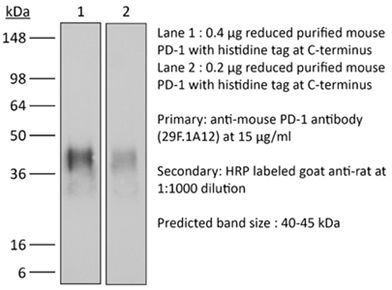
InVivoPlus anti-mouse PD-1 (CD279)
Product Description
Specifications
| Isotype | Rat IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant PD-1-Ig fusion protein |
| Reported Applications |
in vivo blocking of PD-1/PD-L signaling in vitro PD-1 neutralization Immunohistochemistry (frozen) Immunofluorescence Western blot Flow cytometry in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687796 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro Organoids/Organ-on-Chip
Lidström T, Cumming J, Gaur R, Frängsmyr L, Pateras IS, Mickert MJ, Franklin O, Forsell MNE, Arnberg N, Dongre M, Patthey C, Öhlund D (2023). "Extracellular Galectin 4 Drives Immune Evasion and Promotes T-cell Apoptosis in Pancreatic Cancer" Cancer I
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is characterized by rich deposits of extracellular matrix (ECM), affecting the pathophysiology of the disease. Here, we identified galectin 4 (gal 4) as a cancer cell-produced protein that was deposited into the ECM of PDAC tumors and detected high-circulating levels of gal 4 in patients with PDAC. In orthotopic transplantation experiments, we observed increased infiltration of T cells and prolonged survival in immunocompetent mice transplanted with cancer cells with reduced expression of gal 4. Increased survival was not observed in immunodeficient RAG1-/- mice, demonstrating that the effect was mediated by the adaptive immune system. By performing single-cell RNA-sequencing, we found that the myeloid compartment and cancer-associated fibroblast (CAF) subtypes were altered in the transplanted tumors. Reduced gal 4 expression associated with a higher proportion of myofibroblastic CAFs and reduced numbers of inflammatory CAFs. We also found higher proportions of M1 macrophages, T cells, and antigen-presenting dendritic cells in tumors with reduced gal 4 expression. Using a coculture system, we observed that extracellular gal 4 induced apoptosis in T cells by binding N-glycosylation residues on CD3ε/δ. Hence, we show that gal 4 is involved in immune evasion and identify gal 4 as a promising drug target for overcoming immunosuppression in PDAC.
in vitro Organoids/Organ-on-Chip
Lidström T, Cumming J, Gaur R, Frängsmyr L, Pateras IS, Mickert MJ, Franklin O, Forsell MNE, Arnberg N, Dongre M, Patthey C, Öhlund D (2023). "Extracellular Galectin 4 Drives Immune Evasion and Promotes T-cell Apoptosis in Pancreatic Cancer" Cancer I
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is characterized by rich deposits of extracellular matrix (ECM), affecting the pathophysiology of the disease. Here, we identified galectin 4 (gal 4) as a cancer cell-produced protein that was deposited into the ECM of PDAC tumors and detected high-circulating levels of gal 4 in patients with PDAC. In orthotopic transplantation experiments, we observed increased infiltration of T cells and prolonged survival in immunocompetent mice transplanted with cancer cells with reduced expression of gal 4. Increased survival was not observed in immunodeficient RAG1-/- mice, demonstrating that the effect was mediated by the adaptive immune system. By performing single-cell RNA-sequencing, we found that the myeloid compartment and cancer-associated fibroblast (CAF) subtypes were altered in the transplanted tumors. Reduced gal 4 expression associated with a higher proportion of myofibroblastic CAFs and reduced numbers of inflammatory CAFs. We also found higher proportions of M1 macrophages, T cells, and antigen-presenting dendritic cells in tumors with reduced gal 4 expression. Using a coculture system, we observed that extracellular gal 4 induced apoptosis in T cells by binding N-glycosylation residues on CD3ε/δ. Hence, we show that gal 4 is involved in immune evasion and identify gal 4 as a promising drug target for overcoming immunosuppression in PDAC.
in vivo blocking of PD-1/PD-L signaling
Wang, W., et al (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo blocking of PD-1/PD-L signaling
Wang, W., et al (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo blocking of PD-1/PD-L signaling
Wang, W., et al (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo blocking of PD-1/PD-L signaling
Gordon, S. R., et al (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499.
PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vivo blocking of PD-1/PD-L signaling
Gordon, S. R., et al (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499.
PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vivo blocking of PD-1/PD-L signaling
Gordon, S. R., et al (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499.
PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vivo blocking of PD-1/PD-L signaling
Koyama, S., et al (2016). "STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment" Cancer Res 76(5): 999-1008.
PubMed
STK11/LKB1 is among the most commonly inactivated tumor suppressors in non-small cell lung cancer (NSCLC), especially in tumors harboring KRAS mutations. Many oncogenes promote immune escape, undermining the effectiveness of immunotherapies, but it is unclear whether the inactivation of tumor suppressor genes, such as STK11/LKB1, exerts similar effects. In this study, we investigated the consequences of STK11/LKB1 loss on the immune microenvironment in a mouse model of KRAS-driven NSCLC. Genetic ablation of STK11/LKB1 resulted in accumulation of neutrophils with T-cell-suppressive effects, along with a corresponding increase in the expression of T-cell exhaustion markers and tumor-promoting cytokines. The number of tumor-infiltrating lymphocytes was also reduced in LKB1-deficient mouse and human tumors. Furthermore, STK11/LKB1-inactivating mutations were associated with reduced expression of PD-1 ligand PD-L1 in mouse and patient tumors as well as in tumor-derived cell lines. Consistent with these results, PD-1-targeting antibodies were ineffective against Lkb1-deficient tumors. In contrast, treating Lkb1-deficient mice with an IL6-neutralizing antibody or a neutrophil-depleting antibody yielded therapeutic benefits associated with reduced neutrophil accumulation and proinflammatory cytokine expression. Our findings illustrate how tumor suppressor mutations can modulate the immune milieu of the tumor microenvironment, and they offer specific implications for addressing STK11/LKB1-mutated tumors with PD-1-targeting antibody therapies.
in vivo blocking of PD-1/PD-L signaling
Flow Cytometry
Koyama, S., et al (2016). "Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints" Nat Commun 7: 10501.
PubMed
Despite compelling antitumour activity of antibodies targeting the programmed death 1 (PD-1): programmed death ligand 1 (PD-L1) immune checkpoint in lung cancer, resistance to these therapies has increasingly been observed. In this study, to elucidate mechanisms of adaptive resistance, we analyse the tumour immune microenvironment in the context of anti-PD-1 therapy in two fully immunocompetent mouse models of lung adenocarcinoma. In tumours progressing following response to anti-PD-1 therapy, we observe upregulation of alternative immune checkpoints, notably T-cell immunoglobulin mucin-3 (TIM-3), in PD-1 antibody bound T cells and demonstrate a survival advantage with addition of a TIM-3 blocking antibody following failure of PD-1 blockade. Two patients who developed adaptive resistance to anti-PD-1 treatment also show a similar TIM-3 upregulation in blocking antibody-bound T cells at treatment failure. These data suggest that upregulation of TIM-3 and other immune checkpoints may be targetable biomarkers associated with adaptive resistance to PD-1 blockade.
in vivo blocking of PD-1/PD-L signaling
Koyama, S., et al (2016). "STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment" Cancer Res 76(5): 999-1008.
PubMed
STK11/LKB1 is among the most commonly inactivated tumor suppressors in non-small cell lung cancer (NSCLC), especially in tumors harboring KRAS mutations. Many oncogenes promote immune escape, undermining the effectiveness of immunotherapies, but it is unclear whether the inactivation of tumor suppressor genes, such as STK11/LKB1, exerts similar effects. In this study, we investigated the consequences of STK11/LKB1 loss on the immune microenvironment in a mouse model of KRAS-driven NSCLC. Genetic ablation of STK11/LKB1 resulted in accumulation of neutrophils with T-cell-suppressive effects, along with a corresponding increase in the expression of T-cell exhaustion markers and tumor-promoting cytokines. The number of tumor-infiltrating lymphocytes was also reduced in LKB1-deficient mouse and human tumors. Furthermore, STK11/LKB1-inactivating mutations were associated with reduced expression of PD-1 ligand PD-L1 in mouse and patient tumors as well as in tumor-derived cell lines. Consistent with these results, PD-1-targeting antibodies were ineffective against Lkb1-deficient tumors. In contrast, treating Lkb1-deficient mice with an IL6-neutralizing antibody or a neutrophil-depleting antibody yielded therapeutic benefits associated with reduced neutrophil accumulation and proinflammatory cytokine expression. Our findings illustrate how tumor suppressor mutations can modulate the immune milieu of the tumor microenvironment, and they offer specific implications for addressing STK11/LKB1-mutated tumors with PD-1-targeting antibody therapies.
in vivo blocking of PD-1/PD-L signaling
Flow Cytometry
Koyama, S., et al (2016). "Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints" Nat Commun 7: 10501.
PubMed
Despite compelling antitumour activity of antibodies targeting the programmed death 1 (PD-1): programmed death ligand 1 (PD-L1) immune checkpoint in lung cancer, resistance to these therapies has increasingly been observed. In this study, to elucidate mechanisms of adaptive resistance, we analyse the tumour immune microenvironment in the context of anti-PD-1 therapy in two fully immunocompetent mouse models of lung adenocarcinoma. In tumours progressing following response to anti-PD-1 therapy, we observe upregulation of alternative immune checkpoints, notably T-cell immunoglobulin mucin-3 (TIM-3), in PD-1 antibody bound T cells and demonstrate a survival advantage with addition of a TIM-3 blocking antibody following failure of PD-1 blockade. Two patients who developed adaptive resistance to anti-PD-1 treatment also show a similar TIM-3 upregulation in blocking antibody-bound T cells at treatment failure. These data suggest that upregulation of TIM-3 and other immune checkpoints may be targetable biomarkers associated with adaptive resistance to PD-1 blockade.
in vivo blocking of PD-1/PD-L signaling
Flow Cytometry
Koyama, S., et al (2016). "Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints" Nat Commun 7: 10501.
PubMed
Despite compelling antitumour activity of antibodies targeting the programmed death 1 (PD-1): programmed death ligand 1 (PD-L1) immune checkpoint in lung cancer, resistance to these therapies has increasingly been observed. In this study, to elucidate mechanisms of adaptive resistance, we analyse the tumour immune microenvironment in the context of anti-PD-1 therapy in two fully immunocompetent mouse models of lung adenocarcinoma. In tumours progressing following response to anti-PD-1 therapy, we observe upregulation of alternative immune checkpoints, notably T-cell immunoglobulin mucin-3 (TIM-3), in PD-1 antibody bound T cells and demonstrate a survival advantage with addition of a TIM-3 blocking antibody following failure of PD-1 blockade. Two patients who developed adaptive resistance to anti-PD-1 treatment also show a similar TIM-3 upregulation in blocking antibody-bound T cells at treatment failure. These data suggest that upregulation of TIM-3 and other immune checkpoints may be targetable biomarkers associated with adaptive resistance to PD-1 blockade.
in vivo blocking of PD-1/PD-L signaling
Koyama, S., et al (2016). "STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment" Cancer Res 76(5): 999-1008.
PubMed
STK11/LKB1 is among the most commonly inactivated tumor suppressors in non-small cell lung cancer (NSCLC), especially in tumors harboring KRAS mutations. Many oncogenes promote immune escape, undermining the effectiveness of immunotherapies, but it is unclear whether the inactivation of tumor suppressor genes, such as STK11/LKB1, exerts similar effects. In this study, we investigated the consequences of STK11/LKB1 loss on the immune microenvironment in a mouse model of KRAS-driven NSCLC. Genetic ablation of STK11/LKB1 resulted in accumulation of neutrophils with T-cell-suppressive effects, along with a corresponding increase in the expression of T-cell exhaustion markers and tumor-promoting cytokines. The number of tumor-infiltrating lymphocytes was also reduced in LKB1-deficient mouse and human tumors. Furthermore, STK11/LKB1-inactivating mutations were associated with reduced expression of PD-1 ligand PD-L1 in mouse and patient tumors as well as in tumor-derived cell lines. Consistent with these results, PD-1-targeting antibodies were ineffective against Lkb1-deficient tumors. In contrast, treating Lkb1-deficient mice with an IL6-neutralizing antibody or a neutrophil-depleting antibody yielded therapeutic benefits associated with reduced neutrophil accumulation and proinflammatory cytokine expression. Our findings illustrate how tumor suppressor mutations can modulate the immune milieu of the tumor microenvironment, and they offer specific implications for addressing STK11/LKB1-mutated tumors with PD-1-targeting antibody therapies.
in vitro PD-1 neutralization
Park, S. J., et al (2014). "Negative role of inducible PD-1 on survival of activated dendritic cells" J Leukoc Biol 95(4): 621-629.
PubMed
PD-1 is a well-established negative regulator of T cell responses by inhibiting proliferation and cytokine production of T cells via interaction with its ligands, B7-H1 (PD-L1) and B7-DC (PD-L2), expressed on non-T cells. Recently, PD-1 was found to be expressed in innate cells, including activated DCs, and plays roles in suppressing production of inflammatory cytokines. In this study, we demonstrate that PD-1 KO DCs exhibited prolonged longevity compared with WT DCs in the dLNs after transfer of DCs into hind footpads. Interestingly, upon LPS stimulation, WT DCs increased the expression of PD-1 and started to undergo apoptosis. DCs, in spleen of LPS-injected PD-1 KO mice, were more resistant to LPS-mediated apoptosis in vivo than WT controls. Moreover, treatment of blocking anti-PD-1 mAb during DC maturation resulted in enhanced DC survival, suggesting that PD-1:PD-L interactions are involved in DC apoptosis. As a result, PD-1-deficient DCs augmented T cell responses in terms of antigen-specific IFN-gamma production and proliferation of CD4 and CD8 T cells to a greater degree than WT DCs. Moreover, PD-1 KO DCs exhibited increased MAPK1 and CD40-CD40L signaling, suggesting a possible mechanism for enhanced DC survival in the absence of PD-1 expression. Taken together, our findings further extend the function of PD-1, which plays an important role in apoptosis of activated DCs and provides important implications for PD-1-mediated immune regulation.
in vitro PD-1 neutralization
Park, S. J., et al (2014). "Negative role of inducible PD-1 on survival of activated dendritic cells" J Leukoc Biol 95(4): 621-629.
PubMed
PD-1 is a well-established negative regulator of T cell responses by inhibiting proliferation and cytokine production of T cells via interaction with its ligands, B7-H1 (PD-L1) and B7-DC (PD-L2), expressed on non-T cells. Recently, PD-1 was found to be expressed in innate cells, including activated DCs, and plays roles in suppressing production of inflammatory cytokines. In this study, we demonstrate that PD-1 KO DCs exhibited prolonged longevity compared with WT DCs in the dLNs after transfer of DCs into hind footpads. Interestingly, upon LPS stimulation, WT DCs increased the expression of PD-1 and started to undergo apoptosis. DCs, in spleen of LPS-injected PD-1 KO mice, were more resistant to LPS-mediated apoptosis in vivo than WT controls. Moreover, treatment of blocking anti-PD-1 mAb during DC maturation resulted in enhanced DC survival, suggesting that PD-1:PD-L interactions are involved in DC apoptosis. As a result, PD-1-deficient DCs augmented T cell responses in terms of antigen-specific IFN-gamma production and proliferation of CD4 and CD8 T cells to a greater degree than WT DCs. Moreover, PD-1 KO DCs exhibited increased MAPK1 and CD40-CD40L signaling, suggesting a possible mechanism for enhanced DC survival in the absence of PD-1 expression. Taken together, our findings further extend the function of PD-1, which plays an important role in apoptosis of activated DCs and provides important implications for PD-1-mediated immune regulation.
in vivo blocking of PD-1/PD-L signaling
Cooper, Z. A., et al (2014). "Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade" Cancer Immunol Res 2(7): 643-654.
PubMed
BRAF-targeted therapy results in objective responses in the majority of patients; however, the responses are short lived ( approximately 6 months). In contrast, treatment with immune checkpoint inhibitors results in a lower response rate, but the responses tend to be more durable. BRAF inhibition results in a more favorable tumor microenvironment in patients, with an increase in CD8(+) T-cell infiltrate and a decrease in immunosuppressive cytokines. There is also increased expression of the immunomodulatory molecule PDL1, which may contribute to the resistance. On the basis of these findings, we hypothesized that BRAF-targeted therapy may synergize with the PD1 pathway blockade to enhance antitumor immunity. To test this hypothesis, we developed a BRAF(V600E)/Pten(-/-) syngeneic tumor graft immunocompetent mouse model in which BRAF inhibition leads to a significant increase in the intratumoral CD8(+) T-cell density and cytokine production, similar to the effects of BRAF inhibition in patients. In this model, CD8(+) T cells were found to play a critical role in the therapeutic effect of BRAF inhibition. Administration of anti-PD1 or anti-PDL1 together with a BRAF inhibitor led to an enhanced response, significantly prolonging survival and slowing tumor growth, as well as significantly increasing the number and activity of tumor-infiltrating lymphocytes. These results demonstrate synergy between combined BRAF-targeted therapy and immune checkpoint blockade. Although clinical trials combining these two strategies are ongoing, important questions still remain unanswered. Further studies using this new melanoma mouse model may provide therapeutic insights, including optimal timing and sequence of therapy.
in vivo blocking of PD-1/PD-L signaling
Cooper, Z. A., et al (2014). "Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade" Cancer Immunol Res 2(7): 643-654.
PubMed
BRAF-targeted therapy results in objective responses in the majority of patients; however, the responses are short lived ( approximately 6 months). In contrast, treatment with immune checkpoint inhibitors results in a lower response rate, but the responses tend to be more durable. BRAF inhibition results in a more favorable tumor microenvironment in patients, with an increase in CD8(+) T-cell infiltrate and a decrease in immunosuppressive cytokines. There is also increased expression of the immunomodulatory molecule PDL1, which may contribute to the resistance. On the basis of these findings, we hypothesized that BRAF-targeted therapy may synergize with the PD1 pathway blockade to enhance antitumor immunity. To test this hypothesis, we developed a BRAF(V600E)/Pten(-/-) syngeneic tumor graft immunocompetent mouse model in which BRAF inhibition leads to a significant increase in the intratumoral CD8(+) T-cell density and cytokine production, similar to the effects of BRAF inhibition in patients. In this model, CD8(+) T cells were found to play a critical role in the therapeutic effect of BRAF inhibition. Administration of anti-PD1 or anti-PDL1 together with a BRAF inhibitor led to an enhanced response, significantly prolonging survival and slowing tumor growth, as well as significantly increasing the number and activity of tumor-infiltrating lymphocytes. These results demonstrate synergy between combined BRAF-targeted therapy and immune checkpoint blockade. Although clinical trials combining these two strategies are ongoing, important questions still remain unanswered. Further studies using this new melanoma mouse model may provide therapeutic insights, including optimal timing and sequence of therapy.
in vivo blocking of PD-1/PD-L signaling
Cooper, Z. A., et al (2014). "Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade" Cancer Immunol Res 2(7): 643-654.
PubMed
BRAF-targeted therapy results in objective responses in the majority of patients; however, the responses are short lived ( approximately 6 months). In contrast, treatment with immune checkpoint inhibitors results in a lower response rate, but the responses tend to be more durable. BRAF inhibition results in a more favorable tumor microenvironment in patients, with an increase in CD8(+) T-cell infiltrate and a decrease in immunosuppressive cytokines. There is also increased expression of the immunomodulatory molecule PDL1, which may contribute to the resistance. On the basis of these findings, we hypothesized that BRAF-targeted therapy may synergize with the PD1 pathway blockade to enhance antitumor immunity. To test this hypothesis, we developed a BRAF(V600E)/Pten(-/-) syngeneic tumor graft immunocompetent mouse model in which BRAF inhibition leads to a significant increase in the intratumoral CD8(+) T-cell density and cytokine production, similar to the effects of BRAF inhibition in patients. In this model, CD8(+) T cells were found to play a critical role in the therapeutic effect of BRAF inhibition. Administration of anti-PD1 or anti-PDL1 together with a BRAF inhibitor led to an enhanced response, significantly prolonging survival and slowing tumor growth, as well as significantly increasing the number and activity of tumor-infiltrating lymphocytes. These results demonstrate synergy between combined BRAF-targeted therapy and immune checkpoint blockade. Although clinical trials combining these two strategies are ongoing, important questions still remain unanswered. Further studies using this new melanoma mouse model may provide therapeutic insights, including optimal timing and sequence of therapy.
in vitro PD-1 neutralization
Park, S. J., et al (2014). "Negative role of inducible PD-1 on survival of activated dendritic cells" J Leukoc Biol 95(4): 621-629.
PubMed
PD-1 is a well-established negative regulator of T cell responses by inhibiting proliferation and cytokine production of T cells via interaction with its ligands, B7-H1 (PD-L1) and B7-DC (PD-L2), expressed on non-T cells. Recently, PD-1 was found to be expressed in innate cells, including activated DCs, and plays roles in suppressing production of inflammatory cytokines. In this study, we demonstrate that PD-1 KO DCs exhibited prolonged longevity compared with WT DCs in the dLNs after transfer of DCs into hind footpads. Interestingly, upon LPS stimulation, WT DCs increased the expression of PD-1 and started to undergo apoptosis. DCs, in spleen of LPS-injected PD-1 KO mice, were more resistant to LPS-mediated apoptosis in vivo than WT controls. Moreover, treatment of blocking anti-PD-1 mAb during DC maturation resulted in enhanced DC survival, suggesting that PD-1:PD-L interactions are involved in DC apoptosis. As a result, PD-1-deficient DCs augmented T cell responses in terms of antigen-specific IFN-gamma production and proliferation of CD4 and CD8 T cells to a greater degree than WT DCs. Moreover, PD-1 KO DCs exhibited increased MAPK1 and CD40-CD40L signaling, suggesting a possible mechanism for enhanced DC survival in the absence of PD-1 expression. Taken together, our findings further extend the function of PD-1, which plays an important role in apoptosis of activated DCs and provides important implications for PD-1-mediated immune regulation.
in vivo blocking of PD-1/PD-L signaling
in vitro PD-1 neutralization
Duraiswamy, J., et al (2013). "Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors" Cancer Res 73(12): 3591-3603.
PubMed
Tumor progression is facilitated by regulatory T cells (Treg) and restricted by effector T cells. In this study, we document parallel regulation of CD8(+) T cells and Foxp3(+) Tregs by programmed death-1 (PD-1, PDCD1). In addition, we identify an additional role of CTL antigen-4 (CTLA-4) inhibitory receptor in further promoting dysfunction of CD8(+) T effector cells in tumor models (CT26 colon carcinoma and ID8-VEGF ovarian carcinoma). Two thirds of CD8(+) tumor-infiltrating lymphocytes (TIL) expressed PD-1, whereas one third to half of CD8(+) TIL coexpressed PD-1 and CTLA-4. Double-positive (PD-1(+)CTLA-4(+)) CD8(+) TIL had characteristics of more severe dysfunction than single-positive (PD-1(+) or CTLA-4(+)) TIL, including an inability to proliferate and secrete effector cytokines. Blockade of both PD-1 and CTLA-4 resulted in reversal of CD8(+) TIL dysfunction and led to tumor rejection in two thirds of mice. Double blockade was associated with increased proliferation of antigen-specific effector CD8(+) and CD4(+) T cells, antigen-specific cytokine release, inhibition of suppressive functions of Tregs, and upregulation of key signaling molecules critical for T-cell function. When used in combination with GVAX vaccination (consisting of granulocyte macrophage colony-stimulating factor-expressing irradiated tumor cells), inhibitory pathway blockade induced rejection of CT26 tumors in 100% of mice and ID8-VEGF tumors in 75% of mice. Our study indicates that PD-1 signaling in tumors is required for both suppressing effector T cells and maintaining tumor Tregs, and that PD-1/PD-L1 pathway (CD274) blockade augments tumor inhibition by increasing effector T-cell activity, thereby attenuating Treg suppression.
in vivo blocking of PD-1/PD-L signaling
in vitro PD-1 neutralization
Duraiswamy, J., et al (2013). "Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors" Cancer Res 73(12): 3591-3603.
PubMed
Tumor progression is facilitated by regulatory T cells (Treg) and restricted by effector T cells. In this study, we document parallel regulation of CD8(+) T cells and Foxp3(+) Tregs by programmed death-1 (PD-1, PDCD1). In addition, we identify an additional role of CTL antigen-4 (CTLA-4) inhibitory receptor in further promoting dysfunction of CD8(+) T effector cells in tumor models (CT26 colon carcinoma and ID8-VEGF ovarian carcinoma). Two thirds of CD8(+) tumor-infiltrating lymphocytes (TIL) expressed PD-1, whereas one third to half of CD8(+) TIL coexpressed PD-1 and CTLA-4. Double-positive (PD-1(+)CTLA-4(+)) CD8(+) TIL had characteristics of more severe dysfunction than single-positive (PD-1(+) or CTLA-4(+)) TIL, including an inability to proliferate and secrete effector cytokines. Blockade of both PD-1 and CTLA-4 resulted in reversal of CD8(+) TIL dysfunction and led to tumor rejection in two thirds of mice. Double blockade was associated with increased proliferation of antigen-specific effector CD8(+) and CD4(+) T cells, antigen-specific cytokine release, inhibition of suppressive functions of Tregs, and upregulation of key signaling molecules critical for T-cell function. When used in combination with GVAX vaccination (consisting of granulocyte macrophage colony-stimulating factor-expressing irradiated tumor cells), inhibitory pathway blockade induced rejection of CT26 tumors in 100% of mice and ID8-VEGF tumors in 75% of mice. Our study indicates that PD-1 signaling in tumors is required for both suppressing effector T cells and maintaining tumor Tregs, and that PD-1/PD-L1 pathway (CD274) blockade augments tumor inhibition by increasing effector T-cell activity, thereby attenuating Treg suppression.
in vivo blocking of PD-1/PD-L signaling
in vitro PD-1 neutralization
Duraiswamy, J., et al (2013). "Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors" Cancer Res 73(12): 3591-3603.
PubMed
Tumor progression is facilitated by regulatory T cells (Treg) and restricted by effector T cells. In this study, we document parallel regulation of CD8(+) T cells and Foxp3(+) Tregs by programmed death-1 (PD-1, PDCD1). In addition, we identify an additional role of CTL antigen-4 (CTLA-4) inhibitory receptor in further promoting dysfunction of CD8(+) T effector cells in tumor models (CT26 colon carcinoma and ID8-VEGF ovarian carcinoma). Two thirds of CD8(+) tumor-infiltrating lymphocytes (TIL) expressed PD-1, whereas one third to half of CD8(+) TIL coexpressed PD-1 and CTLA-4. Double-positive (PD-1(+)CTLA-4(+)) CD8(+) TIL had characteristics of more severe dysfunction than single-positive (PD-1(+) or CTLA-4(+)) TIL, including an inability to proliferate and secrete effector cytokines. Blockade of both PD-1 and CTLA-4 resulted in reversal of CD8(+) TIL dysfunction and led to tumor rejection in two thirds of mice. Double blockade was associated with increased proliferation of antigen-specific effector CD8(+) and CD4(+) T cells, antigen-specific cytokine release, inhibition of suppressive functions of Tregs, and upregulation of key signaling molecules critical for T-cell function. When used in combination with GVAX vaccination (consisting of granulocyte macrophage colony-stimulating factor-expressing irradiated tumor cells), inhibitory pathway blockade induced rejection of CT26 tumors in 100% of mice and ID8-VEGF tumors in 75% of mice. Our study indicates that PD-1 signaling in tumors is required for both suppressing effector T cells and maintaining tumor Tregs, and that PD-1/PD-L1 pathway (CD274) blockade augments tumor inhibition by increasing effector T-cell activity, thereby attenuating Treg suppression.
Flow Cytometry
Good-Jacobson, K. L., et al (2012). "CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation" J Immunol 188(9): 4217-4225.
PubMed
Germinal center (GC) B cells and T follicular helper (T(FH)) cells interact in the production of high-affinity long-lived plasma cells (PCs) and memory B cells, although the mechanisms regulating the formation of these long-lived populations remain unclear. Because CD80 is one of the few markers shared by human and murine memory B cells, we investigated its role in the development of GCs, memory cells, and PCs. In CD80-deficient mice, fewer long-lived PCs were generated upon immunization compared with that in B6 controls. In concert, the absence of CD80 resulted in an increase in apoptotic GC B cells during the contraction phase of the GC. CD80(-/-) mice had fewer T(FH) cells compared with that of B6, and residual T(FH) cells failed to mature, with decreased ICOS and PD-1 expression and decreased synthesis of IL-21 mRNA. Mixed bone marrow chimeras demonstrated a B cell-intrinsic requirement for CD80 expression for normal T(FH) cell and PC development. Therefore, B cell expression of CD80 plays a critical role in regulating B-T interactions in both early and late GC responses. This, in turn, results in impaired ability to produce long-lived PCs. These data provide new insights into the development of GCs and Ab-forming cells and the functions of CD80 in humoral immunity.
Flow Cytometry
Good-Jacobson, K. L., et al (2012). "CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation" J Immunol 188(9): 4217-4225.
PubMed
Germinal center (GC) B cells and T follicular helper (T(FH)) cells interact in the production of high-affinity long-lived plasma cells (PCs) and memory B cells, although the mechanisms regulating the formation of these long-lived populations remain unclear. Because CD80 is one of the few markers shared by human and murine memory B cells, we investigated its role in the development of GCs, memory cells, and PCs. In CD80-deficient mice, fewer long-lived PCs were generated upon immunization compared with that in B6 controls. In concert, the absence of CD80 resulted in an increase in apoptotic GC B cells during the contraction phase of the GC. CD80(-/-) mice had fewer T(FH) cells compared with that of B6, and residual T(FH) cells failed to mature, with decreased ICOS and PD-1 expression and decreased synthesis of IL-21 mRNA. Mixed bone marrow chimeras demonstrated a B cell-intrinsic requirement for CD80 expression for normal T(FH) cell and PC development. Therefore, B cell expression of CD80 plays a critical role in regulating B-T interactions in both early and late GC responses. This, in turn, results in impaired ability to produce long-lived PCs. These data provide new insights into the development of GCs and Ab-forming cells and the functions of CD80 in humoral immunity.
Flow Cytometry
Good-Jacobson, K. L., et al (2012). "CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation" J Immunol 188(9): 4217-4225.
PubMed
Germinal center (GC) B cells and T follicular helper (T(FH)) cells interact in the production of high-affinity long-lived plasma cells (PCs) and memory B cells, although the mechanisms regulating the formation of these long-lived populations remain unclear. Because CD80 is one of the few markers shared by human and murine memory B cells, we investigated its role in the development of GCs, memory cells, and PCs. In CD80-deficient mice, fewer long-lived PCs were generated upon immunization compared with that in B6 controls. In concert, the absence of CD80 resulted in an increase in apoptotic GC B cells during the contraction phase of the GC. CD80(-/-) mice had fewer T(FH) cells compared with that of B6, and residual T(FH) cells failed to mature, with decreased ICOS and PD-1 expression and decreased synthesis of IL-21 mRNA. Mixed bone marrow chimeras demonstrated a B cell-intrinsic requirement for CD80 expression for normal T(FH) cell and PC development. Therefore, B cell expression of CD80 plays a critical role in regulating B-T interactions in both early and late GC responses. This, in turn, results in impaired ability to produce long-lived PCs. These data provide new insights into the development of GCs and Ab-forming cells and the functions of CD80 in humoral immunity.
Immunofluorescence
Western Blot
Chen, L., et al (2009). "Role of the immune modulator programmed cell death-1 during development and apoptosis of mouse retinal ganglion cells" Invest Ophthalmol Vis Sci 50(10): 4941-4948.
PubMed
PURPOSE: Mammalian programmed cell death (PD)-1 is a membrane-associated receptor regulating the balance between T-cell activation, tolerance, and immunopathology; however, its role in neurons has not yet been defined. The hypothesis that PD-1 signaling actively promotes retinal ganglion cell (RGC) death within the developing mouse retina was investigated. METHODS: Mature retinal cell types expressing PD-1 were identified by immunofluorescence staining of vertical retina sections; developmental expression was localized by immunostaining and quantified by Western blot analysis. PD-1 involvement in developmental RGC survival was assessed in vitro using retinal explants and in vivo using PD-1 knockout mice. PD-1 ligand gene expression was detected by RT-PCR. RESULTS: PD-1 is expressed in most adult RGCs and undergoes dynamic upregulation during the early postnatal window of retinal cell maturation and physiological programmed cell death (PCD). In vitro blockade of PD-1 signaling during this time selectively increases the survival of RGCs. Furthermore, PD-1-deficient mice show a selective increase in RGC number in the neonatal retina at the peak of developmental RGC death. Lastly, gene expression of the immune PD-1 ligand genes Pdcd1lg1 and Pdcd1lg2 was found throughout postnatal retina maturation. CONCLUSIONS: These findings collectively support a novel role for a PD-1-mediated signaling pathway in developmental PCD during postnatal RGC maturation.
Immunofluorescence
Western Blot
Chen, L., et al (2009). "Role of the immune modulator programmed cell death-1 during development and apoptosis of mouse retinal ganglion cells" Invest Ophthalmol Vis Sci 50(10): 4941-4948.
PubMed
PURPOSE: Mammalian programmed cell death (PD)-1 is a membrane-associated receptor regulating the balance between T-cell activation, tolerance, and immunopathology; however, its role in neurons has not yet been defined. The hypothesis that PD-1 signaling actively promotes retinal ganglion cell (RGC) death within the developing mouse retina was investigated. METHODS: Mature retinal cell types expressing PD-1 were identified by immunofluorescence staining of vertical retina sections; developmental expression was localized by immunostaining and quantified by Western blot analysis. PD-1 involvement in developmental RGC survival was assessed in vitro using retinal explants and in vivo using PD-1 knockout mice. PD-1 ligand gene expression was detected by RT-PCR. RESULTS: PD-1 is expressed in most adult RGCs and undergoes dynamic upregulation during the early postnatal window of retinal cell maturation and physiological programmed cell death (PCD). In vitro blockade of PD-1 signaling during this time selectively increases the survival of RGCs. Furthermore, PD-1-deficient mice show a selective increase in RGC number in the neonatal retina at the peak of developmental RGC death. Lastly, gene expression of the immune PD-1 ligand genes Pdcd1lg1 and Pdcd1lg2 was found throughout postnatal retina maturation. CONCLUSIONS: These findings collectively support a novel role for a PD-1-mediated signaling pathway in developmental PCD during postnatal RGC maturation.
Immunofluorescence
Western Blot
Chen, L., et al (2009). "Role of the immune modulator programmed cell death-1 during development and apoptosis of mouse retinal ganglion cells" Invest Ophthalmol Vis Sci 50(10): 4941-4948.
PubMed
PURPOSE: Mammalian programmed cell death (PD)-1 is a membrane-associated receptor regulating the balance between T-cell activation, tolerance, and immunopathology; however, its role in neurons has not yet been defined. The hypothesis that PD-1 signaling actively promotes retinal ganglion cell (RGC) death within the developing mouse retina was investigated. METHODS: Mature retinal cell types expressing PD-1 were identified by immunofluorescence staining of vertical retina sections; developmental expression was localized by immunostaining and quantified by Western blot analysis. PD-1 involvement in developmental RGC survival was assessed in vitro using retinal explants and in vivo using PD-1 knockout mice. PD-1 ligand gene expression was detected by RT-PCR. RESULTS: PD-1 is expressed in most adult RGCs and undergoes dynamic upregulation during the early postnatal window of retinal cell maturation and physiological programmed cell death (PCD). In vitro blockade of PD-1 signaling during this time selectively increases the survival of RGCs. Furthermore, PD-1-deficient mice show a selective increase in RGC number in the neonatal retina at the peak of developmental RGC death. Lastly, gene expression of the immune PD-1 ligand genes Pdcd1lg1 and Pdcd1lg2 was found throughout postnatal retina maturation. CONCLUSIONS: These findings collectively support a novel role for a PD-1-mediated signaling pathway in developmental PCD during postnatal RGC maturation.
Immunohistochemistry (frozen)
Menke, J., et al (2007). "Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles" J Immunol 179(11): 7466-7477.
PubMed
The programmed death 1/programmed death 1 ligand (PD-L) pathway is instrumental in peripheral tolerance. Blocking this pathway exacerbates experimental autoimmune diseases, but its role in autoimmune kidney disease has not been explored. Therefore, we tested the hypothesis that the programmed death 1 ligands (PD-L1 and PD-L2), provide a protective barrier during T cell- and macrophage (Mphi)-dependent autoimmune kidney disease. For this purpose, we compared nephrotoxic serum nephritis (NSN) in mice lacking PD-L1 (PD-L1(-/-)), PD-L2 (PD-L2(-/-)), or both (PD-L1/L2(-/-)) to wild-type (WT) C57BL/6 mice. Kidney pathology, loss of renal function, and intrarenal leukocyte infiltrates were increased in each PD-L(-/-) strain as compared with WT mice. Although the magnitude of renal pathology was similar in PD-L1(-/-) and PD-L2(-/-) mice, our findings suggest that kidney disease in each strain is regulated by distinct mechanisms. Specifically, we detected increased CD68(+) cells along with elevated circulating IgG and IgG deposits in glomeruli in PD-L2(-/-) mice, but not PD-L1(-/-) mice. In contrast, we detected a rise in activated CD8(+) T cells in PD-L1(-/-) mice, but not PD-L2(-/-) mice. Furthermore, since PD-L1 is expressed by parenchymal and hemopoietic cells in WT kidneys, we explored the differential impact of PD-L1 expression on these cell types by inducing NSN in bone marrow chimeric mice. Our results indicate that PD-L1 expression on hemopoietic cells, and not parenchymal cells, is primarily responsible for limiting leukocyte infiltration during NSN. Taken together, our findings indicate that PD-L1 and PD-L2 provide distinct negative regulatory checkpoints poised to suppress autoimmune renal disease.
Immunohistochemistry (frozen)
Menke, J., et al (2007). "Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles" J Immunol 179(11): 7466-7477.
PubMed
The programmed death 1/programmed death 1 ligand (PD-L) pathway is instrumental in peripheral tolerance. Blocking this pathway exacerbates experimental autoimmune diseases, but its role in autoimmune kidney disease has not been explored. Therefore, we tested the hypothesis that the programmed death 1 ligands (PD-L1 and PD-L2), provide a protective barrier during T cell- and macrophage (Mphi)-dependent autoimmune kidney disease. For this purpose, we compared nephrotoxic serum nephritis (NSN) in mice lacking PD-L1 (PD-L1(-/-)), PD-L2 (PD-L2(-/-)), or both (PD-L1/L2(-/-)) to wild-type (WT) C57BL/6 mice. Kidney pathology, loss of renal function, and intrarenal leukocyte infiltrates were increased in each PD-L(-/-) strain as compared with WT mice. Although the magnitude of renal pathology was similar in PD-L1(-/-) and PD-L2(-/-) mice, our findings suggest that kidney disease in each strain is regulated by distinct mechanisms. Specifically, we detected increased CD68(+) cells along with elevated circulating IgG and IgG deposits in glomeruli in PD-L2(-/-) mice, but not PD-L1(-/-) mice. In contrast, we detected a rise in activated CD8(+) T cells in PD-L1(-/-) mice, but not PD-L2(-/-) mice. Furthermore, since PD-L1 is expressed by parenchymal and hemopoietic cells in WT kidneys, we explored the differential impact of PD-L1 expression on these cell types by inducing NSN in bone marrow chimeric mice. Our results indicate that PD-L1 expression on hemopoietic cells, and not parenchymal cells, is primarily responsible for limiting leukocyte infiltration during NSN. Taken together, our findings indicate that PD-L1 and PD-L2 provide distinct negative regulatory checkpoints poised to suppress autoimmune renal disease.
Immunohistochemistry (frozen)
Menke, J., et al (2007). "Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles" J Immunol 179(11): 7466-7477.
PubMed
The programmed death 1/programmed death 1 ligand (PD-L) pathway is instrumental in peripheral tolerance. Blocking this pathway exacerbates experimental autoimmune diseases, but its role in autoimmune kidney disease has not been explored. Therefore, we tested the hypothesis that the programmed death 1 ligands (PD-L1 and PD-L2), provide a protective barrier during T cell- and macrophage (Mphi)-dependent autoimmune kidney disease. For this purpose, we compared nephrotoxic serum nephritis (NSN) in mice lacking PD-L1 (PD-L1(-/-)), PD-L2 (PD-L2(-/-)), or both (PD-L1/L2(-/-)) to wild-type (WT) C57BL/6 mice. Kidney pathology, loss of renal function, and intrarenal leukocyte infiltrates were increased in each PD-L(-/-) strain as compared with WT mice. Although the magnitude of renal pathology was similar in PD-L1(-/-) and PD-L2(-/-) mice, our findings suggest that kidney disease in each strain is regulated by distinct mechanisms. Specifically, we detected increased CD68(+) cells along with elevated circulating IgG and IgG deposits in glomeruli in PD-L2(-/-) mice, but not PD-L1(-/-) mice. In contrast, we detected a rise in activated CD8(+) T cells in PD-L1(-/-) mice, but not PD-L2(-/-) mice. Furthermore, since PD-L1 is expressed by parenchymal and hemopoietic cells in WT kidneys, we explored the differential impact of PD-L1 expression on these cell types by inducing NSN in bone marrow chimeric mice. Our results indicate that PD-L1 expression on hemopoietic cells, and not parenchymal cells, is primarily responsible for limiting leukocyte infiltration during NSN. Taken together, our findings indicate that PD-L1 and PD-L2 provide distinct negative regulatory checkpoints poised to suppress autoimmune renal disease.
in vivo blocking of PD-1/PD-L signaling
Barber, D. L., et al (2006). "Restoring function in exhausted CD8 T cells during chronic viral infection" Nature 439(7077): 682-687.
PubMed
Functional impairment of antigen-specific T cells is a defining characteristic of many chronic infections, but the underlying mechanisms of T-cell dysfunction are not well understood. To address this question, we analysed genes expressed in functionally impaired virus-specific CD8 T cells present in mice chronically infected with lymphocytic choriomeningitis virus (LCMV), and compared these with the gene profile of functional memory CD8 T cells. Here we report that PD-1 (programmed death 1; also known as Pdcd1) was selectively upregulated by the exhausted T cells, and that in vivo administration of antibodies that blocked the interaction of this inhibitory receptor with its ligand, PD-L1 (also known as B7-H1), enhanced T-cell responses. Notably, we found that even in persistently infected mice that were lacking CD4 T-cell help, blockade of the PD-1/PD-L1 inhibitory pathway had a beneficial effect on the ‘helpless’ CD8 T cells, restoring their ability to undergo proliferation, secrete cytokines, kill infected cells and decrease viral load. Blockade of the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) inhibitory pathway had no effect on either T-cell function or viral control. These studies identify a specific mechanism of T-cell exhaustion and define a potentially effective immunological strategy for the treatment of chronic viral infections.
in vivo blocking of PD-1/PD-L signaling
Barber, D. L., et al (2006). "Restoring function in exhausted CD8 T cells during chronic viral infection" Nature 439(7077): 682-687.
PubMed
Functional impairment of antigen-specific T cells is a defining characteristic of many chronic infections, but the underlying mechanisms of T-cell dysfunction are not well understood. To address this question, we analysed genes expressed in functionally impaired virus-specific CD8 T cells present in mice chronically infected with lymphocytic choriomeningitis virus (LCMV), and compared these with the gene profile of functional memory CD8 T cells. Here we report that PD-1 (programmed death 1; also known as Pdcd1) was selectively upregulated by the exhausted T cells, and that in vivo administration of antibodies that blocked the interaction of this inhibitory receptor with its ligand, PD-L1 (also known as B7-H1), enhanced T-cell responses. Notably, we found that even in persistently infected mice that were lacking CD4 T-cell help, blockade of the PD-1/PD-L1 inhibitory pathway had a beneficial effect on the ‘helpless’ CD8 T cells, restoring their ability to undergo proliferation, secrete cytokines, kill infected cells and decrease viral load. Blockade of the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) inhibitory pathway had no effect on either T-cell function or viral control. These studies identify a specific mechanism of T-cell exhaustion and define a potentially effective immunological strategy for the treatment of chronic viral infections.
in vivo blocking of PD-1/PD-L signaling
Barber, D. L., et al (2006). "Restoring function in exhausted CD8 T cells during chronic viral infection" Nature 439(7077): 682-687.
PubMed
Functional impairment of antigen-specific T cells is a defining characteristic of many chronic infections, but the underlying mechanisms of T-cell dysfunction are not well understood. To address this question, we analysed genes expressed in functionally impaired virus-specific CD8 T cells present in mice chronically infected with lymphocytic choriomeningitis virus (LCMV), and compared these with the gene profile of functional memory CD8 T cells. Here we report that PD-1 (programmed death 1; also known as Pdcd1) was selectively upregulated by the exhausted T cells, and that in vivo administration of antibodies that blocked the interaction of this inhibitory receptor with its ligand, PD-L1 (also known as B7-H1), enhanced T-cell responses. Notably, we found that even in persistently infected mice that were lacking CD4 T-cell help, blockade of the PD-1/PD-L1 inhibitory pathway had a beneficial effect on the ‘helpless’ CD8 T cells, restoring their ability to undergo proliferation, secrete cytokines, kill infected cells and decrease viral load. Blockade of the CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) inhibitory pathway had no effect on either T-cell function or viral control. These studies identify a specific mechanism of T-cell exhaustion and define a potentially effective immunological strategy for the treatment of chronic viral infections.
Immunohistochemistry (frozen)
Liang, S. C., et al (2003). "Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses" Eur J Immunol 33(10): 2706-2716.
PubMed
Newer members of the B7-CD28 superfamily include the receptor PD-1 and its two ligands, PD-L1 and PD-L2. Here, we characterize the expression of PD-1, PD-L1, and PD-L2 in tissues of naive miceand in target organs from two models of autoimmunity, the pancreas from non-obese diabetic (NOD) mice and brain from mice with experimental autoimmune encephalomyelitis (EAE). In naive mice, proteiexpression of PD-1, PD-L1, and PD-L2 was detected in the thymus, while PD-1 and PD-L1 were detected in the spleen. PD-L1, but not PD-L2, was also detected at low levels on cardiac endothelium, pancreatic islets, and syncyciotrophoblasts in the placenta. In pre-diabetic NOD mice, PD-1 and PD-L1 were expressed on infiltrating cells in the pancreatic islets. Furthermore, PD-L1 was markedly up-regulated on islet cells. In brains from mice with EAE, PD-1, PD-L1, and PD-L2 were expressed on infiltrating inflammatory cells, and PD-L1 was up-regulated on endothelium within EAE brain. The distinct expression patterns of PD-L1 and PD-L2 led us to compare their transcriptional regulation in STAT4(-/-), STAT6(-/-), or NF-kappaB p50(-/-)p65(+/-) dendritic cells (DC).PD-L2, but not PD-L1, expression was dramatically reduced in p50(-/-)p65(+/-) DC. Thus, PD-L1 and PD-L2 exhibit distinct expression patterns and are differentially regulated on the transcriptional level.
Immunohistochemistry (frozen)
Liang, S. C., et al (2003). "Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses" Eur J Immunol 33(10): 2706-2716.
PubMed
Newer members of the B7-CD28 superfamily include the receptor PD-1 and its two ligands, PD-L1 and PD-L2. Here, we characterize the expression of PD-1, PD-L1, and PD-L2 in tissues of naive miceand in target organs from two models of autoimmunity, the pancreas from non-obese diabetic (NOD) mice and brain from mice with experimental autoimmune encephalomyelitis (EAE). In naive mice, proteiexpression of PD-1, PD-L1, and PD-L2 was detected in the thymus, while PD-1 and PD-L1 were detected in the spleen. PD-L1, but not PD-L2, was also detected at low levels on cardiac endothelium, pancreatic islets, and syncyciotrophoblasts in the placenta. In pre-diabetic NOD mice, PD-1 and PD-L1 were expressed on infiltrating cells in the pancreatic islets. Furthermore, PD-L1 was markedly up-regulated on islet cells. In brains from mice with EAE, PD-1, PD-L1, and PD-L2 were expressed on infiltrating inflammatory cells, and PD-L1 was up-regulated on endothelium within EAE brain. The distinct expression patterns of PD-L1 and PD-L2 led us to compare their transcriptional regulation in STAT4(-/-), STAT6(-/-), or NF-kappaB p50(-/-)p65(+/-) dendritic cells (DC).PD-L2, but not PD-L1, expression was dramatically reduced in p50(-/-)p65(+/-) DC. Thus, PD-L1 and PD-L2 exhibit distinct expression patterns and are differentially regulated on the transcriptional level.
Immunohistochemistry (frozen)
Liang, S. C., et al (2003). "Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses" Eur J Immunol 33(10): 2706-2716.
PubMed
Newer members of the B7-CD28 superfamily include the receptor PD-1 and its two ligands, PD-L1 and PD-L2. Here, we characterize the expression of PD-1, PD-L1, and PD-L2 in tissues of naive miceand in target organs from two models of autoimmunity, the pancreas from non-obese diabetic (NOD) mice and brain from mice with experimental autoimmune encephalomyelitis (EAE). In naive mice, proteiexpression of PD-1, PD-L1, and PD-L2 was detected in the thymus, while PD-1 and PD-L1 were detected in the spleen. PD-L1, but not PD-L2, was also detected at low levels on cardiac endothelium, pancreatic islets, and syncyciotrophoblasts in the placenta. In pre-diabetic NOD mice, PD-1 and PD-L1 were expressed on infiltrating cells in the pancreatic islets. Furthermore, PD-L1 was markedly up-regulated on islet cells. In brains from mice with EAE, PD-1, PD-L1, and PD-L2 were expressed on infiltrating inflammatory cells, and PD-L1 was up-regulated on endothelium within EAE brain. The distinct expression patterns of PD-L1 and PD-L2 led us to compare their transcriptional regulation in STAT4(-/-), STAT6(-/-), or NF-kappaB p50(-/-)p65(+/-) dendritic cells (DC).PD-L2, but not PD-L1, expression was dramatically reduced in p50(-/-)p65(+/-) DC. Thus, PD-L1 and PD-L2 exhibit distinct expression patterns and are differentially regulated on the transcriptional level.
Product Citations
-
-
Cancer Research
DPP7 promotes fatty acid β-oxidation in tumor-associated macrophages and determines immunosuppressive microenvironment in colorectal cancer.
In Int J Biol Sci on 10 November 2025 by Chang, J., Niu, Y., et al.
PubMed
Background: Tumor-associated macrophages (TAMs) are pivotal mediators of the immunosuppressive tumor immune microenvironment (TIME) in colorectal cancer (CRC). However, genes of TAMs that potentiate immunotherapy remain to be explored. Methods: Single-cell RNA sequencing (scRNA-seq) data were analyzed to identify TAM molecular signatures, which were validated in patient cohorts from Huadong Hospital and TCGA to explore their clinical significance. Multidimensional characterization of CRC TIME and Dipeptidyl peptidase VII (DPP7)-positive TAMs functional state was achieved through cytometry by time-of-flight, multiplex immunofluorescence, in vitro and in vivo experiments. Mechanistic investigations integrating RNA-seq, Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)-based proteomics, and targeted lipid metabolomics have revealed the reprogramming of key metabolic pathways. Finally, the therapeutic potential of DPP7, which targets the enhancement of anti-PD-1 immunotherapy efficacy, was demonstrated. Results: DPP7 was identified as the key gene in TAMs, and DPP7+TAMs correlated with metastasis and worse overall survival in multiple clinical cohorts. Functional characterization demonstrated that DPP7+TAMs drove the immunosuppressive TIME and promoted the exhaustion of CD8+T cells, thus exhibiting M2-polarized features. Mechanistically, DPP7 reduced ubiquitination-induced degradation of Carnitine Palmitoyltransferase 1A (CPT1A) by binding to CPT1A in a mutually exclusive manner with TRIM25, thus enhancing fatty acid oxidation (FAO) in TAMs. This metabolic reprogramming consumes lipids (including triglycerides and free fatty acids), elevates adenosine triphosphate (ATP) generation, and induces an immunosuppressive phenotype. In vivo, DPP7 knockdown in bone marrow-derived macrophages (BMDMs) synergized with anti-PD-1 therapy, achieving significant suppression of subcutaneous xenograft tumor growth and liver metastatic burden by reversing the immunosuppressive TIME. Conclusions: DPP7 is mainly expressed in TAMs and DPP7+TAMs are strongly associated with adverse prognosis in CRC. Mechanistically, DPP7 enhances FAO to promote the M2-polarized phenotype in TAMs, leading to an immunosuppressive TIME. Targeting DPP7+TAMs may potentiate the efficacy of immunotherapy for CRC.
-
-
-
Immunology and Microbiology
-
Cancer Research
DAPK1-positve macrophages facilitate immunosuppressive microenvironment and determine immunotherapy efficacy in colorectal cancer.
In J Transl Med on 4 November 2025 by Chang, J., Niu, Y., et al.
PubMed
Colorectal cancer (CRC) is a highly immunosuppressive malignancy characterized by limited therapeutic options and a poor prognosis. Within the CRC tumor immune microenvironment (TIME), tumor-associated macrophages (TAMs) represent the predominant immune cell population. This study aimed to characterize the specific macrophage subsets contributing to CRC progression and resistance to immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
HMOX1+ macrophages determine immunosuppressive microenvironment and immunotherapy efficacy in hepatocellular carcinoma.
In Hepatol Commun on 1 November 2025 by Du, Y., Xu, W., et al.
PubMed
HCC is a notably immunosuppressive malignancy with limited therapeutic options and poor prognosis. Macrophages, as major immune cell populations within the tumor microenvironment, significantly influence disease progression and therapy efficacy. We aim to identify pivotal macrophage subsets associated with HCC progression and resistance to immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
FSTL3 is a biomarker of poor prognosis and associated with immunotherapy resistance in ovarian cancer.
In J Exp Clin Cancer Res on 30 September 2025 by Chauvin, M., Tromelin, E., et al.
PubMed
High-grade serous ovarian carcinoma (HGSOC) is associated with high mortality rates due to late-stage diagnosis and limited treatment options. We investigated the role of FSTL3 in ovarian cancer progression both as a prognostic biomarker and as a potential therapeutic target.We measured levels of follistatin (FST) and follistatin-like 3 (FSTL3) in 96 ovarian cancer patient ascites samples and found that FSTL3 overexpression was more predominant than FST and associated with poorer survival outcomes. Mice implanted with an HGSOC syngeneic cell line bearing common alterations in ovarian cancer (KRASG12 V, P53R172H, CCNE1oe, AKT2oe) had increasing levels of FST and FSTL3 in serum during tumor growth. Further alteration of this model to generate a knockout of FST (KPCA.FSTKO) and an overexpression of human FSTL3 (KPCA.FSTKO_hFSTL3) revealed that FSTL3 expression was associated with a more fibrotic tumor microenvironment, correlating with an increased abundance of cancer-associated myofibroblasts (myCAFs), and cancer cells with a more mesenchymal phenotype. Tumors overexpressing FSTL3 also had significantly less immunocyte infiltration, reduced intratumoral T-cell abundance, and increased CD8+ T cell exhaustion. FSTL3 overexpression completely abrogated tumor response to PPC treatment (Prexasertib combined with PD-1 and CTLA-4 blockade) compared to controls, suggesting that FSTL3 may be involved in immunotherapy resistance. In conclusion, this study suggests a role for FSTL3 as a prognostic marker and as therapeutic target in HGSOC, where it may play a role in promoting a mesenchymal tumor phenotype, maintaining an immunosuppressive tumor microenvironment, and driving immunotherapy resistance.
-
-
-
Cancer Research
-
Immunology and Microbiology
Olaparib Combined with Anti-PD1 Enhances Immunotherapy of Gastric Cancer Via NF-κB/c-Myc/PD-L1 Signaling.
In Dig Dis Sci on 1 June 2025 by Zheng, W., Ge, Z., et al.
PubMed
PARP inhibitors, effective in BRCA-mutated cancers, show potential in gastric cancer (GC) where homologous recombination defects (e.g., BRCA1/2 mutations) are common. Olaparib, a PARP inhibitor, upregulates PD-L1, suggesting synergy with PD-1 inhibitors for enhanced GC therapy.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Dual Ribosome Profiling reveals metabolic limitations of cancer and stromal cells in the tumor microenvironment.
In Nat Commun on 19 May 2025 by Aviles-Huerta, D., Del Pizzo, R., et al.
PubMed
The tumor microenvironment (TME) influences cancer cell metabolism and survival. However, how immune and stromal cells respond to metabolic stress in vivo, and how nutrient limitations affect therapy, remains poorly understood. Here, we introduce Dual Ribosome Profiling (DualRP) to simultaneously monitor translation and ribosome stalling in multiple tumor cell populations. DualRP reveals that cancer-fibroblast interactions trigger an inflammatory program that reduces amino acid shortages during glucose starvation. In immunocompetent mice, we show that serine and glycine are essential for optimal T cell function and that their deficiency impairs T cell fitness. Importantly, immune checkpoint blockade therapy imposes amino acid restrictions specifically in T cells, demonstrating that therapies create distinct metabolic demands across TME cell types. By mapping codon-resolved ribosome stalling in a cell‑type‑specific manner, DualRP uncovers metabolic crosstalk that shapes translational programs. DualRP thus offers a powerful, innovative approach for dissecting tumor cell metabolic interplay and guiding combined metabolic-immunotherapeutic strategies.
-
-
-
Cancer Research
-
Immunology and Microbiology
Sleep deprivation-induced sympathetic activation promotes pro-tumoral macrophage phenotype via the ADRB2/KLF4 pathway to facilitate NSCLC metastasis.
In iScience on 16 May 2025 by Yin, S., Wang, J., et al.
PubMed
Sleep deprivation is one of concomitant symptoms of cancer patients, particularly those with non-small cell lung cancer (NSCLC). The potential effect of sleep deprivation on tumor progression and underlying mechanisms remain to be fully investigated. Using a sleep-deprived tumor-bearing mouse model, we found that sleep deprivation altered immune cell composition and regulated pro-tumoral M2 macrophage polarization by the sympathetic nervous system. Furthermore, we identified a role of catecholaminergic neurons in the rostral ventrolateral medulla (RVLM) in influencing NSCLC metastasis. Clinical analyses revealed a correlation between sympathetic-related indicators and poor prognosis. Mechanistically, our findings indicate that sleep deprivation facilitates the polarization of pro-tumoral macrophages by upregulating β2-adrenergic receptor (ADRB2), which subsequently enhances the expression of Kruppel-like transcription factor 4 (KLF4) through the JAK1/STAT6 phosphorylation pathway. These findings highlight a neuro-immune mechanism linking sleep deprivation to NSCLC metastasis, suggesting that targeting the ADRB2/KLF4 axis could improve outcomes for sleep-deprived NSCLC patients.
-
-
-
Cancer Research
-
Immunology and Microbiology
Propionyl-CoA carboxylase subunit B regulates anti-tumor T cells in a pancreatic cancer mouse model.
In Elife on 11 March 2025 by Han, H. V., Efem, R., et al.
PubMed
Most human pancreatic ductal adenocarcinoma (PDAC) are not infiltrated with cytotoxic T cells and are highly resistant to immunotherapy. Over 90% of PDAC have oncogenic KRAS mutations, and phosphoinositide 3-kinases (PI3Ks) are direct effectors of KRAS. Our previous study demonstrated that ablation of Pik3ca in KPC (KrasG12D; Trp53R172H; Pdx1-Cre) pancreatic cancer cells induced host T cells to infiltrate and completely eliminate the tumors in a syngeneic orthotopic implantation mouse model. Now, we show that implantation of Pik3ca-/- KPC (named αKO) cancer cells induces clonal enrichment of cytotoxic T cells infiltrating the pancreatic tumors. To identify potential molecules that can regulate the activity of these anti-tumor T cells, we conducted an in vivo genome-wide gene-deletion screen using αKO cells implanted in the mouse pancreas. The result shows that deletion of propionyl-CoA carboxylase subunit B gene (Pccb) in αKO cells (named p-αKO) leads to immune evasion, tumor progression, and death of host mice. Surprisingly, p-αKO tumors are still infiltrated with clonally enriched CD8+ T cells but they are inactive against tumor cells. However, blockade of PD-L1/PD1 interaction reactivated these clonally enriched T cells infiltrating p-αKO tumors, leading to slower tumor progression and improve survival of host mice. These results indicate that Pccb can modulate the activity of cytotoxic T cells infiltrating some pancreatic cancers and this understanding may lead to improvement in immunotherapy for this difficult-to-treat cancer.
-
-
-
Cancer Research
-
Immunology and Microbiology
Integrative analysis of immunogenic PANoptosis and experimental validation of cinobufagin-induced activation to enhance glioma immunotherapy.
In J Exp Clin Cancer Res on 3 February 2025 by Cai, Y., Xiao, H., et al.
PubMed
Glioma, particularly glioblastoma (GBM), is a highly aggressive tumor with limited responsiveness to immunotherapy. PANoptosis, a form of programmed cell death merging pyroptosis, apoptosis, and necroptosis, plays an important role in reshaping the tumor microenvironment (TME) and enhancing immunotherapy effectiveness. This study investigates PANoptosis dynamics in glioma and explores the therapeutic potential of its activation, particularly through natural compounds such as cinobufagin.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Dual Ribosome Profiling reveals metabolic limitations of cancer and stromal cells in the tumor microenvironment
In bioRxiv on 8 January 2025 by Aviles-Huerta, D., Rossella, D. P., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Systemic IFN-I combined with topical TLR7/8 agonists promotes distant tumor suppression by c-Jun-dependent IL-12 expression in dendritic cells.
In Nat Cancer on 1 January 2025 by Sanlorenzo, M., Novoszel, P., et al.
PubMed
Dendritic cell (DC) activation by pattern recognition receptors like Toll-like-receptors (TLRs) is crucial for cancer immunotherapies. Here, we demonstrate the effectiveness of the TLR7/8 agonist imiquimod (IMQ) in treating both local tumors and distant metastases. Administered orally, IMQ activates plasmacytoid DCs (pDCs) to produce systemic type I interferons (IFN-I) required for TLR7/8 upregulation in DCs and macrophages, sensitizing them to topical IMQ treatment, which is essential for therapeutic efficacy. The mechanism involves c-Jun/AP-1 mediating TLR7/8 signaling in IFN-I-primed DCs, upregulating the pDC-recruiting chemokine CCL2 and the anti-angiogenic cytokine interleukin-12, which suppresses VEGF-A production leading to tumor necrosis and regression. Combining topical and systemic IMQ or IFN-I generates a CD8+ T cell-dependent response at metastatic sites, reinforced by PD-1 blockade, leading to long-lasting memory. Analysis of cohorts of patients with melanoma demonstrates DC-specific TLR7/8 upregulation by IFN-I, supporting the translational potential of combining systemic IFN-I and topical IMQ to improve immunotherapy of topically accessible tumors.
-
-
-
Cancer Research
-
Immunology and Microbiology
Enhancing immune response and survival in hepatocellular carcinoma with novel oncolytic Jurona virus and immune checkpoint blockade.
In Mol Ther Oncol on 19 December 2024 by Tesfay, M. Z., Zhang, Y., et al.
PubMed
Members of the Vesiculovirus genus including Jurona virus (JURV) have emerged as promising immunotherapeutic agents, characterized by their tumor selectivity, fast kinetics, low seroprevalence, and minimal toxicity in humans. Here, we demonstrate that the administration of JURV leads to tumor regression in both hepatocellular carcinoma (HCC) xenograft and syngeneic models. Furthermore, our findings indicate that combining JURV and anti-PD-1 therapy reduced tumor burden and improved survival rates over JURV or anti-PD-1 alone in an orthotopic HCC model. Proteogenomic analysis of JURV-treated, murine HCC tumors demonstrates that the therapeutic effects of the combination of JURV and anti-PD-1 are predominantly driven by coordinated activation of immune effectors, which modulate the tumor microenvironment into a state conducive to anti-tumor activity. Our results establish JURV as a potent candidate for immunovirotherapy in HCC, capable of modulating immune response and synergizing with standard of care for HCC to prolong survival in preclinical models. Further, this research deepens our understanding of JURV's anti-tumoral mechanisms and highlights its potential as a novel approach to HCC treatment strategies.
-
-
-
Cancer Research
-
Immunology and Microbiology
FSTL3 is a biomarker of poor prognosis and is associated with immunotherapy resistance in ovarian cancer
In bioRxiv on 12 December 2024 by Chauvin, M., Tromelin, E., et al.
-
-
-
Immunology and Microbiology
-
AKAP12 positive fibroblast determines immunosuppressive contexture and immunotherapy response in patients with TNBC by promoting macrophage M2 polarization.
In J Immunother Cancer on 23 October 2024 by Liu, Z., Hu, S., et al.
PubMed
Triple-negative breast cancer (TNBC) is a molecular subtype of breast cancer with high aggressiveness and poor prognosis. Cancer-associated fibroblasts (CAFs) are major components of the TNBC microenvironment and play an important role in tumor progression and treatment responses. Our goal is to identify specific CAFs subpopulations contributing to TNBC development.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
53BP1 loss elicits cGAS-STING-dependent antitumor immunity in ovarian and pancreatic cancer.
In Nat Commun on 6 August 2024 by Sun, Y., Patterson-Fortin, J., et al.
PubMed
53BP1 nucleates the anti-end resection machinery at DNA double-strand breaks, thereby countering BRCA1 activity. Loss of 53BP1 leads to DNA end processing and homologous recombination in BRCA1-deficient cells. Consequently, BRCA1-mutant tumors, typically sensitive to PARP inhibitors (PARPi), become resistant in the absence of 53BP1. Here, we demonstrate that the 'leaky' DNA end resection in the absence of 53BP1 results in increased micronuclei and cytoplasmic double-stranded DNA, leading to activation of the cGAS-STING pathway and pro-inflammatory signaling. This enhances CD8+ T cell infiltration, activates macrophages and natural killer cells, and impedes tumor growth. Loss of 53BP1 correlates with a response to immune checkpoint blockade (ICB) and improved overall survival. Immunohistochemical assessment of 53BP1 in two malignancies, high grade serous ovarian cancer and pancreatic ductal adenocarcinoma, which are refractory to ICBs, reveals that lower 53BP1 levels correlate with an increased adaptive and innate immune response. Finally, BRCA1-deficient tumors that develop resistance to PARPi due to the loss of 53BP1 are susceptible to ICB. Therefore, we conclude that 53BP1 is critical for tumor immunogenicity and underpins the response to ICB. Our results support including 53BP1 expression as an exploratory biomarker in ICB trials for malignancies typically refractory to immunotherapy.
-
-
Copy number gain of FAM131B-AS2 promotes the progression of glioblastoma by mitigating replication stress.
In Neuro Oncol on 3 June 2024 by Wang, S., Qi, Y., et al.
PubMed
Glioblastoma (GBM) is characterized by chromosome 7 copy number gains, notably 7q34, potentially contributing to therapeutic resistance, yet the underlying oncogenes have not been fully characterized. Pertinently, the significance of long noncoding RNAs (lncRNAs) in this context has gained attention, necessitating further exploration.
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Exogenous non-coding dsDNA-dependent trans-activation of phagocytes augments anti-tumor immunity.
In Cell Rep Med on 21 May 2024 by Delaunay, T., Son, S., et al.
PubMed
Stimulator of interferon genes (STING)-dependent signaling is requisite for effective anti-microbial and anti-tumor activity. STING signaling is commonly defective in cancer cells, which enables tumor cells to evade the immunosurveillance system. We evaluate here whether intrinsic STING signaling in such tumor cells could be reconstituted by creating recombinant herpes simplex viruses (rHSVs) that express components of the STING signaling pathway. We observe that rHSVs expressing STING and/or cGAS replicate inefficiently yet retain in vivo anti-tumor activity, independent of oncolytic activity requisite on the trans-activation of extrinsic STING signaling in phagocytes by engulfed microbial dsDNA species. Accordingly, the in vivo effects of virotherapy could be simulated by nanoparticles incorporating non-coding dsDNA species, which comparably elicit the trans-activation of phagocytes and augment the efficacy of established cancer treatments including checkpoint inhibition and radiation therapy. Our results help elucidate mechanisms of virotherapeutic anti-tumor activity as well as provide alternate strategies to treat cancer.
-
-
-
Cancer Research
-
Immunology and Microbiology
Chemokine CCL21 determines immunotherapy response in hepatocellular carcinoma by affecting neutrophil polarization.
In Cancer Immunol Immunother on 17 February 2024 by Xu, W., Weng, J., et al.
PubMed
The efficacy of immune checkpoint inhibitors (ICIs) in hepatocellular carcinoma (HCC) is poor and great heterogeneity among individuals. Chemokines are highly correlated with tumor immune response. Here, we aimed to identify an effective chemokine for predicting the efficacy of immunotherapy in HCC.
-
-
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
ZNF689 deficiency promotes intratumor heterogeneity and immunotherapy resistance in triple-negative breast cancer.
In Cell Res on 1 January 2024 by Ge, L. P., Jin, X., et al.
PubMed
Triple-negative breast cancer (TNBC) is an aggressive disease characterized by remarkable intratumor heterogeneity (ITH), which poses therapeutic challenges. However, the clinical relevance and key determinant of ITH in TNBC are poorly understood. Here, we comprehensively characterized ITH levels using multi-omics data across our center's cohort (n = 260), The Cancer Genome Atlas cohort (n = 134), and four immunotherapy-treated cohorts (n = 109). Our results revealed that high ITH was associated with poor patient survival and immunotherapy resistance. Importantly, we identified zinc finger protein 689 (ZNF689) deficiency as a crucial determinant of ITH formation. Mechanistically, the ZNF689-TRIM28 complex was found to directly bind to the promoter of long interspersed element-1 (LINE-1), inducing H3K9me3-mediated transcriptional silencing. ZNF689 deficiency reactivated LINE-1 retrotransposition to exacerbate genomic instability, which fostered ITH. Single-cell RNA sequencing, spatially resolved transcriptomics and flow cytometry analysis confirmed that ZNF689 deficiency-induced ITH inhibited antigen presentation and T-cell activation, conferring immunotherapy resistance. Pharmacological inhibition of LINE-1 significantly reduced ITH, enhanced antitumor immunity, and eventually sensitized ZNF689-deficient tumors to immunotherapy in vivo. Consistently, ZNF689 expression positively correlated with favorable prognosis and immunotherapy response in clinical samples. Altogether, our study uncovers a previously unrecognized mechanism underlying ZNF689 deficiency-induced ITH and suggests LINE-1 inhibition combined with immunotherapy as a novel treatment strategy for TNBC.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Aldehyde dehydrogenase 2-mediated aldehyde metabolism promotes tumor immune evasion by regulating the NOD/VISTA axis.
In J Immunother Cancer on 7 December 2023 by Chen, Y., Sun, J., et al.
PubMed
Aldehyde dehydrogenase 2 (ALDH2) is a crucial enzyme involved in endogenous aldehyde detoxification and has been implicated in tumor progression. However, its role in tumor immune evasion remains unclear.
-