Catalog #SIM0010
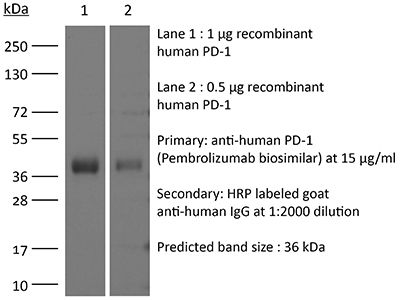
InVivoSIM anti-human PD-1 (Pembrolizumab Biosimilar)
Clone
Pembrolizumab
Reactivities
Human
Product Citations
11
Isotype
Human IgG4