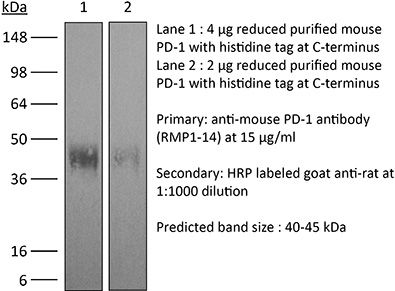
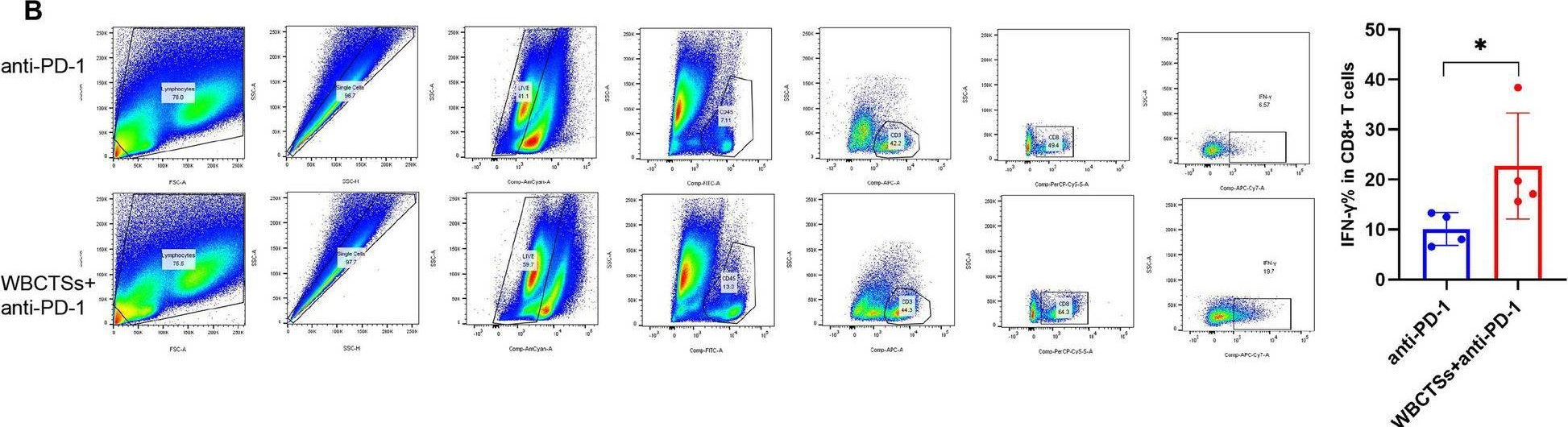
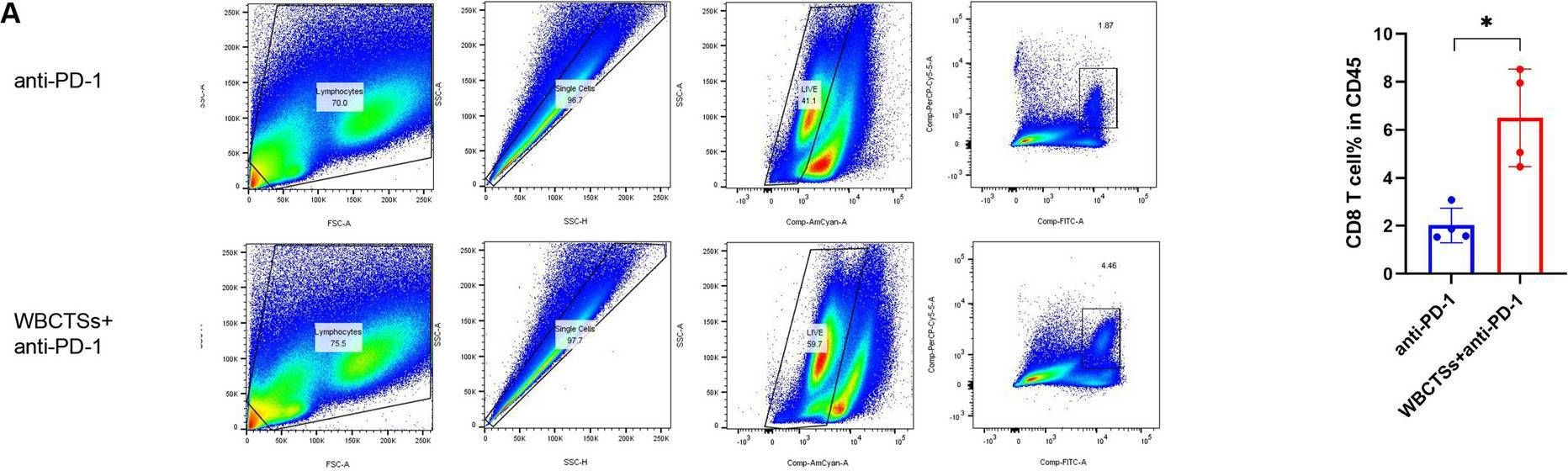
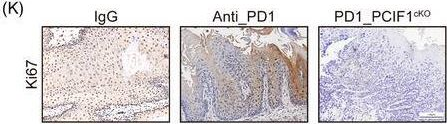
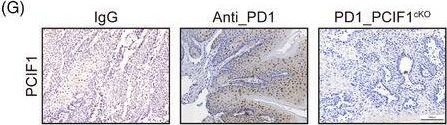
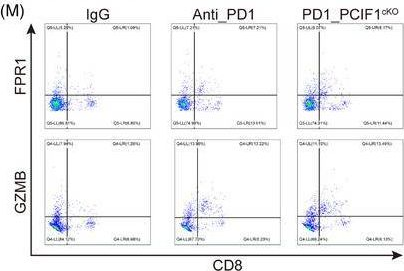
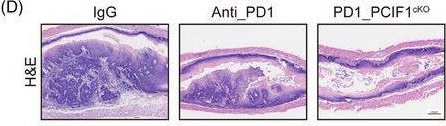
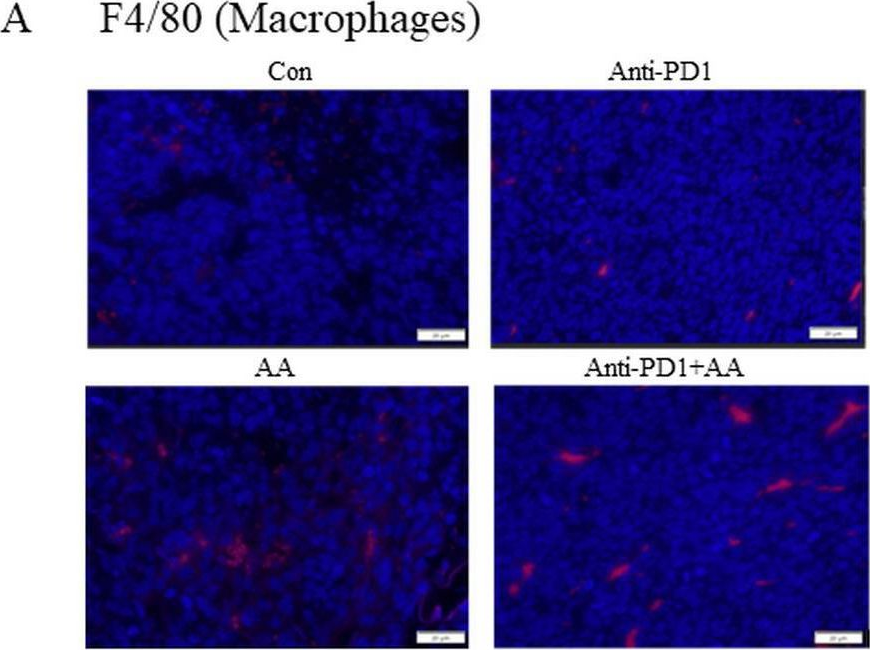
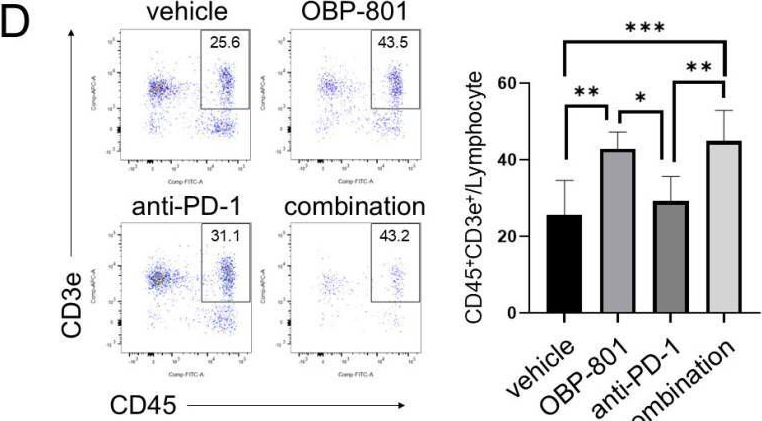
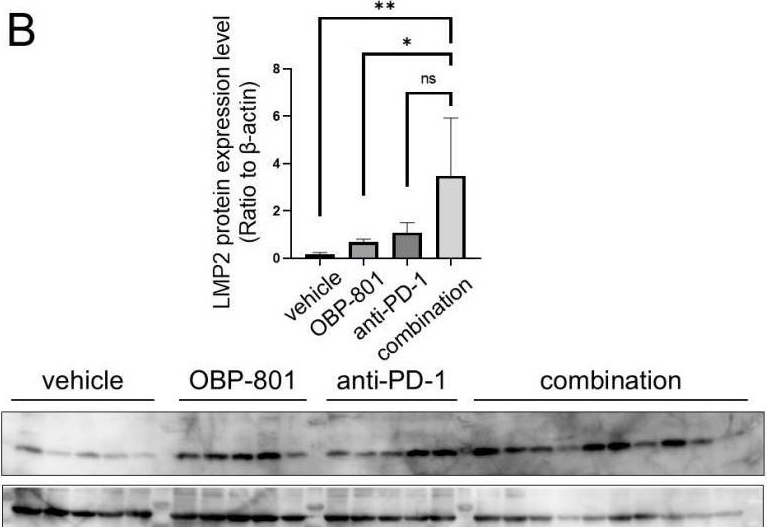
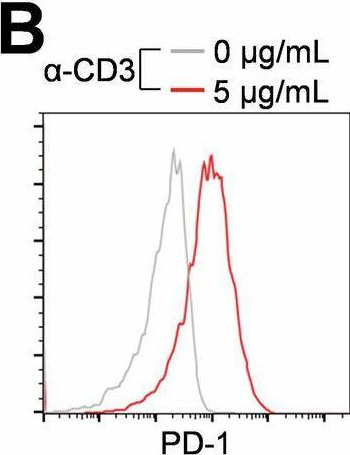
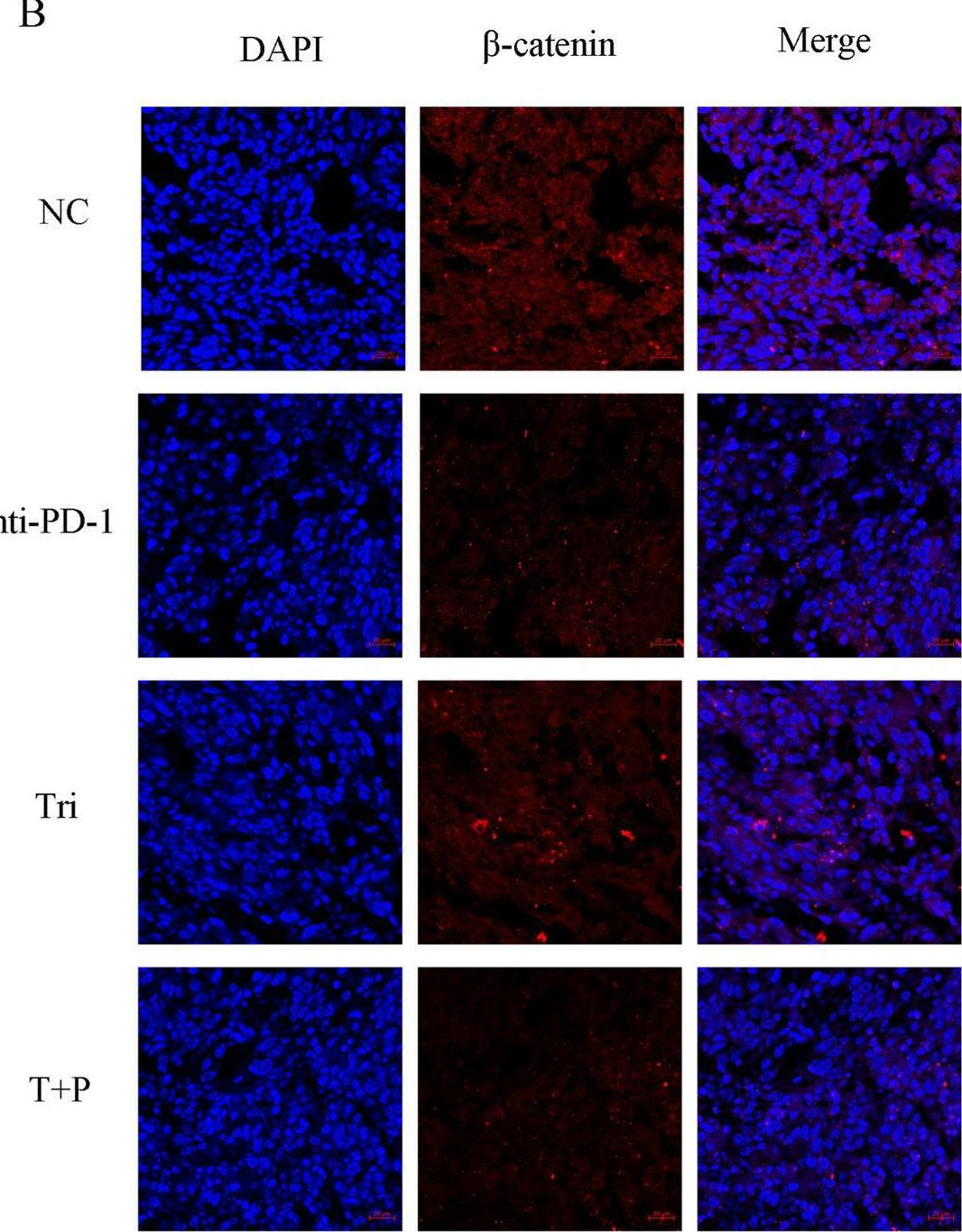
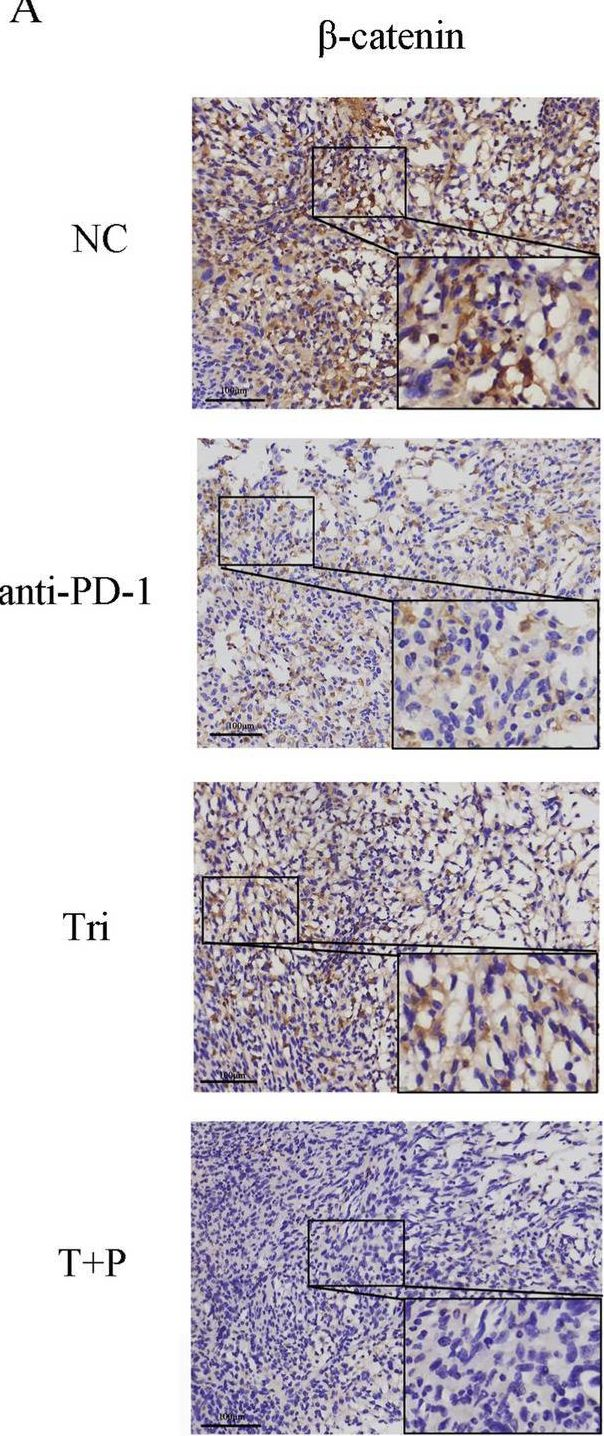
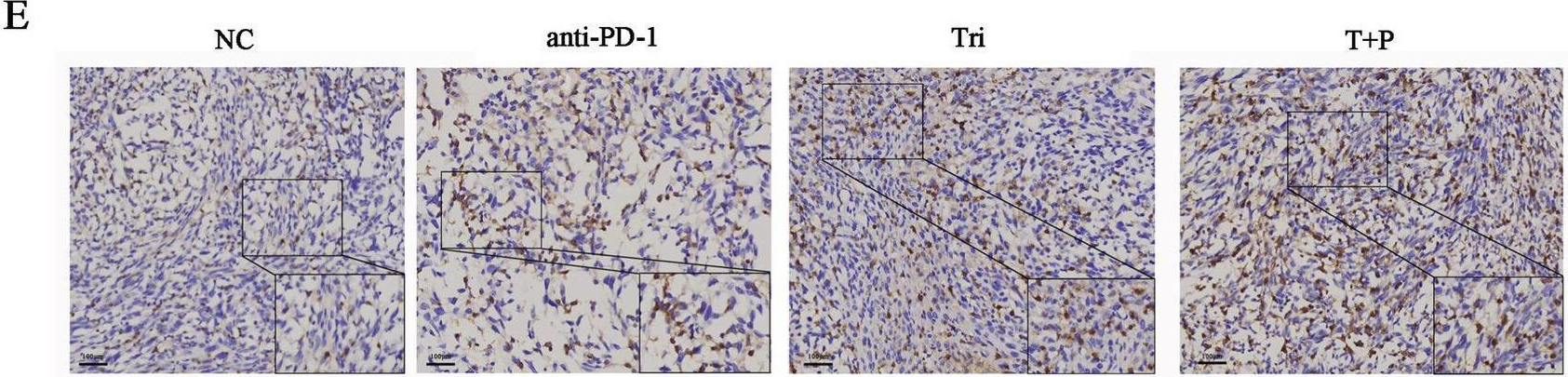
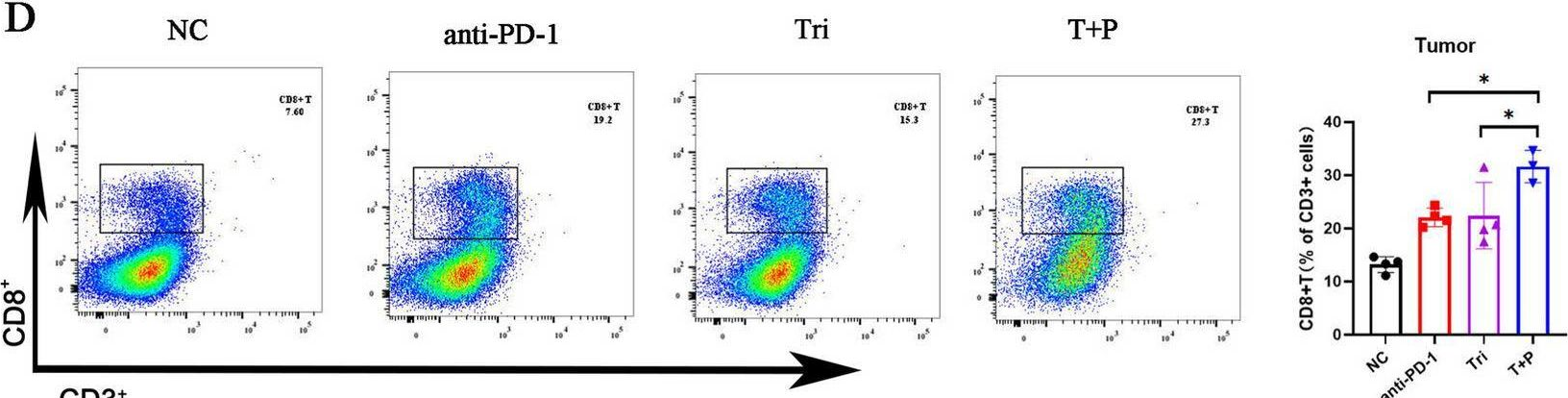
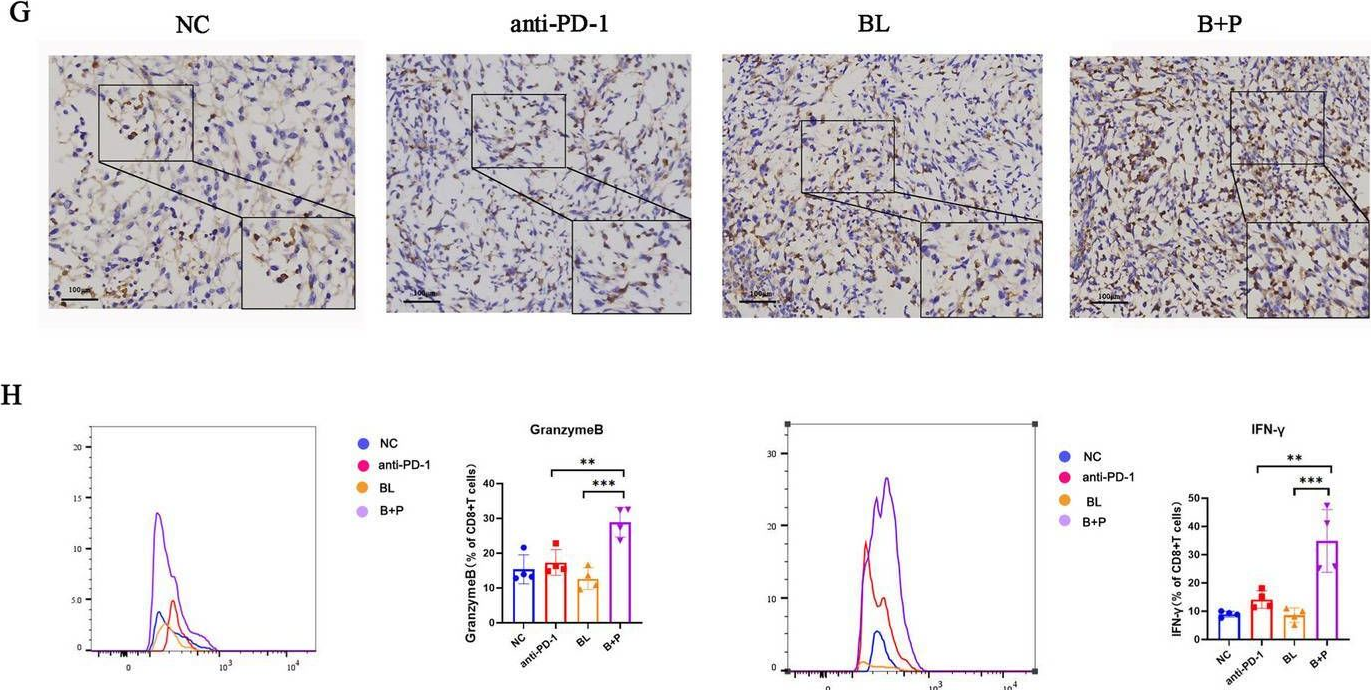
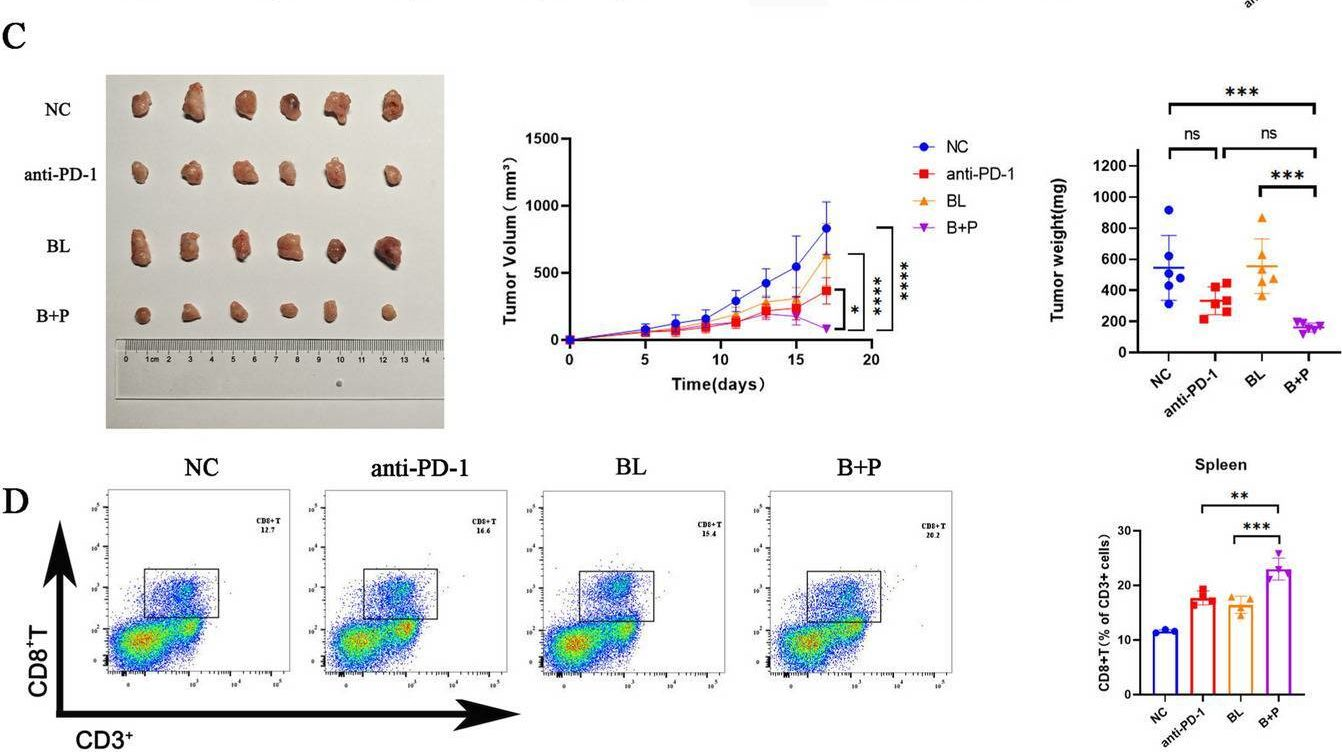
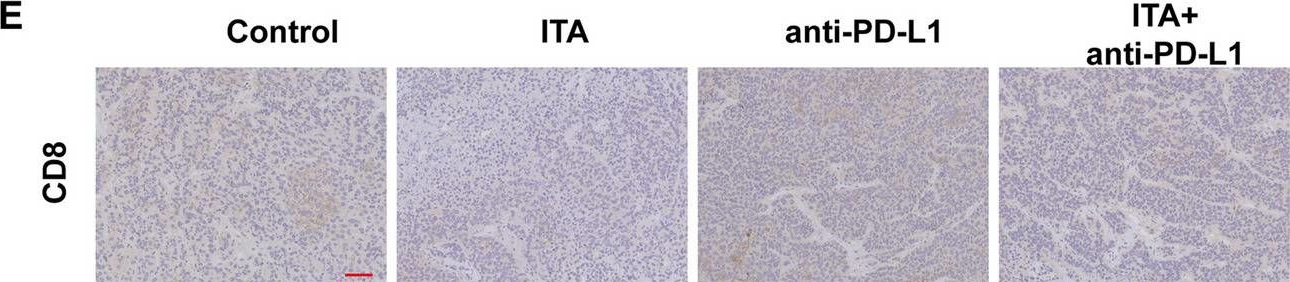
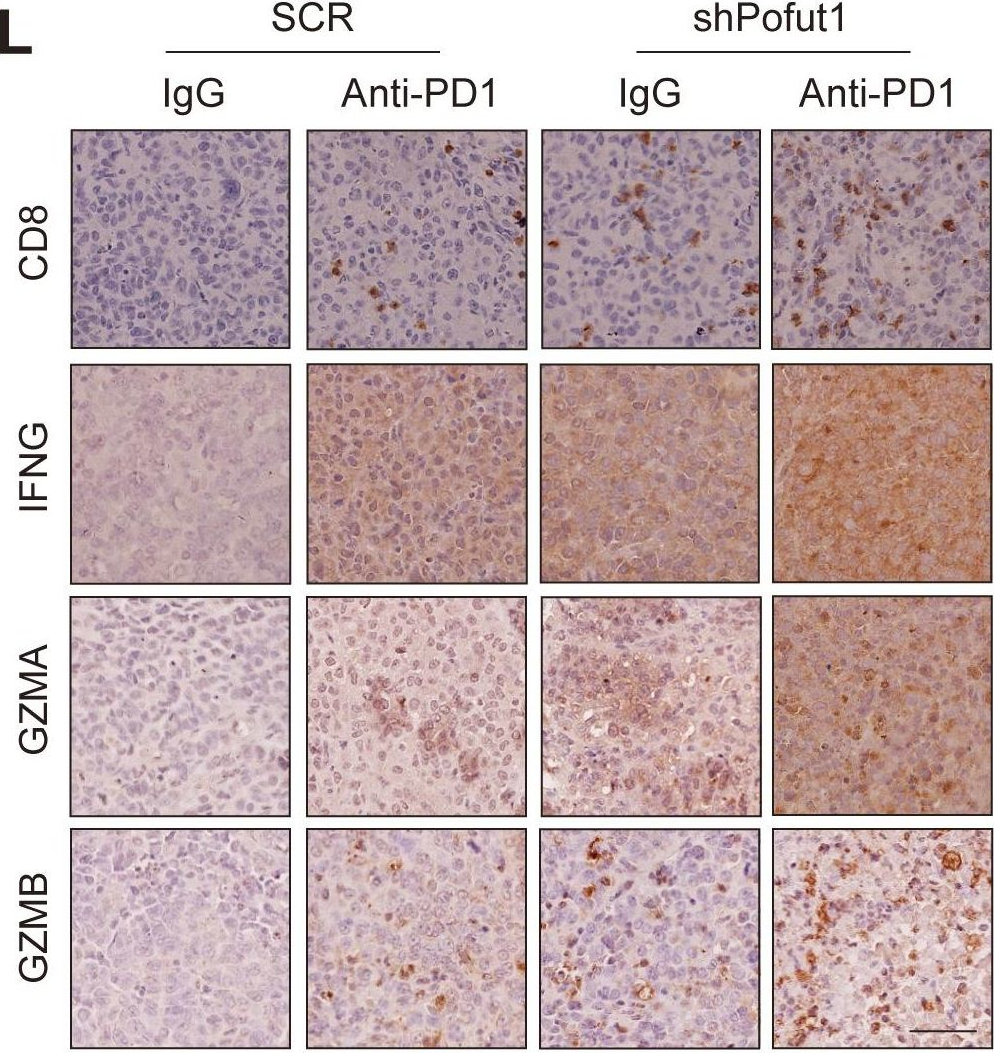
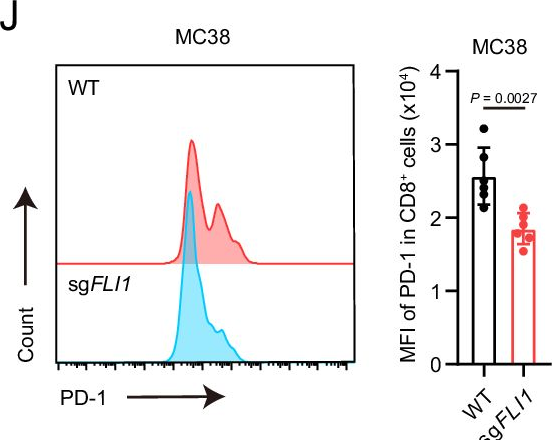
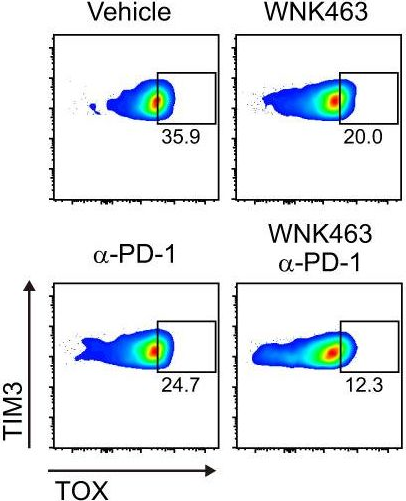
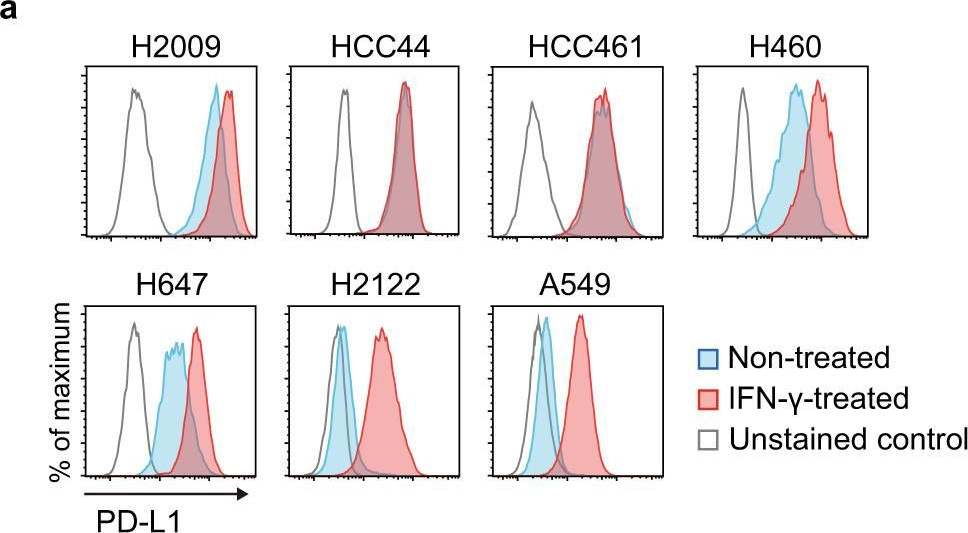
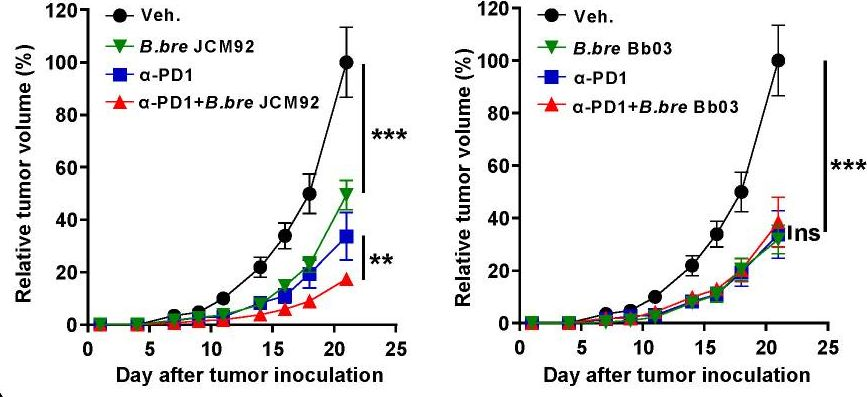
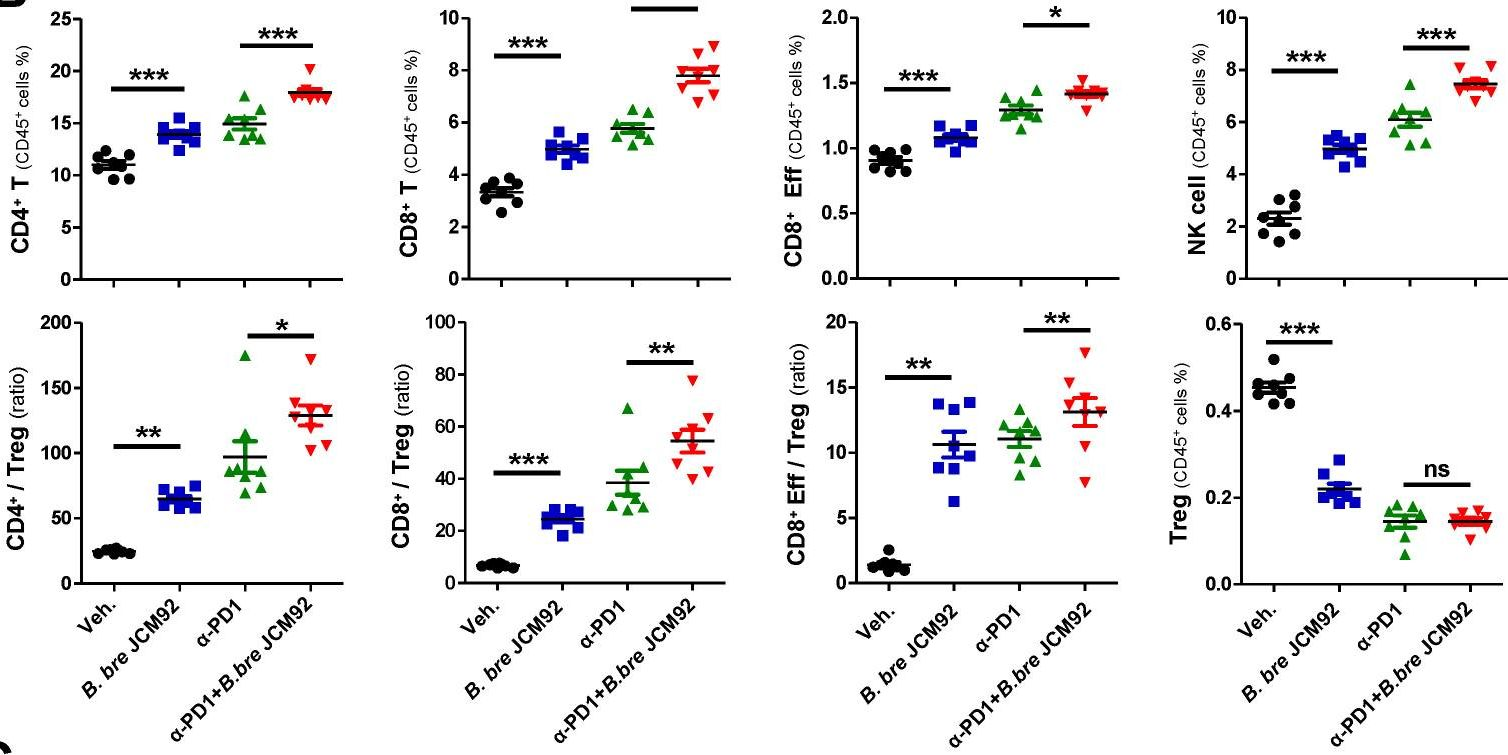
InVivoMAb anti-mouse PD-1 (CD279)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| Reported Applications |
in vivo blocking of PD-1/PD-L signaling in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949053 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of PD-1/PD-L signaling
Nakazawa Y, Miyano M, Tsukamoto S, Kogai H, Yamamoto A, Iso K, Inoue S, Yamane Y, Yabe Y, Umihara H, Taguchi J, Akagi T, Yamaguchi A, Koga M, Toshimitsu K, Hirayama T, Mukai Y, Machinaga A (2024). "Delivery of a BET protein degrader via a CEACAM6-tar
PubMed
Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis of all cancers. To improve PDAC therapy, we establish screening systems based on organoid and co-culture technologies and find a payload of antibody-drug conjugate (ADC), a bromodomain and extra-terminal (BET) protein degrader named EBET. We select CEACAM6/CD66c as an ADC target and developed an antibody, #84.7, with minimal reactivity to CEACAM6-expressing normal cells. EBET-conjugated #84.7 (84-EBET) has lethal effects on various PDAC organoids and bystander efficacy on CEACAM6-negative PDAC cells and cancer-associated fibroblasts. In mouse studies, a single injection of 84-EBET induces marked tumor regression in various PDAC-patient-derived xenografts, with a decrease in the inflammatory phenotype of stromal cells and without significant body weight loss. Combination with standard chemotherapy or PD-1 antibody induces more profound and sustained regression without toxicity enhancement. Our preclinical evidence demonstrates potential efficacy by delivering BET protein degrader to PDAC and its microenvironment via CEACAM6-targeted ADC.
in vitro Organoids/Organ-on-Chip
in vivo blocking of PD-1/PD-L signaling
Holokai L, Chakrabarti J, Lundy J, Croagh D, Adhikary P, Richards SS, Woodson C, Steele N, Kuester R, Scott A, Khreiss M, Frankel T, Merchant J, Jenkins BJ, Wang J, Shroff RT, Ahmad SA, Zavros Y (2020). "Murine- and Human-Derived Autologous Organoid/
PubMed
Purpose: Pancreatic ductal adenocarcinoma (PDAC) has the lowest five-year survival rate of all cancers in the United States. Programmed death 1 receptor (PD-1)-programmed death ligand 1 (PD-L1) immune checkpoint inhibition has been unsuccessful in clinical trials. Myeloid-derived suppressor cells (MDSCs) are known to block anti-tumor CD8+ T cell immune responses in various cancers including pancreas. This has led us to our objective that was to develop a clinically relevant in vitro organoid model to specifically target mechanisms that deplete MDSCs as a therapeutic strategy for PDAC. Method: Murine and human pancreatic ductal adenocarcinoma (PDAC) autologous organoid/immune cell co-cultures were used to test whether PDAC can be effectively treated with combinatorial therapy involving PD-1 inhibition and MDSC depletion. Results: Murine in vivo orthotopic and in vitro organoid/immune cell co-culture models demonstrated that polymorphonuclear (PMN)-MDSCs promoted tumor growth and suppressed cytotoxic T lymphocyte (CTL) proliferation, leading to diminished efficacy of checkpoint inhibition. Mouse- and human-derived organoid/immune cell co-cultures revealed that PD-L1-expressing organoids were unresponsive to nivolumab in vitro in the presence of PMN-MDSCs. Depletion of arginase 1-expressing PMN-MDSCs within these co-cultures rendered the organoids susceptible to anti-PD-1/PD-L1-induced cancer cell death. Conclusions: Here we use mouse- and human-derived autologous pancreatic cancer organoid/immune cell co-cultures to demonstrate that elevated infiltration of polymorphonuclear (PMN)-MDSCs within the PDAC tumor microenvironment inhibit T cell effector function, regardless of PD-1/PD-L1 inhibition. We present a pre-clinical model that may predict the efficacy of targeted therapies to improve the outcome of patients with this aggressive and otherwise unpredictable malignancy.
in vivo blocking of PD-1/PD-L signaling
Triplett, T. A., et al (2018). "Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme" Nat Biotechnol 36(8): 758-764.
PubMed
Increased tryptophan (Trp) catabolism in the tumor microenvironment (TME) can mediate immune suppression by upregulation of interferon (IFN)-gamma-inducible indoleamine 2,3-dioxygenase (IDO1) and/or ectopic expression of the predominantly liver-restricted enzyme tryptophan 2,3-dioxygenase (TDO). Whether these effects are due to Trp depletion in the TME or mediated by the accumulation of the IDO1 and/or TDO (hereafter referred to as IDO1/TDO) product kynurenine (Kyn) remains controversial. Here we show that administration of a pharmacologically optimized enzyme (PEGylated kynureninase; hereafter referred to as PEG-KYNase) that degrades Kyn into immunologically inert, nontoxic and readily cleared metabolites inhibits tumor growth. Enzyme treatment was associated with a marked increase in the tumor infiltration and proliferation of polyfunctional CD8(+) lymphocytes. We show that PEG-KYNase administration had substantial therapeutic effects when combined with approved checkpoint inhibitors or with a cancer vaccine for the treatment of large B16-F10 melanoma, 4T1 breast carcinoma or CT26 colon carcinoma tumors. PEG-KYNase mediated prolonged depletion of Kyn in the TME and reversed the modulatory effects of IDO1/TDO upregulation in the TME.
in vivo blocking of PD-1/PD-L signaling
Grasselly, C., et al (2018). "The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent" Front Immunol 9: 2100.
PubMed
In spite of impressive response rates in multiple cancer types, immune checkpoint inhibitors (ICIs) are active in only a minority of patients. Alternative strategies currently aim to combine immunotherapies with conventional agents such as cytotoxic chemotherapies. Here, we performed a study of PD-1 or PDL-1 blockade in combination with reference chemotherapies in four fully immunocompetent mouse models of cancer. We analyzed both the in vivo antitumor response, and the tumor immune infiltrate 4 days after the first treatment. in vivo tumor growth experiments revealed variable responsiveness to ICIs between models. We observed enhanced antitumor effects of the combination of immunotherapy with chemotherapy in the MC38 colon and MB49 bladder models, a lack of response in the 4T1 breast model, and an inhibition of ICIs activity in the MBT-2 bladder model. Flow cytometry analysis of tumor samples showed significant differences in all models between untreated and treated mice. At baseline, all the tumor models studied were predominantly infiltrated with cells harboring an immunosuppressive phenotype. Early alterations of the tumor immune infiltrate after treatment were found to be highly variable. We found that the balance between effector cells and immunosuppressive cells in the tumor microenvironment could be altered with some treatment combinations, but this effect was not always correlated with an impact on in vivo tumor growth. These results show that the combination of cytotoxic chemotherapy with ICIs may result in enhanced, similar or reduced antitumor activity, in a model- and regimen-dependent fashion. The present investigations should help to select appropriate combination regimens for ICIs.
in vivo blocking of PD-1/PD-L signaling
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo blocking of PD-1/PD-L signaling
Twyman-Saint Victor, C., et al (2015). "Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer" Nature 520(7547): 373-377.
PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
in vivo blocking of PD-1/PD-L signaling
Zelenay, S., et al (2015). "Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity" Cell 162(6): 1257-1270.
PubMed
The mechanisms by which melanoma and other cancer cells evade anti-tumor immunity remain incompletely understood. Here, we show that the growth of tumors formed by mutant Braf(V600E) mouse melanoma cells in an immunocompetent host requires their production of prostaglandin E2, which suppresses immunity and fuels tumor-promoting inflammation. Genetic ablation of cyclooxygenases (COX) or prostaglandin E synthases in Braf(V600E) mouse melanoma cells, as well as in Nras(G12D) melanoma or in breast or colorectal cancer cells, renders them susceptible to immune control and provokes a shift in the tumor inflammatory profile toward classic anti-cancer immune pathways. This mouse COX-dependent inflammatory signature is remarkably conserved in human cutaneous melanoma biopsies, arguing for COX activity as a driver of immune suppression across species. Pre-clinical data demonstrate that inhibition of COX synergizes with anti-PD-1 blockade in inducing eradication of tumors, implying that COX inhibitors could be useful adjuvants for immune-based therapies in cancer patients.
in vivo blocking of PD-1/PD-L signaling
Vanpouille-Box, C., et al (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo blocking of PD-1/PD-L signaling
Evans, E. E., et al (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo blocking of PD-1/PD-L signaling
Ngiow, S. F., et al (2015). "A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1" Cancer Res 75(18): 3800-3811.
PubMed
Despite successes, thus far, a significant proportion of the patients treated with anti-PD1 antibodies have failed to respond. We use mouse tumor models of anti-PD1 sensitivity and resistance and flow cytometry to assess tumor-infiltrating immune cells immediately after therapy. We demonstrate that the expression levels of T-cell PD1 (PD1(lo)), myeloid, and T-cell PDL1 (PDL1(hi)) in the tumor microenvironment inversely correlate and dictate the efficacy of anti-PD1 mAb and function of intratumor CD8(+) T cells. In sensitive tumors, we reveal a threshold for PD1 downregulation on tumor-infiltrating CD8(+) T cells below which the release of adaptive immune resistance is achieved. In contrast, PD1(hi) T cells in resistant tumors fail to be rescued by anti-PD1 therapy and remain dysfunctional unless intratumor PDL1(lo) immune cells are targeted. Intratumor Tregs are partly responsible for the development of anti-PD1-resistant tumors and PD1(hi) CD8(+) T cells. Our analyses provide a framework to interrogate intratumor CD8(+) T-cell PD1 and immune PDL1 levels and response in human cancer. Cancer Res; 75(18); 3800-11. (c)2015 AACR.
in vivo blocking of PD-1/PD-L signaling
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo blocking of PD-1/PD-L signaling
McGray, A. J., et al (2014). "Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor" Mol Ther 22(1): 206-218.
PubMed
Despite clear evidence of immunogenicity, cancer vaccines only provide a modest clinical benefit. To evaluate the mechanisms that limit tumor regression following vaccination, we have investigated the weak efficacy of a highly immunogenic experimental vaccine using a murine melanoma model. We discovered that the tumor adapts rapidly to the immune attack instigated by tumor-specific CD8+ T cells in the first few days following vaccination, resulting in the upregulation of a complex set of biological networks, including multiple immunosuppressive processes. This rapid adaptation acts to prevent sustained local immune attack, despite continued infiltration by increasing numbers of tumor-specific T cells. Combining vaccination with adoptive transfer of tumor-specific T cells produced complete regression of the treated tumors but did not prevent the adaptive immunosuppression. In fact, the adaptive immunosuppressive pathways were more highly induced in regressing tumors, commensurate with the enhanced level of immune attack. Examination of tumor infiltrating T-cell functionality revealed that the adaptive immunosuppression leads to a progressive loss in T-cell function, even in tumors that are regressing. These novel observations that T cells produced by therapeutic intervention can instigate a rapid adaptive immunosuppressive response within the tumor have important implications for clinical implementation of immunotherapies.
in vivo blocking of PD-1/PD-L signaling
Mittal, D., et al (2014). "Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor" Cancer Res 74(14): 3652-3658.
PubMed
Adenosine targeting is an attractive new approach to cancer treatment, but no clinical study has yet examined adenosine inhibition in oncology despite the safe clinical profile of adenosine A2A receptor inhibitors (A2ARi) in Parkinson disease. Metastasis is the main cause of cancer-related deaths worldwide, and therefore we have studied experimental and spontaneous mouse models of melanoma and breast cancer metastasis to demonstrate the efficacy and mechanism of a combination of A2ARi in combination with anti-PD-1 monoclonal antibody (mAb). This combination significantly reduces metastatic burden and prolongs the life of mice compared with either monotherapy alone. Importantly, the combination was only effective when the tumor expressed high levels of CD73, suggesting a tumor biomarker that at a minimum could be used to stratify patients that might receive this combination. The mechanism of the combination therapy was critically dependent on NK cells and IFNgamma, and to a lesser extent, CD8(+) T cells and the effector molecule, perforin. Overall, these results provide a strong rationale to use A2ARi with anti-PD-1 mAb for the treatment of minimal residual and metastatic disease.
in vivo blocking of PD-1/PD-L signaling
John, L. B., et al (2013). "Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells" Clin Cancer Res 19(20): 5636-5646.
PubMed
PURPOSE: To determine the antitumor efficacy and toxicity of a novel combination approach involving adoptive T-cell immunotherapy using chimeric antigen receptor (CAR) T cells with an immunomodulatory reagent for blocking immunosuppression. EXPERIMENTAL DESIGN: We examined whether administration of a PD-1 blocking antibody could increase the therapeutic activity of CAR T cells against two different Her-2(+) tumors. The use of a self-antigen mouse model enabled investigation into the efficacy, mechanism, and toxicity of this combination approach. RESULTS: In this study, we first showed a significant increase in the level of PD-1 expressed on transduced anti-Her-2 CD8(+) T cells following antigen-specific stimulation with PD-L1(+) tumor cells and that markers of activation and proliferation were increased in anti-Her-2 T cells in the presence of anti-PD-1 antibody. In adoptive transfer studies in Her-2 transgenic recipient mice, we showed a significant improvement in growth inhibition of two different Her-2(+) tumors treated with anti-Her-2 T cells in combination with anti-PD-1 antibody. The therapeutic effects observed correlated with increased function of anti-Her-2 T cells following PD-1 blockade. Strikingly, a significant decrease in the percentage of Gr1(+) CD11b(+) myeloid-derived suppressor cells (MDSC) was observed in the tumor microenvironment of mice treated with the combination therapy. Importantly, increased antitumor effects were not associated with any autoimmune pathology in normal tissue expressing Her-2 antigen. CONCLUSION: This study shows that specifically blocking PD-1 immunosuppression can potently enhance CAR T-cell therapy that has significant implications for potentially improving therapeutic outcomes of this approach in patients with cancer.
in vivo blocking of PD-1/PD-L signaling
van der Werf, N., et al (2013). "Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection" PLoS Pathog 9(3): e1003215.
PubMed
The suppression of protective Type 2 immunity is a principal factor driving the chronicity of helminth infections, and has been attributed to a range of Th2 cell-extrinsic immune-regulators. However, the intrinsic fate of parasite-specific Th2 cells within a chronic immune down-regulatory environment, and the resultant impact such fate changes may have on host resistance is unknown. We used IL-4gfp reporter mice to demonstrate that during chronic helminth infection with the filarial nematode Litomosoides sigmodontis, CD4(+) Th2 cells are conditioned towards an intrinsically hypo-responsive phenotype, characterised by a loss of functional ability to proliferate and produce the cytokines IL-4, IL-5 and IL-2. Th2 cell hypo-responsiveness was a key element determining susceptibility to L. sigmodontis infection, and could be reversed in vivo by blockade of PD-1 resulting in long-term recovery of Th2 cell functional quality and enhanced resistance. Contrasting with T cell dysfunction in Type 1 settings, the control of Th2 cell hypo-responsiveness by PD-1 was mediated through PD-L2, and not PD-L1. Thus, intrinsic changes in Th2 cell quality leading to a functionally hypo-responsive phenotype play a key role in determining susceptibility to filarial infection, and the therapeutic manipulation of Th2 cell-intrinsic quality provides a potential avenue for promoting resistance to helminths.
in vivo blocking of PD-1/PD-L signaling
Holmgaard, R. B., et al (2013). "Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4" J Exp Med 210(7): 1389-1402.
PubMed
The cytotoxic T lymphocyte antigen-4 (CTLA-4)-blocking antibody ipilimumab results in durable responses in metastatic melanoma, though therapeutic benefit has been limited to a fraction of patients. This calls for identification of resistance mechanisms and development of combinatorial strategies. Here, we examine the inhibitory role of indoleamine 2,3-dioxygenase (IDO) on the antitumor efficacy of CTLA-4 blockade. In IDO knockout mice treated with anti-CTLA-4 antibody, we demonstrate a striking delay in B16 melanoma tumor growth and increased overall survival when compared with wild-type mice. This was also observed with antibodies targeting PD-1-PD-L1 and GITR. To highlight the therapeutic relevance of these findings, we show that CTLA-4 blockade strongly synergizes with IDO inhibitors to mediate rejection of both IDO-expressing and nonexpressing poorly immunogenic tumors, emphasizing the importance of the inhibitory role of both tumor- and host-derived IDO. This effect was T cell dependent, leading to enhanced infiltration of tumor-specific effector T cells and a marked increase in the effector-to-regulatory T cell ratios in the tumors. Overall, these data demonstrate the immunosuppressive role of IDO in the context of immunotherapies targeting immune checkpoints and provide a strong incentive to clinically explore combination therapies using IDO inhibitors irrespective of IDO expression by the tumor cells.
in vivo blocking of PD-1/PD-L signaling
Curran, M. A., et al (2010). "PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors" Proc Natl Acad Sci U S A 107(9): 4275-4280.
PubMed
Vaccination with irradiated B16 melanoma cells expressing either GM-CSF (Gvax) or Flt3-ligand (Fvax) combined with antibody blockade of the negative T-cell costimulatory receptor cytotoxic T-lymphocyte antigen-4 (CTLA-4) promotes rejection of preimplanted tumors. Despite CTLA-4 blockade, T-cell proliferation and cytokine production can be inhibited by the interaction of programmed death-1 (PD-1) with its ligands PD-L1 and PD-L2 or by the interaction of PD-L1 with B7-1. Here, we show that the combination of CTLA-4 and PD-1 blockade is more than twice as effective as either alone in promoting the rejection of B16 melanomas in conjunction with Fvax. Adding alphaPD-L1 to this regimen results in rejection of 65% of preimplanted tumors vs. 10% with CTLA-4 blockade alone. Combination PD-1 and CTLA-4 blockade increases effector T-cell (Teff) infiltration, resulting in highly advantageous Teff-to-regulatory T-cell ratios with the tumor. The fraction of tumor-infiltrating Teffs expressing CTLA-4 and PD-1 increases, reflecting the proliferation and accumulation of cells that would otherwise be anergized. Combination blockade also synergistically increases Teff-to-myeloid-derived suppressor cell ratios within B16 melanomas. IFN-gamma production increases in both the tumor and vaccine draining lymph nodes, as does the frequency of IFN-gamma/TNF-alpha double-producing CD8(+) T cells within the tumor. These results suggest that combination blockade of the PD-1/PD-L1- and CTLA-4-negative costimulatory pathways allows tumor-specific T cells that would otherwise be inactivated to continue to expand and carry out effector functions, thereby shifting the tumor microenvironment from suppressive to inflammatory.
Product Citations
-
-
Immunology and Microbiology
Daytime SHP2 inhibitor dosing, when immune cell numbers are elevated, shrinks neurofibromas.
In Life Sci Alliance on 1 December 2025 by Ahmari, N., Choi, K., et al.
PubMed
Loss of NF1 in Schwann cells leads to activation of the RAS-MAPK pathway, followed by immune cell recruitment and development of benign nerve tumors (PNFs). MEK inhibitors, which shrink most PNFs, also reduce tumor-associated myeloid cells. We tested whether SHP2 inhibition, predicted to block RAS-MAPK signaling and exert immunomodulatory effects, alters tumor volume or the immune microenvironment in PNFs, using flow cytometry and single-cell RNA sequencing. We found that both cobimetinib and daytime RMC-4550 similarly reduced tumor volume. The abundance of CD163-negative PNF-associated macrophages, derived from circulating monocytes, correlated with tumor size. Combining SHP2 inhibition with anti-PD1 altered tumor monocyte phenotype and reversed SHP2-mediated tumor shrinkage. Diurnal patterns of monocyte trafficking were disrupted in tumor-bearing mice, and SHP2 inhibition reduced tumor volume only when administered during the day, when myeloid infiltration was low. These findings suggest that SHP2 inhibitor-driven tumor shrinkage requires targeting monocyte-derived macrophages and is influenced by the timing of drug administration.
-
-
-
Immunology and Microbiology
Targeting hepatocytic TβRI ameliorates liver metastatic outcomes by revitalizing stem-like CD8+ Tex subsets.
In Nat Commun on 27 November 2025 by Wang, H., Zhou, Y., et al.
PubMed
Stem-like CD8⁺ exhausted T cells (Tex) sustain antitumor immunity, whereas TGFβ signaling acts as a major immunosuppressive pathway. In patients with colorectal liver metastases, we observe that elevated TβRI expression in peri-metastatic hepatocytes correlates with poor prognosis. We therefore investigate whether disrupting hepatocytic TGFβ signaling can reinvigorate stem-like CD8⁺ Tex cells to restrict liver metastasis. In support of this hypothesis, mice with hepatocyte-specific TβRI depletion exhibit reduced liver metastatic burden across multiple tumor models. Mechanistically, hepatocytic TβRI blockade suppresses Galectin-9 secretion, which reshapes the transcriptional program of intra-tumoral CD8⁺ T cells. This reprogramming promotes a phenotypic transition from terminal exhaustion toward stem-like and effector states, yielding T cell subsets with enhanced metastasis-control capacity. Importantly, this axis functions independently of macrophages and CD4⁺ T cells. Furthermore, therapeutic delivery of Galunisertib using choline-modified lipid nanoparticles synergizes with αPD-1, fostering the conversion of exhausted CD8⁺ T cells into responsive Ly108⁺CX3CR1⁺ subsets and suppressing liver metastases. Collectively, our results identify hepatocyte TGFβ signaling as a targetable checkpoint against liver metastases.
-
-
-
Cancer Research
-
Immunology and Microbiology
Cancer cell-derived IL-1β reverses chemo-immunotherapy resistance in non-small cell lung cancer.
In Nat Commun on 21 November 2025 by Perrichet, A., Lecuelle, J., et al.
PubMed
Many non-small cell lung cancer (NSCLC) patients remain unresponsive to the current standard of care, which includes chemotherapy and immune checkpoint inhibitors, like anti-PD-1/PD-L1 antibodies. While interleukin (IL)-1β is known to promote lung cancer growth in humans and mice, we show here that IL-1β administration or overexpression overcomes resistance to classical chemo-immunotherapy (cisplatin/pemetrexed/anti-PD-1) in mouse lung cancer models. The antitumor effects of IL-1β rely on cancer cell-derived CXCL10 which mediates CD8 T cell recruitment at the tumor site. In lung cancer cells, Thioredoxin Interacting Protein (TXNIP) induces mitochondrial DNA (mtDNA) release in the cytosol, activating Absence in Melanoma 2 (AIM2) inflammasome, which subsequently triggers IL-1β and CXCL10 secretion, thereby reversing chemo-immunotherapy resistance. The clinical relevance of our findings is supported by the transcriptomic analysis of patient tumors, showing that high expression of IL1B, IL1R1, AIM2 and/or TXNIP is associated with better response to immunotherapy in NSCLC patients. Additionally, drug screening identifies MEK and MDM2 inhibitors as inducers of TXNIP expression capable of reversing resistance to chemo-immunotherapy. This study highlights a positive role of IL-1β in lung cancer treatment and suggests that enhancing IL-1β production at the tumor site can overcome resistance to chemo-immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
β-adrenergic signaling blockade attenuates metastasis through activation of cytotoxic CD4 T cells.
In Nat Commun on 17 November 2025 by Fjæstad, K. Y., Johansen, A. Z., et al.
PubMed
β-adrenergic signaling has been suggested to promote tumor growth, and β-blockers are being evaluated for repurposing for cancer treatment. Here, we identify a β-adrenergic signaling axis involved in metastasis formation. We show that the β-blocker propranolol has strong anti-metastatic activity in multiple murine models, with this effect being completely dependent on CD4 + T cells and independent of NK or CD8 + T cells. We also observe that CD4 + T cells are required for the anti-tumor effect of propranolol in a syngeneic subcutaneous model of colon cancer. Mechanistically, propranolol induces a Th1-polarized and cytotoxic CD4 + T cell response, which requires MHC class II expression by cancer cells for full efficacy. We also report propanolol-driven systemic changes in the monocyte compartment, and upon depletion of monocytes, propranolol loses its anti-tumor effects. Finally, we show that propranolol treatment synergizes with anti-CTLA-4 therapy to further enhance CD4 + T cell infiltration and control metastasis. Thus, we show that β-adrenergic signaling limits CD4 T cell-mediated anti-tumor immunity, highlighting the potential of repurposing β-blockers for cancer treatment.
-
-
-
Cancer Research
-
Immunology and Microbiology
Development of the rationale of a personalized cancer vaccine based on the in situ vaccine effect of radiotherapy: a mechanistic study of the POLARSTAR trial.
In Cancer Immunol Immunother on 12 November 2025 by Pang, K., Sun, P., et al.
PubMed
Radiotherapy induces multiple forms of tumor cell death, including immunogenic cell death (ICD) like GSDME-mediated pyroptosis and MLKL-mediated necroptosis; and ICD has been increasingly accepted as a crucial element leading to enhanced anti-tumor adaptive immunity. We aim to clarify whether a vaccine-like effect is intrinsic for radiation-induced tumor cell death, and to explore potential applications.
-
-
-
Immunology and Microbiology
-
Cancer Research
CBX6 induces CD8+ T cell exhaustion and tumor development in esophageal squamous cell carcinoma through SMARCD1-mediated CCL8 secretion and lactate efflux.
In Cell Biol Toxicol on 12 November 2025 by Wang, L., Liu, G., et al.
PubMed
This study investigates the functions of chromobox 6 (CBX6) in esophageal squamous cell carcinoma (ESCC) and delves into its functional mechanisms. The bioinformatics insights suggested that CBX6 was overexpressed in ESCC and linked to dismal prognosis. Cbx6 knockdown was induced in mouse mEC25 cells. This procedure curbed the proliferation and migration of mEC25 cells and reduced exhaustion of the co-cultured CD8+ T cells. In vivo, Cbx6 knockdown in mEC25 cells reduced tumorigenesis while enhancing immune activity in mice. Further experiments showed that CBX6 reduced CD8+ T cell cytotoxicity by secreting C-C motif chemokine ligand 8 (CCL8) and promoting monocarboxylate transporter 4 (MCT4)-mediated lactate transport. Regarding the mechanism, CBX6 regulated the expression of SWI/SNF related BAF chromatin remodeling complex subunit D1 (Smarcd1) to modulate chromatin remodeling, thus promoting transcription of Ccl8 and Slc16a3 (encoding MCT4). Smarcd1 overexpression restored metabolic activity in mEC25 cells, reduced activity of co-cultured CD8+ T cells, and promoted tumorigenesis in vivo. Tissue microarrays analysis suggested that CBX6 and SMARCD1 were linked to immunosuppression and poor prognosis in clinical samples. In conclusion, this study suggests that CBX6 induces CD8+ T cell exhaustion and tumor development in ESCC through SMARCD1-mediated CCL8 secretion and lactate efflux.
-
-
-
Cancer Research
-
Immunology and Microbiology
Wild-type KRAS activation drives evasion of interferon-mediated immunity and resistance to immunotherapy in hepatocellular carcinoma.
In Nat Commun on 11 November 2025 by Lei, M. M. L., Leung, C. O. N., et al.
PubMed
Increasing evidence indicates that activation of oncogenic pathways contributes to an unfavourable tumour immune microenvironment (TIME), ultimately resulting in resistance to immunotherapy. Here, we aim to identify a critical oncogenic pathway involved in an antigen-expressing c-MYC-lucOSOE/Tp53KO hepatocellular carcinoma (HCC) mouse model that simulates immune response against tumour-associated antigens. Using data-independent acquisition proteomics, we reveal the role of wild-type KRAS in immune escaped mouse HCC tumours, with EGF concurrently activating EGFR/MEK/ERK signalling. Single cell RNA sequencing data analysis reveals that KRAS signalling intrinsically inhibits interferon-mediated MHC-I expression and extrinsically impairs CD8+ T cell activity due to the suppression of CXCL9 through the EGFR/MEK/ERK pathway. We observe KRAS activation in HCC patients who received immune checkpoint inhibitor (ICI) treatments, where it correlates with poor clinical outcomes. Notably, combination therapy with SOS1 inhibitor MRTX0902, Trametinib, and anti-PD-1 antibody effectively increased intratumoural CD8+ T cell infiltration and improved survival. Our study thus reveals that targeting wild-type KRAS signalling in combination with ICIs may serve as an effective treatment strategy for advanced HCC patients.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Cancer Research
-
Biochemistry and Molecular biology
Tumoral ALOX5 mediated arachidonic acid metabolism regulates immune response in non-small cell lung cancer.
In Cell Oncol (Dordr) on 5 November 2025 by Gao, Y., Xia, Y., et al.
PubMed
Tumor cells reprogram their fatty acid metabolism to meet the demands for their rapid proliferation. However, the interplay between fatty acid metabolism and the tumor microenvironment (TME) in lung cancer remains poorly defined. This study aims to elucidate how arachidonic acid (AA) metabolism, specifically via the enzyme 5-lipoxygenase (ALOX5), modulates anti-tumor immunity in non-small cell lung cancer (NSCLC).
-
-
-
Cancer Research
Cryoablation plus sintilimab and lenvatinib in advanced or metastatic intrahepatic cholangiocarcinoma: a phase 2 trial.
In Nat Cancer on 1 November 2025 by Gu, S., Luo, Q., et al.
PubMed
Treatment options for advanced or metastatic intrahepatic cholangiocarcinoma (ICC) are limited. In this single-arm, phase 2 trial (CASTLE-01, NCT05010668 ), 28 participants with advanced or metastatic ICC who have progressed after chemotherapy were treated with cryoablation, followed by anti-PD1 sintilimab (200 mg every 3 weeks) plus lenvatinib (8-12 mg per day) 2 weeks later. The objective response rate assessed by Response Evaluation Criteria in Solid Tumors version 1.1 was 75.0% (95% confidence interval (CI): 59-91%), meeting the prespecified primary endpoint. Secondary endpoints of disease control rate, median progression-free survival and overall survival were respectively 100% (95% CI: 100-100%), 16.8 months (95% CI: 11.5-not reached (NR)) and 25.4 months (95% CI: 13.3-NR). Treatment was well tolerated. Post hoc multiomics analysis of paired pretreatment and on-treatment tumor biopsies suggested that cryoablation increased the tumor immunogenicity and dendritic cell activation, followed by triggering continuous replenishment of intratumoral CD8+PD1hi effectors from peripheral blood. The addition of lenvatinib transitioned endothelial cells into inflamed venules to boost lymphocyte influx and targeted tumor stroma to promote CD8+PD1hi effectors penetrating into tumor cell nests. Therefore, cryoablation combined with sintilimab plus lenvatinib represents a promising approach for the treatment of advanced or metastatic ICC. These findings also support the notion that cryoablation may trigger abscopal antitumor immunity in ICC when combined with lenvatinib and PD1 blockade. ClinicalTrials.gov registration: NCT05010668 .
-
-
-
Immunology and Microbiology
-
Cancer Research
-
COVID-19
-
Genetics
SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade.
In Nature on 1 November 2025 by Grippin, A., Marconi, C., et al.
PubMed
Immune checkpoint inhibitors (ICIs) extend survival in many patients with cancer but are ineffective in patients without pre-existing immunity1-9. Although personalized mRNA cancer vaccines sensitize tumours to ICIs by directing immune attacks against preselected antigens, personalized vaccines are limited by complex and time-intensive manufacturing processes10-14. Here we show that mRNA vaccines targeting SARS-CoV-2 also sensitize tumours to ICIs. In preclinical models, SARS-CoV-2 mRNA vaccines led to a substantial increase in type I interferon, enabling innate immune cells to prime CD8+ T cells that target tumour-associated antigens. Concomitant ICI treatment is required for maximal efficacy in immunologically cold tumours, which respond by increasing PD-L1 expression. Similar correlates of vaccination response are found in humans, including increases in type I interferon, myeloid-lymphoid activation in healthy volunteers and PD-L1 expression on tumours. Moreover, receipt of SARS-CoV-2 mRNA vaccines within 100 days of initiating ICI is associated with significantly improved median and three-year overall survival in multiple large retrospective cohorts. This benefit is similar among patients with immunologically cold tumours. Together, these results demonstrate that clinically available mRNA vaccines targeting non-tumour-related antigens are potent immune modulators capable of sensitizing tumours to ICIs.
-
-
-
Immunology and Microbiology
Novel anti-LAG-3 antibody LBL-007 with anti-PD-1 blockade enhances antitumor immunity by promoting T cell-induced apoptosis.
In Sci Rep on 27 October 2025 by Qin, K., Zhou, H., et al.
PubMed
Immune checkpoint combination therapy, particularly dual LAG-3/PD-1 blockade, demonstrates superior clinical efficacy over monotherapy in cancers like melanoma, yet its mechanistic synergy requires further validation. In this study, we established a cell co-culture model by co-culturing LAG-3+PD-1+ Jurkat cells induced by phytohemagglutinin (PHA) and human tumor cells with high expression of LAG-3 and PD-1 major ligands to characterize the combination effect of LBL-007 with anti-PD-1 antibodies and the mechanism of action in cancer immunotherapy. The results showed that the combination of LBL-007 and anti-PD-1 antibodies in the cell co-culture model enhanced the ability of activated Jurkat cells to kill tumor cells compared with monotherapy. Furthermore, this combination also inhibited the apoptosis of Jurkat cells and promoted IL-2, IL-10, and TNF secretion from Jurkat cells. Tumor cell death via apoptosis induced by activated Jurkat cells was observed, which was enhanced by combined LBL-007 and anti-PD-1 antibody treatment. The combination of LBL-007 and anti-PD-1 antibodies delayed tumor growth and promoted tumor cell apoptosis compared with monotherapy in human LAG-3 transgenic mice subjected to transplantation with colorectal tumor cells. Taken together, the combination of LBL-007 and anti-PD-1 antibodies plays an enhanced antitumor role by improving T cell viability and activity as well as by promoting T cell-induced apoptosis, thereby suggesting this combination as a potential effective strategy for cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
VEGFR2 blockade converts thermally ablative focused ultrasound into a potent driver of T cell-dependent anti-tumor immunity
In bioRxiv on 24 October 2025 by Schwartz, M. R., Anwar, N. Z., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
OCA-B/Pou2af1 Expression in Mouse T Cells Promotes PD-1 Blockade-Induced Autoimmunity but is Dispensable for Anti-Tumor Immunity
In bioRxiv on 23 October 2025 by Du, J., Manna, A. K., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Genetics
Epigenetic suppression of Nrf2-Slc40a1 axis induces ferroptosis and enhances immunotherapy in pancreatic cancer.
In J Immunother Cancer on 23 October 2025 by Zhang, Y., Yu, R., et al.
PubMed
Despite progress in immunotherapy for several solid tumors, pancreatic ductal adenocarcinoma (PDAC) remains largely unresponsive, primarily due to its profoundly immunosuppressive tumor microenvironment (TME) characterized by limited CD8+ T cell infiltration. Novel strategies are needed to overcome this immune resistance and enhance the efficacy of checkpoint blockade.
-
-
-
Immunology and Microbiology
Improving immunotherapy responses by dual inhibition of macrophage migration inhibitory factor and PD-1.
In JCI Insight on 22 October 2025 by Tran, T. T., Sánchez-Zuno, G. A., et al.
PubMed
Macrophage migration inhibitory factor (MIF) is an upstream regulatory cytokine that is associated with advanced disease and poor outcomes in multiple cancer types, including melanoma. We investigated whether anti-MIF therapy could enhance the antitumor effects of the immune checkpoint inhibitor anti-programmed cell death 1 (anti-PD-1) in 2 murine tumor models. The therapeutic efficacy of anti-MIF, alone or combined with anti-PD-1, was tested in the YUMMER1.7 melanoma and MC38 colorectal cancer models. Tumor growth and survival were assessed in untreated Mif-knockout (KO) and low-expression human MIF allele (CATT5) mice and compared with wild-type (WT) or high-expression MIF allele (CATT7) mice. Tumor-bearing animals underwent cytokine profiling, tumor immunohistochemistry, flow cytometry, and scRNA-Seq. We also correlated functional variant MIF alleles with melanoma incidence and progression in patients. Our results showed that combined anti-MIF and anti-PD-1 significantly reduced tumor growth, improved survival, and promoted tumor regression, accompanied by enhanced TH1 cytokine levels, increased macrophage activation-related cytokines, and increased type 1 conventional dendritic cells. scRNA-Seq analysis revealed an expansion of intratumor Cd74/C1q/Aif1-expressing macrophages, which exhibited an antitumor phenotype, in response to anti-MIF therapy. MIF-KO and CATT5 mice exhibited reduced tumor burdens compared with WT or CATT7 mice alone and in the presence of anti-PD-1. In patients with melanoma, the high-MIF expression genotype (-173C/C) occurred at higher frequencies compared with healthy controls. These findings highlight that the addition of anti-MIF to anti-PD-1 reduces tumor growth, enhances antitumor responses, prolongs survival, and augments key intratumor immune cell populations involved in immune activation against tumors. This approach merits further consideration for clinical trial development.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor-intrinsic MHC-II activation in pancreatic ductal adenocarcinoma enhances immune response and treatment efficacy
In bioRxiv on 22 October 2025 by Chen, C., Gribbin, K. P., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
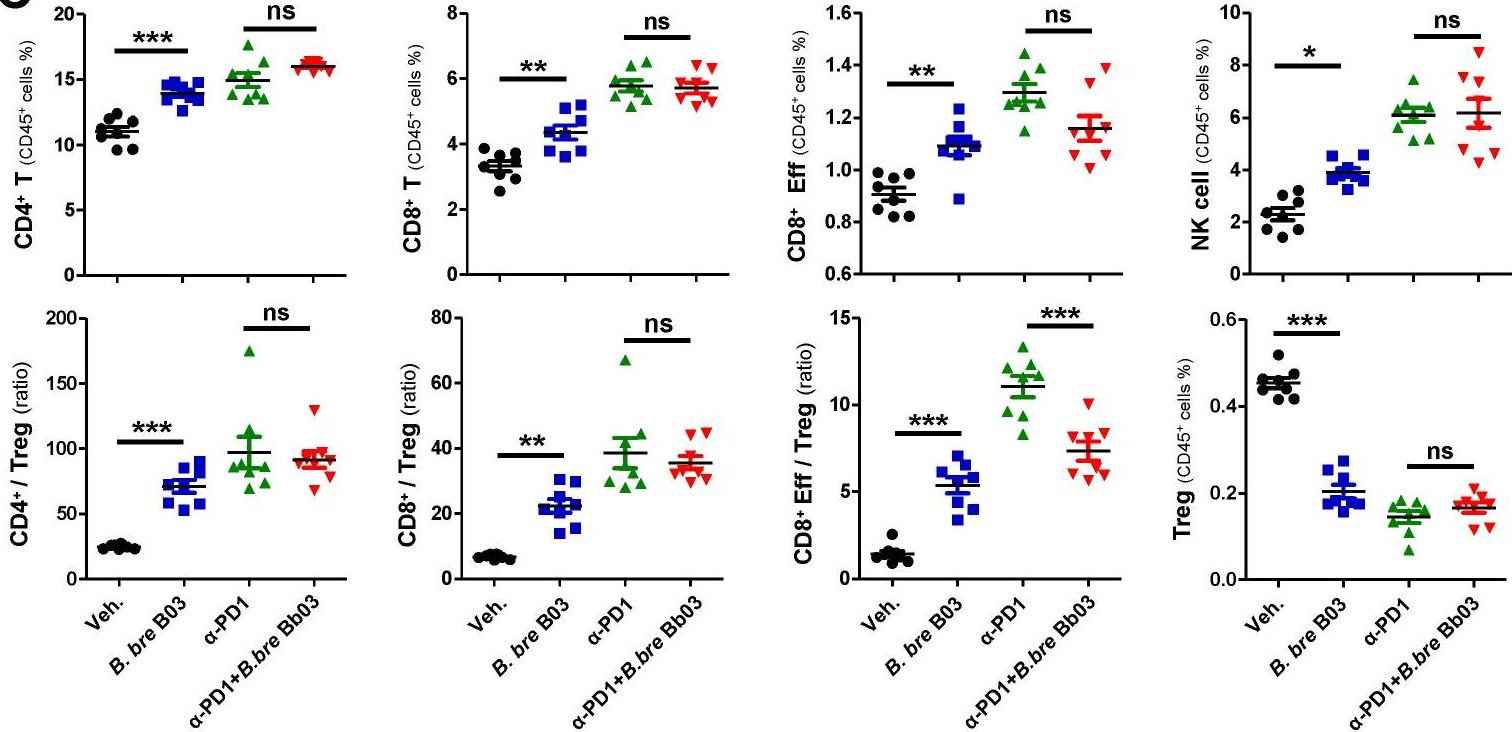
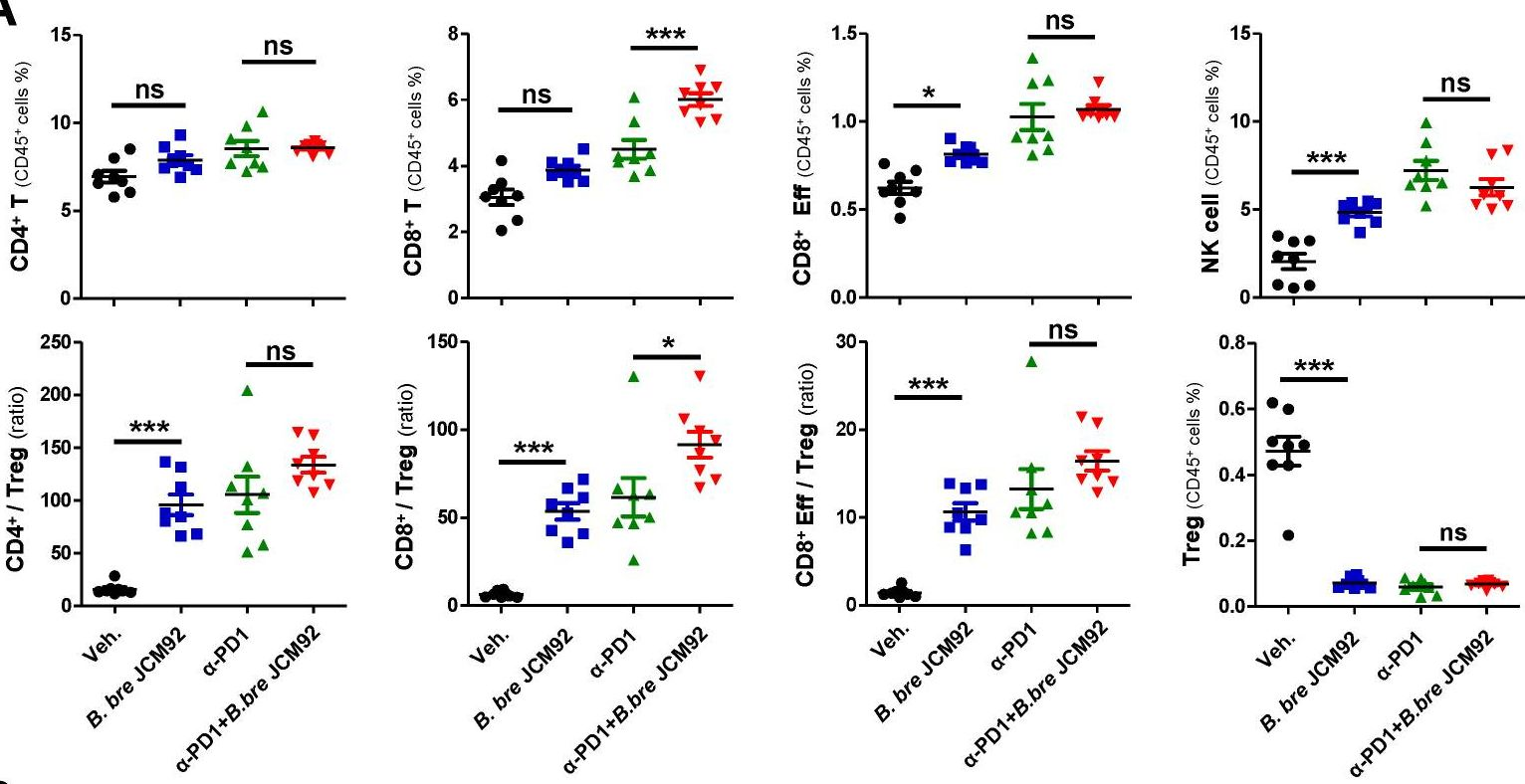
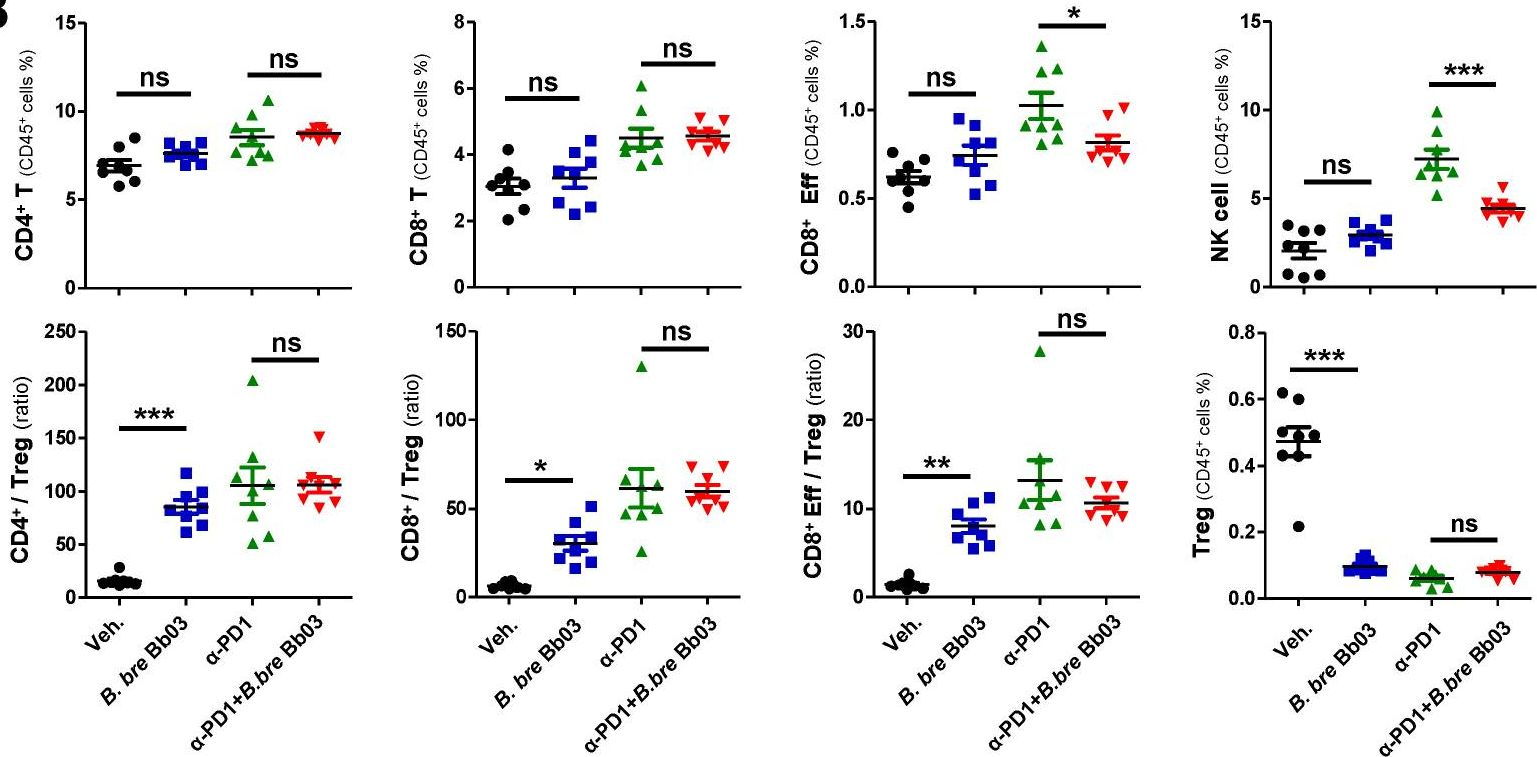
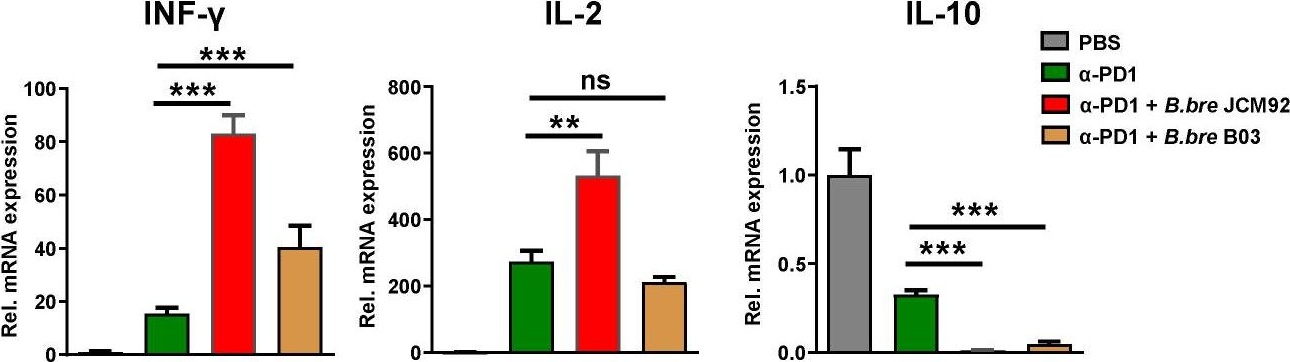
Enhancing melanoma treatment through systemic delivery of an immune boosting Staphylococcus epidermidis strain.
In Sci Rep on 21 October 2025 by Hwang, J., Kim, G., et al.
PubMed
A unique strain of Staphylococcus epidermidis, AIT01 (AIT, Airway Immune Trainer), identified in our previous research, has demonstrated immune-boosting properties. This study aimed to evaluate the systemic immune-modulatory effects and potential anti-tumor properties of this immune-enhancing skin microbiota strain. A series of ex vivo and in vivo experiments were conducted to assess immune cell proliferation, cytokine production, and anti-tumor efficacy. In ex vivo studies, splenocytes treated with the bacterial lysate or culture supernatant of the strain showed significantly increased viability in a concentration-dependent manner. Flow cytometry analysis revealed increased populations of dendritic cells, NK cells (Natural killer cells), and γδ T cells, with enhanced cytokine production, particularly IFN-γ (Interferon-γ) and perforin, in the lysate-treated group. When administered via intraperitoneal and intravenous routes in vivo, mice showed significant inhibition of melanoma growth upon receiving the bacterial lysate. Notably, pre-treatment demonstrated superior efficacy compared to post-treatment. Furthermore, the combination of the bacterial lysate with anti-PD-1 (anti-Programmed cell death protein-1) monoclonal antibody further suppressed tumor growth compared to anti-PD-1 monotherapy. These findings suggest that the AIT01 lysate enhances immune cell proliferation and cytokine production, contributing to its potent anti-tumor effects. The systemic delivery of this immune-boosting skin microbiota strain, particularly in combination with anti-PD-1 therapy, holds promise as an effective immunotherapeutic strategy against melanoma.
-
-
-
Cancer Research
-
Immunology and Microbiology
ACOX2 destabilizes the MRE11-RAD50-NBS1 complex and boosts anticancer immunity via the cGAS-STING pathway in clear cell renal cell carcinoma.
In Mol Cancer on 21 October 2025 by Ye, S., Xu, W., et al.
PubMed
The rapid development of ICI-based immunotherapy has ushered in a promising era for clear cell renal cell carcinoma (ccRCC). However, durable clinical responses remain limited to a subset of patients. Therefore, identifying novel predictive biomarkers and developing effective combination immunotherapies are critical for advancing personalized ccRCC management. In this study, we report that ccRCC patients exhibiting elevated ACOX2 expression may benefit from PARPi in combination with ICI. Multi-omics cohorts show ACOX2 is significantly downregulated in ccRCC and correlated with improved clinical prognosis. ACOX2 inhibits the growth of ccRCC both in vitro and in vivo. Mechanistically, ACOX2 interacts with MRE11 and inhibits the binding of MRE11 and RAD50, thereby destabilizing the MRE11-RAD50-NBS1 (MRN) complex. Furthermore, ACOX2 activates the cGAS-STING pathway, correlates with more mature tertiary lymphoid structures (TLS), and enhances CD8+ T cell infiltration and activity. Therapeutically, preclinical ccRCC models with high ACOX2 expression, including ccRCC cells, cell-derived xenograft (CDX), patient-derived organoid (PDO), patient-derived xenograft (PDX), and immunocompetent mouse models show increased sensitivity to PARPi plus anti-PD-1 therapy. In conclusion, our findings elucidate a pivotal role of ACOX2 in inhibiting HRR and propose that PARPi, either alone or in combination with anti-PD-1 therapy, represents a promising treatment strategy for ccRCC with elevated ACOX2 expression.
-
-
-
Cancer Research
-
Immunology and Microbiology
αTIGIT-IL2 achieves tumor regression by promoting tumor-infiltrating regulatory T cell fragility in mouse models.
In Nat Commun on 17 October 2025 by Wang, T., Xu, Y., et al.
PubMed
Administration of IL-2 may promote the suppressive function and proliferation of Treg cells that cause immune tolerance in patients with cancer, which causes low-dose IL-2 to fail in achieving an optimal anti-tumor effect. Here, we designed an immunocytokine by fusing IL-2 and an anti-TIGIT monoclonal antibody, named αTIGIT-IL2, that targets Treg cells and promotes their fragility in the tumor milieu. These fragile-like Treg cells show impaired suppressive function and high IFN-γ production, triggering an immune-reactive tumor microenvironment. Such inflammation leads to the recruitment and functional reprogramming of intratumoral neutrophils, improving cross-talk between neutrophils and CD8+ T cells and enhancing the antitumor ability of CD8+ T cells. Combination therapy with αTIGIT-IL2 and PD-1 blocker could eliminate triple-negative breast cancer (TNBC) tumors resistant to immune checkpoint blockade (ICB) therapy. These findings provide the basis for developing a new generation of immunocytokines that target Treg cells and promote their fragility in the tumor milieu, resulting in robust antitumor immunity.
-
-
-
Immunology and Microbiology
-
Cancer Research
Macrophage repolarization by immune checkpoint blockade drives T cell engagement in the tumor microenvironment.
In iScience on 17 October 2025 by Kwok, T., Silva-Junior, I. A., et al.
PubMed
Immunotherapy combinations can improve patient outcomes, yet the interactions within the tumor microenvironment (TME) that drive therapeutic synergy are poorly understood. Tumor establishment drives monocyte recruitment and differentiation into tumor-associated macrophages (TAMs), which have essential roles in coordinating immune responses and are thus attractive targets for therapeutic modulation. In a murine model of combination anti-programmed cell death protein 1 (PD-1) and its ligand (anti-PD-L1) checkpoint blockade, tumor control was associated with increased infiltration of CD8+ T cells and M1-like repolarization of TAMs. Live-cell imaging of the tumor microenvironment revealed close contacts between tumor-infiltrating CD8+ T cells and TAMs, in which the extent of the contact interfaces increased with combination immunotherapy. Treatment with anti-PD-L1 was able to increase macrophage expression of pro-inflammatory factors and phagocytic activity, suggesting a role for TAMs in reactivating CD8+ T cells in the TME. However, co-treatment with anti-PD-1 was ultimately necessary for tumor control, indicating the need for combination targeting of the TME.
-