InVivoSIM anti-human PD-1 (Tislelizumab Biosimilar)
Product Description
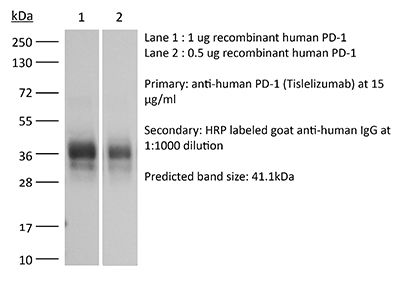
Specifications
| Isotype | Human IgG4, κ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG4 (S228P/R409K) isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Mutations | S228P/E233P/F234V/L235A/D265A/R409K |
| Immunogen | Human PD-1 |
| Reported Applications |
Blocking of PD-1/PD-L signaling Functional assays ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Interferon-α and thymosin-α1 plus tislelizumab enhance CD8+ T cell cytotoxicity toward pancreatic ductal adenocarcinoma.
In iScience on 15 August 2025 by Deng, S., Deng, R., et al.
PubMed
The strong immunosuppression and immune evasion of pancreatic ductal adenocarcinoma (PDAC) result in poor efficacy of immune checkpoint blockade. In this study, the PD-1 level on CD8+ T cells in the peripheral blood of patients with PDAC was significantly greater than that in the peripheral blood of healthy individuals. To enhance the anticancer activity of adoptive CD8+ T cells toward PDAC, interferon-α (IFN-α) and thymosin-α1 (Tα1) plus tislelizumab were preclinically explored. Compared with those of tislelizumab monotherapy, the proliferation and cytokine secretion of CD8+ T cells and the cytotoxic activity toward PDAC cells were significantly greater with the combination treatment of IFN-α and Tα1 plus tislelizumab. Moreover, the growth of PDAC tumors was inhibited by CD8+ T cells with high efficacy under the combination treatment. Thus, IFN-α and Tα1 plus tislelizumab enhance the anticancer activity of CD8+ T cells toward PDAC, representing an alternative strategy for improving cancer immunotherapy.
-