosis, setting the stage for designing ICI strategies that minimize cardiovascular disease risk in cancer survivors.
-
-
-
Immunology and Microbiology
Modeling immune checkpoint inhibitor associated myocarditis in vitro and its therapeutic implications.
In J Mol Cell Cardiol Plus on 1 December 2024 by Jensen, G., Wang, X., et al.
PubMed
Immune checkpoint inhibitor-associated myocarditis is the most lethal side effect of immune checkpoint blockade. Myocarditis leads to persistently increased mortality and lacks effective treatments. The development of patient-relevant disease models may enable disease prediction, increased understanding of disease pathophysiology, and the development of effective treatment strategies. Here, we report a new method to model immune checkpoint inhibitor-associated myocarditis in vitro via a co-culture of activated primary human immune cells, human induced pluripotent stem cell-derived cardiomyocytes, and FDA-approved immune checkpoint inhibitors to recapitulate myocarditis in vitro. Significant cardiomyocyte necrosis, arrhythmia development, and sarcomere destruction occur, replicating clinical findings from myocarditis. This tissue culture myocarditis phenotype may rely on an induced pluripotent stem cell-derived cardiomyocyte antigen-specific CD8+ T cell response. The administration of dexamethasone rescued cardiomyocyte viability, morphology, and electrophysiology and suppressed inflammatory cytokine production. In conclusion, we detail how this platform can effectively model and provide critical information about the morphological and electrophysiological changes induced by immune checkpoint inhibitor-associated myocarditis. We have also validated the ability of this platform to screen potential medications to treat immune checkpoint inhibitor-associated myocarditis. This work establishes a robust, scalable model for identifying new therapies and risk factors, which is valuable in delineating the nature of interactions between the immune system and the heart during myocarditis.
-
-
-
Cancer Research
-
Immunology and Microbiology
ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis.
In iScience on 19 April 2024 by Deng, R., Tian, R., et al.
PubMed
Hepatitis B virus (HBV) infection generally elicits weak type-I interferon (IFN) immune response in hepatocytes, covering the regulatory effect of IFN-stimulated genes. In this study, low level of IFN-stimulated gene 12a (ISG12a) predicted malignant transformation and poor prognosis of HBV-associated hepatocellular carcinoma (HCC), whereas high level of ISG12a indicated active NK cell phenotypes. ISG12a interacts with TRIM21 to inhibit the phosphorylation activation of protein kinase B (PKB, also known as AKT) and β-catenin, suppressing PD-L1 expression to block PD-1/PD-L1 signaling, thereby enhancing the anticancer effect of NK cells. The suppression of PD-1-deficient NK-92 cells on HBV-associated tumors was independent of ISG12a expression, whereas the anticancer effect of PD-1-expressed NK-92 cells on HBV-associated tumors was enhanced by ISG12a and treatments of atezolizumab and nivolumab. Thus, tumor intrinsic ISG12a promotes the anticancer effect of NK cells by regulating PD-1/PD-L1 signaling, presenting the significant role of innate immunity in defending against HBV-associated HCC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy.
In Front Immunol on 30 May 2023 by Das, S., Valton, J., et al.
PubMed
Adoptive cell therapy based on chimeric antigen receptor (CAR)-engineered T-cells has proven to be lifesaving for many cancer patients. However, its therapeutic efficacy has so far been restricted to only a few malignancies, with solid tumors proving to be especially recalcitrant to efficient therapy. Poor intra-tumor infiltration by T cells and T cell dysfunction due to a desmoplastic, immunosuppressive microenvironment are key barriers for CAR T-cell success against solid tumors. Cancer-associated fibroblasts (CAFs) are critical components of the tumor stroma, evolving specifically within the tumor microenvironment (TME) in response to tumor cell cues. The CAF secretome is a significant contributor to the extracellular matrix and a plethora of cytokines and growth factors that induce immune suppression. Together they form a physical and chemical barrier which induces a T cell-excluding 'cold' TME. CAF depletion in stroma rich solid tumors can thus provide an opportunity to convert immune evasive tumors susceptible to tumor-antigen CAR T-cell cytotoxicity. Using our TALEN-based gene editing platform we engineered non-alloreactive, immune evasive CAR T-cells (termed UCAR T-cells) targeting the unique CAF marker Fibroblast Activation Protein, alpha (FAP). In an orthotopic mouse model of triple-negative breast cancer (TNBC) composed of patient derived-CAFs and tumor cells, we demonstrate the efficacy of our engineered FAP UCAR T-cells in CAF depletion, reduction of desmoplasia and successful tumor infiltration. Furthermore, while previously resistant, pre-treatment with FAP UCAR T-cells now sensitized these tumors to Mesothelin (Meso) UCAR T-cell infiltration and anti-tumor cytotoxicity. Combination therapy of FAP UCAR, Meso UCAR T cells and the checkpoint inhibitor anti-PD-1 significantly reduced tumor burden and prolonged mice survival. Our study thus proposes a novel treatment paradigm for successful CAR T-cell immunotherapy against stroma-rich solid tumors.
-
-
-
Cancer Research
-
Immunology and Microbiology
Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy
In bioRxiv on 27 February 2023 by Das, S., Valton, J., et al.
-
-
-
Immunology and Microbiology
-
Pharmacology
YIV-906 enhances nuclear factor of activated T-cells (NFAT) activity of T cells and promotes immune checkpoint blockade antibody action and CAR T-cell activity.
In Front Pharmacol on 24 January 2023 by Lam, W., Hu, R., et al.
PubMed
YIV-906 is a systems biology botanical cancer drug, inspired by a traditional Chinese herbal formulation. Results from eight Phase I/II to II clinical studies demonstrated the potential of YIV-906 to prolong survival and improve the quality of life of cancer patients. As an immunomodulator in the tumor microenvironment, YIV-906 can turn cold tumors hot and potentiate anti-tumor activity for different classes of anticancer agents; and as a cytoprotector in the GI, YIV-906 can reduce non-hematological side effects and speed up damaged tissue recovery. YIV-906 enhanced anti-PD1 action against hepatoma in mice by stimulating both innate and adaptive immunity. In a Jurkat cell-staphylococcal superantigen E (SEE)-Raji cell culture model, YIV-906 promoted T cell activation with upregulation of CD69 by enhancing NFAT activity, with or without PD1-PD-L1 interaction. YIV-906 could trigger the phosphorylation of TCR downstream signaling cascades without the involvement of TCR. YIV-906 could inhibit SHP1 and SHP2 activities, which dephosphorylates TCR downstream proteins due to the PD1-PD-L1 interaction. Therefore, YIV-906 could enhance anti-PD1 action to rescue the depressed NFAT activity of Jurkat cells due to the PD1-PD-L1 interaction. In addition, YIV-906 enhanced the NFAT activity and killing capability of Jurkat cells expressing chimeric antigen receptor (CAR-CD19-CD3z) toward CD19 expressing cells, such as Raji cells, with or without PD1-PD-L1 overexpression. Ingredient herb S (Scutellaria baicalensis Georgi) of YIV-906 and some S compounds were found to play key roles in these activities. In conclusion, YIV-906 modulates adaptive immunity by activating T effector cells mainly through its action on SHP1/2. YIV-906 could also facilitate immune checkpoint blockade therapy or CAR-T cell therapy for cancer treatment.
-
-
-
Cancer Research
-
Immunology and Microbiology
Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties.
In Cancer Lett on 10 October 2018 by Qiu, H., Zmina, P. M., et al.
PubMed
We have previously shown that Notch1 plays a critical role in modulating melanoma tumor cell growth and survival. Here we show that Notch1 also contributes to an immune-suppressive tumor microenvironment (TME). Notch1 inhibition reduces immune suppressive cells (i.e. MDSCs and Tregs) while allowing the recruitment of functional CD8(+) T cells, leading to a decrease in the Tregs/CD8(+) ratio, a key parameter in assessing positive responses to immune-checkpoint inhibitors. Inhibition of Notch1 improves the antitumor activity of nivolumab and ipilimumab, particularly when given in combination. Mechanistically, tumor-associated Notch1 regulates the expression of several chemokines involved in MDSCs and Tregs recruitment. Among them, CCL5, IL6 and IL8, or MIP2 in mouse, were consistently reduced by Notch1 depletion in several human and mouse melanoma cell lines. Notch1 controls the transcription of IL8 and IL6; and the secretion of CCL5 likely by inhibiting the expression of SNAP23, a member of the SNAREs family of proteins involved in cell exocytosis. Inhibition of SNAP23 decreases CCL5 secretion similarly to Notch1 inhibition. Hence, targeting Notch1 would affect both melanoma intrinsic growth/survival properties, and provide an immune-responsive TME, thus improving immune therapy efficacy.
-