InVivoMAb anti-human PD-1 (CD279)
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vitro PD-1 neutralization in vivo PD-1 blockade in humanized mice in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950318 |
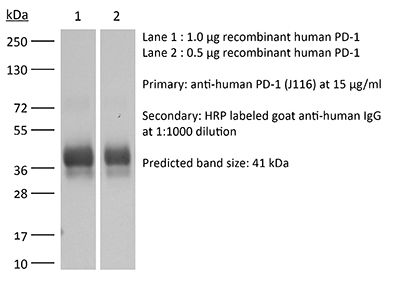
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro PD-1 neutralization
Nakazawa Y, Miyano M, Tsukamoto S, Kogai H, Yamamoto A, Iso K, Inoue S, Yamane Y, Yabe Y, Umihara H, Taguchi J, Akagi T, Yamaguchi A, Koga M, Toshimitsu K, Hirayama T, Mukai Y, Machinaga A (2024). "Delivery of a BET protein degrader via a CEACAM6-tar
PubMed
Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis of all cancers. To improve PDAC therapy, we establish screening systems based on organoid and co-culture technologies and find a payload of antibody-drug conjugate (ADC), a bromodomain and extra-terminal (BET) protein degrader named EBET. We select CEACAM6/CD66c as an ADC target and developed an antibody, #84.7, with minimal reactivity to CEACAM6-expressing normal cells. EBET-conjugated #84.7 (84-EBET) has lethal effects on various PDAC organoids and bystander efficacy on CEACAM6-negative PDAC cells and cancer-associated fibroblasts. In mouse studies, a single injection of 84-EBET induces marked tumor regression in various PDAC-patient-derived xenografts, with a decrease in the inflammatory phenotype of stromal cells and without significant body weight loss. Combination with standard chemotherapy or PD-1 antibody induces more profound and sustained regression without toxicity enhancement. Our preclinical evidence demonstrates potential efficacy by delivering BET protein degrader to PDAC and its microenvironment via CEACAM6-targeted ADC.
in vitro PD-1 neutralization
Tkachev, V., et al (2015). "Programmed death-1 controls T cell survival by regulating oxidative metabolism" J Immunol 194(12): 5789-5800.
PubMed
The coinhibitory receptor programmed death-1 (PD-1) maintains immune homeostasis by negatively regulating T cell function and survival. Blockade of PD-1 increases the severity of graft-versus-host disease (GVHD), but the interplay between PD-1 inhibition and T cell metabolism is not well studied. We found that both murine and human alloreactive T cells concomitantly upregulated PD-1 expression and increased levels of reactive oxygen species (ROS) following allogeneic bone marrow transplantation. This PD-1(Hi)ROS(Hi) phenotype was specific to alloreactive T cells and was not observed in syngeneic T cells during homeostatic proliferation. Blockade of PD-1 signaling decreased both mitochondrial H2O2 and total cellular ROS levels, and PD-1-driven increases in ROS were dependent upon the oxidation of fatty acids, because treatment with etomoxir nullified changes in ROS levels following PD-1 blockade. Downstream of PD-1, elevated ROS levels impaired T cell survival in a process reversed by antioxidants. Furthermore, PD-1-driven changes in ROS were fundamental to establishing a cell’s susceptibility to subsequent metabolic inhibition, because blockade of PD-1 decreased the efficacy of later F1F0-ATP synthase modulation. These data indicate that PD-1 facilitates apoptosis in alloreactive T cells by increasing ROS in a process dependent upon the oxidation of fat. In addition, blockade of PD-1 undermines the potential for subsequent metabolic inhibition, an important consideration given the increasing use of anti-PD-1 therapies in the clinic.
in vivo PD-1 blockade in humanized mice
Tsukahara, T., et al (2015). "The Tol2 transposon system mediates the genetic engineering of T-cells with CD19-specific chimeric antigen receptors for B-cell malignancies" Gene Ther 22(2): 209-215.
PubMed
Engineered T-cell therapy using a CD19-specific chimeric antigen receptor (CD19-CAR) is a promising strategy for the treatment of advanced B-cell malignancies. Gene transfer of CARs to T-cells has widely relied on retroviral vectors, but transposon-based gene transfer has recently emerged as a suitable nonviral method to mediate stable transgene expression. The advantages of transposon vectors compared with viral vectors include their simplicity and cost-effectiveness. We used the Tol2 transposon system to stably transfer CD19-CAR into human T-cells. Normal human peripheral blood lymphocytes were co-nucleofected with the Tol2 transposon donor plasmid carrying CD19-CAR and the transposase expression plasmid and were selectively propagated on NIH3T3 cells expressing human CD19. Expanded CD3(+) T-cells with stable and high-level transgene expression (~95%) produced interferon-gamma upon stimulation with CD19 and specifically lysed Raji cells, a CD19(+) human B-cell lymphoma cell line. Adoptive transfer of these T-cells suppressed tumor progression in Raji tumor-bearing Rag2(-/-)gammac(-/-) immunodeficient mice compared with control mice. These results demonstrate that the Tol2 transposon system could be used to express CD19-CAR in genetically engineered T-cells for the treatment of refractory B-cell malignancies.
in vivo PD-1 blockade in humanized mice
Wang, C., et al (2013). "Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells" J Clin Invest 123(4): 1677-1693.
PubMed
Human graft endothelial cells (ECs) can act as antigen-presenting cells to initiate allograft rejection by host memory T cells. Rapamycin, an mTOR inhibitor used clinically to suppress T cell responses, also acts on DCs, rendering them tolerogenic. Here, we report the effects of rapamycin on EC alloimmunogenicity. Compared with mock-treated cells, rapamycin-pretreated human ECs (rapa-ECs) stimulated less proliferation and cytokine secretion from allogeneic CD4+ memory cells, an effect mimicked by shRNA knockdown of mTOR or raptor in ECs. The effects of rapamycin persisted for several days and were linked to upregulation of the inhibitory molecules PD-L1 and PD-L2 on rapa-ECs. Additionally, rapa-ECs produced lower levels of the inflammatory cytokine IL-6. CD4+ memory cells activated by allogeneic rapa-ECs became hyporesponsive to restimulation in an alloantigen-specific manner and contained higher percentages of suppressive CD4+CD25(hi)CD127(lo)FoxP3+ cells that did not produce effector cytokines. In a human-mouse chimeric model of allograft rejection, rapamycin pretreatment of human arterial allografts increased graft EC expression of PD-L1 and PD-L2 and reduced subsequent infiltration of allogeneic effector T cells into the artery intima and intimal expansion. Preoperative conditioning of allograft ECs with rapamycin could potentially reduce immune-mediated rejection.
in vitro PD-1 neutralization
Singh, A., et al (2012). "Foxp3+ regulatory T cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN-gamma producing T cells" PLoS One 7(9): e44728.
PubMed
CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg) and programmed death-1 (PD-1) molecules have emerged as pivotal players in immune suppression of chronic diseases. However, their impact on the disease severity, therapeutic response and restoration of immune response in human tuberculosis remains unclear. Here, we describe the possible role of Treg cells, their M. tuberculosis driven expansion and contribution of PD-1 pathway to the suppressive function of Treg cells among pulmonary tuberculosis (PTB) patients. Multicolor flow cytometry, cell culture, cells sorting and ELISA were employed to execute the study. Our results showed significant increase in frequency of antigen-reactive Treg cells, which gradually declined during successful therapy and paralleled with decline of M. tuberculosis-specific IL-10 along with elevation of IFN-gamma production, and raising the IFN-gamma/IL-4 ratio. Interestingly, persistence of Treg cells tightly correlated with MDR tuberculosis. Also, we show that blocking PD-1/PD-L1 pathway abrogates Treg-mediated suppression, suggesting that the PD-1/PD-L1 pathway is required for Treg-mediated suppression of the antigen-specific T cells. Treg cells possibly play a role in dampening the effector immune response and abrogating PD-1 pathway on Treg cells significantly rescued protective T cell response, suggesting its importance in immune restoration among tuberculosis patients.
in vitro PD-1 neutralization
Rosignoli, G., et al (2009). "Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals" Clin Exp Immunol 157(1): 90-97.
PubMed
Human immunodeficiency virus (HIV)-1 causes T cell anergy and affects T cell maturation. Various mechanisms are responsible for impaired anti-HIV-1-specific responses: programmed death (PD)-1 molecule and its ligand PD-L1 are negative regulators of T cell activity and their expression is increased during HIV-1 infection. This study examines correlations between T cell maturation, expression of PD-1 and PD-L1, and the effects of their blockade. Peripheral blood mononuclear cells (PBMC) from 24 HIV-1(+) and 17 uninfected individuals were phenotyped for PD-1 and PD-L1 expression on CD4(+) and CD8(+) T cell subsets. The effect of PD-1 and PD-L1 blockade on proliferation and interferon (IFN)-gamma production was tested on eight HIV-1(+) patients. Naive (CCR7(+)CD45RA(+)) CD8(+) T cells were reduced in HIV-1 aviraemic (P = 0.0065) and viraemic patients (P = 0.0130); CD8 T effector memory subsets [CCR7(-)CD45RA(-)(T(EM))] were increased in HIV-1(+) aviraemic (P = 0.0122) and viraemic (P = 0.0023) individuals versus controls. PD-1 expression was increased in CD4 naive (P = 0.0496), central memory [CCR7(+)CD45RA(-) (T(CM)); P = 0.0116], T(EM) (P = 0.0037) and CD8 naive T cells (P = 0.0133) of aviraemic HIV-1(+) versus controls. PD-L1 was increased in CD4 T(EMRA) (CCR7(-)CD45RA(+), P = 0.0119), CD8 T(EM) (P = 0.0494) and CD8 T(EMRA) (P = 0.0282) of aviraemic HIV-1(+)versus controls. PD-1 blockade increased HIV-1-specific proliferative responses in one of eight patients, whereas PD-L1 blockade restored responses in four of eight patients, but did not increase IFN-gamma-production. Alteration of T cell subsets, accompanied by increased PD-1 and PD-L1 expression in HIV-1 infection contributes to anergy and impaired anti-HIV-1-specific responses which are not rescued when PD-1 is blocked, in contrast to when PD-L1 is blocked, due possibly to an ability to bind to receptors other than PD-1.
in vitro PD-1 neutralization
urado, J. O., et al (2008). "Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis" J Immunol 181(1): 116-125.
PubMed
Protective immunity against Mycobacterium tuberculosis requires the generation of cell-mediated immunity. We investigated the expression and role of programmed death 1 (PD-1) and its ligands, molecules known to modulate T cell activation, in the regulation of IFN-gamma production and lytic degranulation during human tuberculosis. We demonstrated that specific Ag-stimulation increased CD3+PD-1+ lymphocytes in peripheral blood and pleural fluid from tuberculosis patients in direct correlation with IFN-gamma production from these individuals. Moreover, M. tuberculosis-induced IFN-gamma participated in the up-regulation of PD-1 expression. Blockage of PD-1 or PD-1 and its ligands (PD-Ls: PD-L1, PD-L2) enhanced the specific degranulation of CD8+ T cells and the percentage of specific IFN-gamma-producing lymphocytes against the pathogen, demonstrating that the PD-1:PD-Ls pathway inhibits T cell effector functions during active M. tuberculosis infection. Furthermore, the simultaneous blockage of the inhibitory receptor PD-1 together with the activation of the costimulatory protein signaling lymphocytic activation molecule led to the promotion of protective IFN-gamma responses to M. tuberculosis, even in patients with weak cell-mediated immunity against the bacteria. Together, we demonstrated that PD-1 interferes with T cell effector functions against M. tuberculosis, suggesting that PD-1 has a key regulatory role during the immune response of the host to the pathogen.
Product Citations
-
-
Cancer Research
Humanized mouse models of KRAS-mutated colorectal and pancreatic cancers with HLA-class-I match for pre-clinical evaluation of cancer immunotherapies.
In Oncoimmunology on 1 December 2025 by Dávola, M. E., Cormier, O., et al.
PubMed
Cancer immunotherapy promises to treat challenging cancers including KRAS-mutated colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC). However, pre-clinical animal models that better mimic patient tumor and immune system interactions are required. While humanized mice are promising vehicles for pre-clinical immunotherapy testing, currently used cancer models retain limitations, such as lack of a human thymus for human leukocyte antigen (HLA)-based education of human T cells. As cytotoxic T lymphocyte (CTL) activity underlies many immunotherapies, we developed more clinically relevant KRAS-mutated CRC and PDAC humanized cancer models using transgenic NRG-A2 mice expressing HLA-A2.1 to enable HLA-class-I match between mouse tissues (including the thymus), the humanized immune system and human tumors. Using these novel humanized cancer models and a CTL-mediated combination (immuno)therapy with clinical potential, we were able to recapitulate the complexity and therapy-induced changes reported in patient biopsies, demonstrating the use of these HLA-matched models for pre-clinical validation of novel immunotherapies.
-
-
-
Immunology and Microbiology
-
Cancer Research
Enhanced anti-tumor efficacy of tumor-infiltrating lymphocytes by GITR agonist in ovarian cancer.
In Front Immunol on 24 November 2025 by Jung, D., Goh, A. R., et al.
PubMed
Adoptive cell therapy (ACT) using autologous tumor-infiltrating lymphocytes (TILs) is a personalized immunotherapy that has shown promising clinical results in various tumor types. Although TILs are associated with improved survival in patients with ovarian cancer (OC), their therapeutic efficacy remains limited. Therefore, novel strategies to enhance the anti-tumor activity of TILs are needed to improve outcomes in OC treatment.
-
-
-
Immunology and Microbiology
Novel anti-LAG-3 antibody LBL-007 with anti-PD-1 blockade enhances antitumor immunity by promoting T cell-induced apoptosis.
In Sci Rep on 27 October 2025 by Qin, K., Zhou, H., et al.
PubMed
Immune checkpoint combination therapy, particularly dual LAG-3/PD-1 blockade, demonstrates superior clinical efficacy over monotherapy in cancers like melanoma, yet its mechanistic synergy requires further validation. In this study, we established a cell co-culture model by co-culturing LAG-3+PD-1+ Jurkat cells induced by phytohemagglutinin (PHA) and human tumor cells with high expression of LAG-3 and PD-1 major ligands to characterize the combination effect of LBL-007 with anti-PD-1 antibodies and the mechanism of action in cancer immunotherapy. The results showed that the combination of LBL-007 and anti-PD-1 antibodies in the cell co-culture model enhanced the ability of activated Jurkat cells to kill tumor cells compared with monotherapy. Furthermore, this combination also inhibited the apoptosis of Jurkat cells and promoted IL-2, IL-10, and TNF secretion from Jurkat cells. Tumor cell death via apoptosis induced by activated Jurkat cells was observed, which was enhanced by combined LBL-007 and anti-PD-1 antibody treatment. The combination of LBL-007 and anti-PD-1 antibodies delayed tumor growth and promoted tumor cell apoptosis compared with monotherapy in human LAG-3 transgenic mice subjected to transplantation with colorectal tumor cells. Taken together, the combination of LBL-007 and anti-PD-1 antibodies plays an enhanced antitumor role by improving T cell viability and activity as well as by promoting T cell-induced apoptosis, thereby suggesting this combination as a potential effective strategy for cancer immunotherapy.
-
-
-
Immunology and Microbiology
-
Cancer Research
-
Biochemistry and Molecular biology
The transcription factor Blimp-1 is suppressed by SLAMF1 and drives Treg cell-mediated immune evasion in non-small cell lung cancer.
In BJC Rep on 21 October 2025 by Finotto, S., Trufa, D. I., et al.
PubMed
Immune checkpoint inhibitors targeting the interaction between PD1 and PDL1 are effective for immunotherapy in non-small cell lung cancer (NSCLC). However, only subgroups of patients respond to therapy, suggesting the existence of resistance mechanisms.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Cancer Research
-
Biochemistry and Molecular biology
SOX4-ZIP14-zinc metabolism mediates oncogenesis and suppresses T cell immunity in nasopharyngeal carcinoma.
In Cell Rep Med on 16 September 2025 by Yang, Y., Liu, Q., et al.
PubMed
Subtle variations of micronutrients in the tumor microenvironment often coincide with tumor progression and immune disorders. Nevertheless, the underlying mechanisms of how micronutrients, such as metal ions, influence tumor-intrinsic properties and tumor-immune crosstalk remain inadequately characterized. Here, our integrative analysis of multi-center single-cell, spatial transcriptome sequencing, and bulk RNA sequencing (RNA-seq) cohorts reveals that nasopharyngeal carcinoma (NPC)-specific SRY-box transcription factor 4 (SOX4) governs microenvironmental and cellular zinc metabolism through its downstream target, SLC39A14 (ZIP14), a membrane zinc uptake transporter. Mechanistically, NPC cells enhance zinc uptake and activate Wnt/β-catenin signaling to initiate tumor growth, creating a zinc-deficient niche hostile to T cells. Zinc deficiency of tumor-infiltrating CD8+ T cells impairs LCK phosphorylation and T cell receptor (TCR) signaling, compromising their effector function. Our study elucidates the idea that the SOX4-ZIP14-zinc metabolism axis has a multifactorial effect in NPC, fostering the malignant phenotypes of NPC and suppressing the T cell response through the deprivation of zinc metabolism.
-
-
-
Immunology and Microbiology
Identification and validation of intratumoral microbiome associated with sensitization to immune checkpoint inhibitors.
In Cell Rep Med on 16 September 2025 by Chen, J., Gao, Y., et al.
PubMed
As a part of the commensal microbiome, the regulatory role of the intratumoral microbiome in tumor immunity is gradually revealed. However, the relationship between the intratumoral microbiome and the efficacy of immune checkpoint inhibitors (ICIs) clinical treatment remains unclear. Here, we collect RNA sequencing (RNA-seq) data and clinical information from publicly available ICIs therapy cohorts. By developing an improved bioinformatics pipeline to identify the intratumoral microbiome and performing a comprehensive association analysis, we find that the intratumoral microbiome is associated with response to ICIs and characteristics of the tumor microenvironment (TME). In vivo experiments demonstrate that intratumoral injection of Burkholderia cepacia, Priestia megaterium, or Corynebacterium kroppenstedtii, which were selected from our analysis results, would synergize with anti-PD-1 therapy to inhibit tumor growth and enhance antitumor immunity. Our findings highlight the essential role of the intratumoral microbiome in the clinical effectiveness differences of ICIs, suggesting its potential in future ICIs combination therapy.
-
-
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Genetics
-
Cancer Research
-
Biochemistry and Molecular biology
WTAP Accelerates Exhaustion of CD8+ T Cells and Progression of Hepatocellular Carcinoma by Promoting m6A Modification and Translation of PD1 mRNA.
In Mediators Inflamm on 26 June 2025 by Li, R., Li, S., et al.
PubMed
The N6-methyladenosine (m6A) methylase WTAP has been identified as a proto-oncogene in multiple cancers, including hepatocellular carcinoma (HCC). Interestingly, although WTAP expression does not differ between normal liver and HCC tissues or across different stages of HCC, patients with higher WTAP expression exhibit significantly shorter median survival times (MSTs). Here, we found that WTAP was upregulated in tumor-infiltrating CD8+ T cells, which were more enriched in HCC patients compared to the controls. HCC patients also displayed higher PD1 levels and a greater proportion of exhausted CD8+ T cells (TCF+ PD1+). Moreover, WTAP promoted PD1 expression and suppressed the proliferation and immune activity of CD8+ T cells. In the co-culture system, WTAP-overexpressing CD8+ T cells enhanced the malignancy of HCC cells. Notably, WTAP silencing further augmented the boosting effect of PD1 silencing on CD8+ T cell immune activity and strengthened its inhibitory effect on HCC cell growth. As an m6A "writer", WTAP increased the m6A level of PD1 mRNA, thereby promoting YTHDF1-mediated translation of PD1. Finally, in the HuNSG xenograft tumor model, WTAP knockdown not only alleviated CD8+ T cell exhaustion and inhibited tumor progression but also synergistically enhanced the antitumor efficacy of anti-PD1 therapy. In conclusion, WTAP promoted CD8+ T cell exhaustion and HCC progression by facilitating the m6A modification and translation of PD1 mRNA.
-
-
-
Cancer Research
-
Immunology and Microbiology
Enhancing T-cell recruitment in renal cell carcinoma with cytokine-armed adenoviruses.
In Oncoimmunology on 1 October 2024 by Feodoroff, M., Hamdan, F., et al.
PubMed
Immunotherapy has emerged as a promising approach for cancer treatment, with oncolytic adenoviruses showing power as immunotherapeutic agents. In this study, we investigated the immunotherapeutic potential of an adenovirus construct expressing CXCL9, CXCL10, or IL-15 in clear cell renal cell carcinoma (ccRCC) tumor models. Our results demonstrated robust cytokine secretion upon viral treatment, suggesting effective transgene expression. Subsequent analysis using resistance-based transwell migration and microfluidic chip assays demonstrated increased T-cell migration in response to chemokine secretion by infected cells in both 2D and 3D cell models. Flow cytometry analysis revealed CXCR3 receptor expression across T-cell subsets, with the highest percentage found on CD8+ T-cells, underscoring their key role in immune cell migration. Alongside T-cells, we also detected NK-cells in the tumors of immunocompromised mice treated with cytokine-encoding adenoviruses. Furthermore, we identified potential immunogenic antigens that may enhance the efficacy and specificity of our armed oncolytic adenoviruses in ccRCC. Overall, our findings using ccRCC cell line, in vivo humanized mice, physiologically relevant PDCs in 2D and patient-derived organoids (PDOs) in 3D suggest that chemokine-armed adenoviruses hold promise for enhancing T-cell migration and improving immunotherapy outcomes in ccRCC. Our study contributes to the development of more effective ccRCC treatment strategies by elucidating immune cell infiltration and activation mechanisms within the tumor microenvironment (TME) and highlights the usefulness of PDOs for predicting clinical relevance and validating novel immunotherapeutic approaches. Overall, our research offers insights into the rational design and optimization of viral-based immunotherapies for ccRCC.
-
-
-
Cancer Research
-
Cell Biology
Tumor cell-intrinsic PD-1 promotes Merkel cell carcinoma growth by activating downstream mTOR-mitochondrial ROS signaling.
In Sci Adv on 19 January 2024 by Martins, C., Rasbach, E., et al.
PubMed
Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer. Inhibitors targeting the programmed cell death 1 (PD-1) immune checkpoint have improved MCC patient outcomes by boosting antitumor T cell immunity. Here, we identify PD-1 as a growth-promoting receptor intrinsic to MCC cells. In human MCC lines and clinical tumors, RT-PCR-based sequencing, immunoblotting, flow cytometry, and immunofluorescence analyses demonstrated PD-1 gene and protein expression by MCC cells. MCC-PD-1 ligation enhanced, and its inhibition or silencing suppressed, in vitro proliferation and in vivo tumor xenograft growth. Consistently, MCC-PD-1 binding to PD-L1 or PD-L2 induced, while antibody-mediated PD-1 blockade inhibited, protumorigenic mTOR signaling, mitochondrial (mt) respiration, and ROS generation. Last, pharmacologic inhibition of mTOR or mtROS reversed MCC-PD-1:PD-L1-dependent proliferation and synergized with PD-1 checkpoint blockade in suppressing tumorigenesis. Our results identify an MCC-PD-1-mTOR-mtROS axis as a tumor growth-accelerating mechanism, the blockade of which might contribute to clinical response in patients with MCC.
-
-
-
Cell Biology
-
Immunology and Microbiology
Genome scale CRISPR screens identify actin capping proteins as key modulators of therapeutic responses to radiation and immunotherapy
In bioRxiv on 15 January 2024 by Verma, N., Renauer, P. A., et al.
-
-
-
Immunology and Microbiology
-
Cancer Research
Genomic Profiling of Radiation-Induced Sarcomas Reveals the Immunologic Characteristics and Its Response to Immune Checkpoint Blockade.
In Clin Cancer Res on 1 August 2023 by Hong, D. C., Yang, J., et al.
PubMed
Radiation-induced sarcomas (RIS) have a poor prognosis and lack effective treatments. Its genome and tumor microenvironment are not well characterized and need further exploration.
-
-
-
Cancer Research
Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma.
In JCI Insight on 10 April 2023 by Tran, T. T., Caulfield, J., et al.
PubMed
Targeting tumor-associated blood vessels to increase immune infiltration may enhance treatment effectiveness, yet limited data exist regarding anti-angiogenesis effects on the tumor microenvironment (TME). We hypothesized that dual targeting of angiogenesis with immune checkpoints would improve both intracranial and extracranial disease. We used subcutaneous and left ventricle melanoma models to evaluate anti-PD-1/anti-VEGF and anti-PD-1/lenvatinib (pan-VEGFR inhibitor) combinations. Cytokine/chemokine profiling and flow cytometry were performed to assess signaling and immune-infiltrating populations. An in vitro blood-brain barrier (BBB) model was utilized to study intracranial treatment effects on endothelial integrity and leukocyte transmigration. Anti-PD-1 with either anti-VEGF or lenvatinib improved survival and decreased tumor growth in systemic melanoma murine models; treatment increased Th1 cytokine/chemokine signaling. Lenvatinib decreased tumor-associated macrophages but increased plasmacytoid DCs early in treatment; this effect was not evident with anti-VEGF. Both lenvatinib and anti-VEGF resulted in decreased intratumoral blood vessels. Although anti-VEGF promoted endothelial stabilization in an in vitro BBB model, while lenvatinib did not, both regimens enabled leukocyte transmigration. The combined targeting of PD-1 and VEGF or its receptors promotes enhanced melanoma antitumor activity, yet their effects on the TME are quite different. These studies provide insights into dual anti-PD-1 and anti-angiogenesis combinations.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Low-density lipoprotein balances T cell metabolism and enhances response to anti-PD-1 blockade in a HCT116 spheroid model.
In Front Oncol on 14 February 2023 by Babl, N., Hofbauer, J., et al.
PubMed
The discovery of immune checkpoints and the development of their specific inhibitors was acclaimed as a major breakthrough in cancer therapy. However, only a limited patient cohort shows sufficient response to therapy. Hence, there is a need for identifying new checkpoints and predictive biomarkers with the objective of overcoming immune escape and resistance to treatment. Having been associated with both, treatment response and failure, LDL seems to be a double-edged sword in anti-PD1 immunotherapy. Being embedded into complex metabolic conditions, the impact of LDL on distinct immune cells has not been sufficiently addressed. Revealing the effects of LDL on T cell performance in tumor immunity may enable individual treatment adjustments in order to enhance the response to routinely administered immunotherapies in different patient populations. The object of this work was to investigate the effect of LDL on T cell activation and tumor immunity in-vitro.
-
-
-
Cancer Research
-
Immunology and Microbiology
Preclinical Platform Using a Triple-negative Breast Cancer Syngeneic Murine Model to Evaluate Immune Checkpoint Inhibitors.
In Anticancer Res on 1 January 2023 by Katuwal, N. B., Park, N., et al.
PubMed
To evaluate the feasibility of syngeneic mouse models of breast cancer by analyzing the efficacy of immune checkpoint inhibitors (ICIs) and potential predictive biomarkers.
-
-
-
Cancer Research
-
Immunology and Microbiology
FGFR4-Targeted Chimeric Antigen Receptors Combined with Anti-Myeloid Polypharmacy Effectively Treat Orthotopic Rhabdomyosarcoma.
In Mol Cancer Ther on 7 October 2022 by Sullivan, P. M., Kumar, R., et al.
PubMed
Rhabdomyosarcoma (RMS) is the most common soft tissue cancer in children. Treatment outcomes, particularly for relapsed/refractory or metastatic disease, have not improved in decades. The current lack of novel therapies and low tumor mutational burden suggest that chimeric antigen receptor (CAR) T-cell therapy could be a promising approach to treating RMS. Previous work identified FGF receptor 4 (FGFR4, CD334) as being specifically upregulated in RMS, making it a candidate target for CAR T cells. We tested the feasibility of an FGFR4-targeted CAR for treating RMS using an NSG mouse with RH30 orthotopic (intramuscular) tumors. The first barrier we noted was that RMS tumors produce a collagen-rich stroma, replete with immunosuppressive myeloid cells, when T-cell therapy is initiated. This stromal response is not seen in tumor-only xenografts. When scFV-based binders were selected from phage display, CARs targeting FGFR4 were not effective until our screening approach was refined to identify binders to the membrane-proximal domain of FGFR4. Having improved the CAR, we devised a pharmacologic strategy to augment CAR T-cell activity by inhibiting the myeloid component of the T-cell-induced tumor stroma. The combined treatment of mice with anti-myeloid polypharmacy (targeting CSF1R, IDO1, iNOS, TGFbeta, PDL1, MIF, and myeloid misdifferentiation) allowed FGFR4 CAR T cells to successfully clear orthotopic RMS tumors, demonstrating that RMS tumors, even with very low copy-number targets, can be targeted by CAR T cells upon reversal of an immunosuppressive microenvironment.
-
-
-
Immunology and Microbiology
Targeting cathepsin B by cycloastragenol enhances antitumor immunity of CD8 T cells via inhibiting MHC-I degradation.
In J Immunother Cancer on 1 October 2022 by Deng, G., Zhou, L., et al.
PubMed
The loss of tumor antigens and depletion of CD8 T cells caused by the PD-1/PD-L1 pathway are important factors for tumor immune escape. In recent years, there has been increasing research on traditional Chinese medicine in tumor treatment. Cycloastragenol (CAG), an effective active molecule in Astragalus membranaceus, has been found to have antiviral, anti-aging, anti-inflammatory, and other functions. However, its antitumor effect and mechanism are not clear.
-
-
-
Cancer Research
-
Immunology and Microbiology
Preclinical platform using a triple-negative breast cancer syngeneic murine model to evaluate immune checkpoint inhibitors
In Research Square on 8 September 2022 by Bahadur, N. B., Park, N., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Breast cancer cell-derived extracellular vesicles promote CD8+ T cell exhaustion via TGF-β type II receptor signaling.
In Nat Commun on 1 August 2022 by Xie, F., Zhou, X., et al.
PubMed
Cancer immunotherapies have shown clinical success in various types of tumors but the patient response rate is low, particularly in breast cancer. Here we report that malignant breast cancer cells can transfer active TGF-β type II receptor (TβRII) via tumor-derived extracellular vesicles (TEV) and thereby stimulate TGF-β signaling in recipient cells. Up-take of extracellular vesicle-TβRII (EV-TβRII) in low-grade tumor cells initiates epithelial-to-mesenchymal transition (EMT), thus reinforcing cancer stemness and increasing metastasis in intracardial xenograft and orthotopic transplantation models. EV-TβRII delivered as cargo to CD8+ T cells induces the activation of SMAD3 which we demonstrated to associate and cooperate with TCF1 transcription factor to impose CD8+ T cell exhaustion, resulting in failure of immunotherapy. The levels of TβRII+ circulating extracellular vesicles (crEV) appears to correlate with tumor burden, metastasis and patient survival, thereby serve as a non-invasive screening tool to detect malignant breast tumor stages. Thus, our findings not only identify a possible mechanism by which breast cancer cells can promote T cell exhaustion and dampen host anti-tumor immunity, but may also identify a target for immune therapy against the most devastating breast tumors.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
IFNα Potentiates Anti-PD-1 Efficacy by Remodeling Glucose Metabolism in the Hepatocellular Carcinoma Microenvironment.
In Cancer Discov on 6 July 2022 by Hu, B., Yu, M., et al.
PubMed
The overall response rate for anti-PD-1 therapy remains modest in hepatocellular carcinoma (HCC). We found that a combination of IFNα and anti-PD-1-based immunotherapy resulted in enhanced antitumor activity in patients with unresectable HCC. In both immunocompetent orthotopic and spontaneous HCC models, IFNα therapy synergized with anti-PD-1 and the combination treatment led to significant enrichment of cytotoxic CD27+CD8+ T cells. Mechanistically, IFNα suppressed HIF1α signaling by inhibiting FosB transcription in HCC cells, resulting in reduced glucose consumption capacity and consequentially establishing a high-glucose microenvironment that fostered transcription of the T-cell costimulatory molecule Cd27 via mTOR-FOXM1 signaling in infiltrating CD8+ T cells. Together, these data reveal that IFNα reprograms glucose metabolism within the HCC tumor microenvironment, thereby liberating T-cell cytotoxic capacities and potentiating the PD-1 blockade-induced immune response. Our findings suggest that IFNα and anti-PD-1 cotreatment is an effective novel combination strategy for patients with HCC.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
In vivo experiments
-
In vivo experiments
-
In vivo experiments
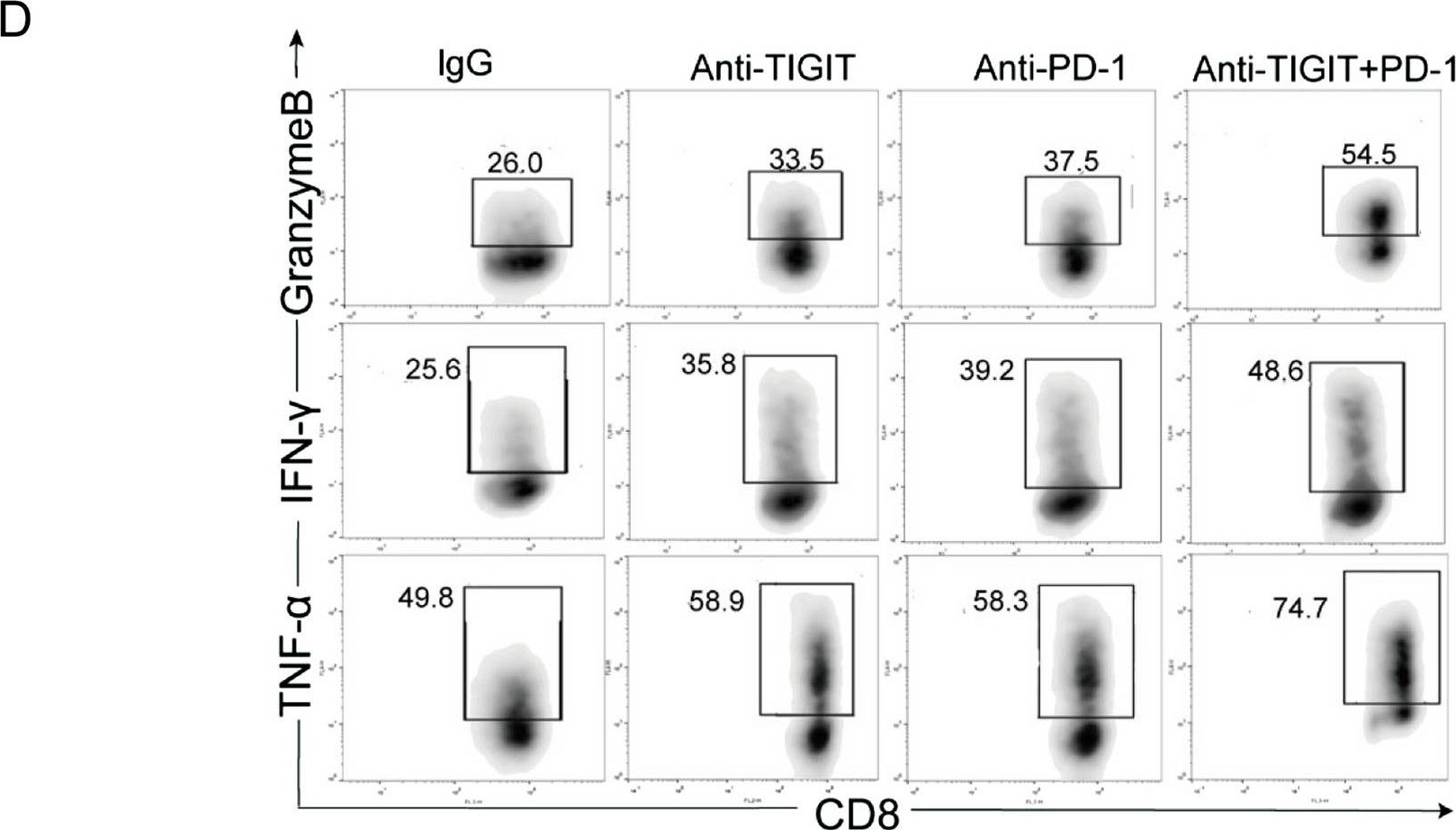
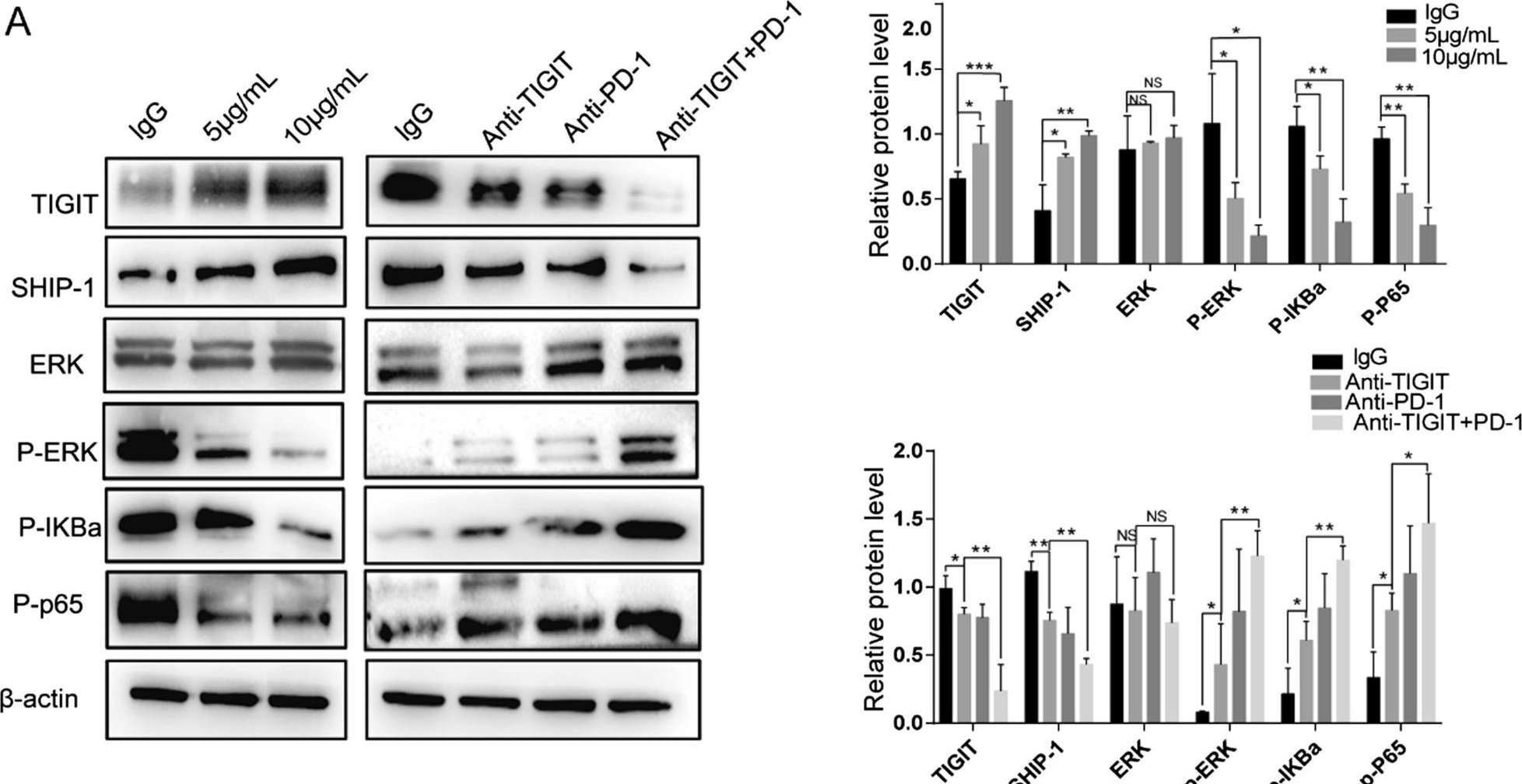
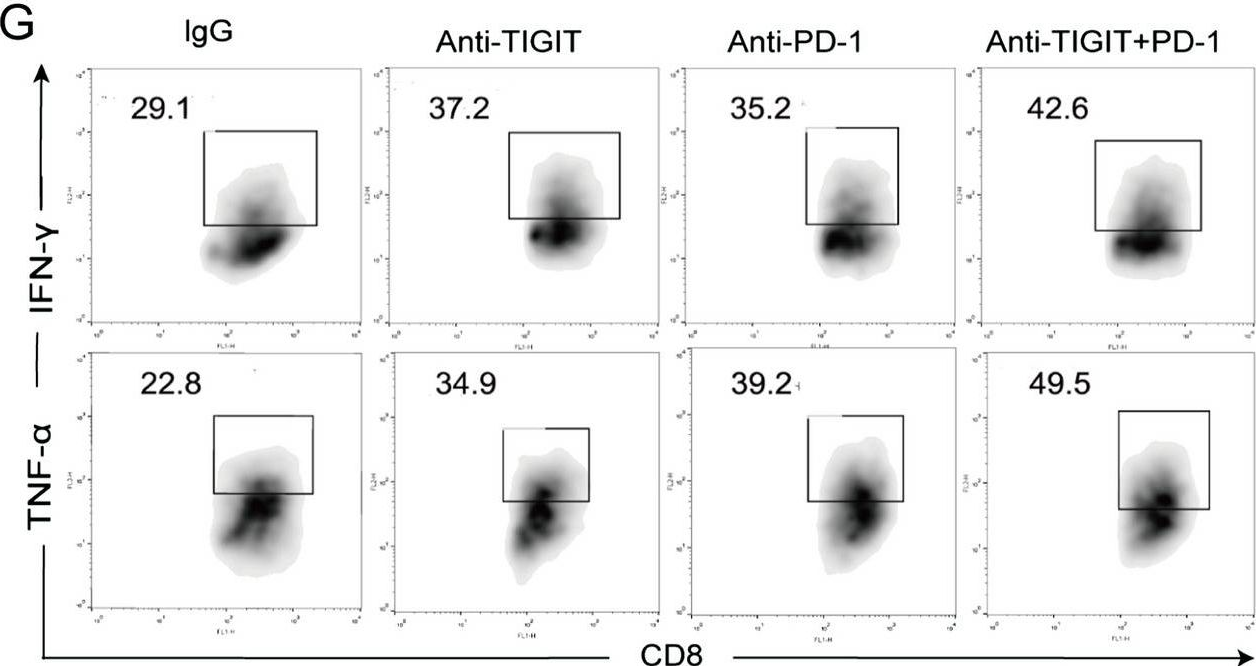
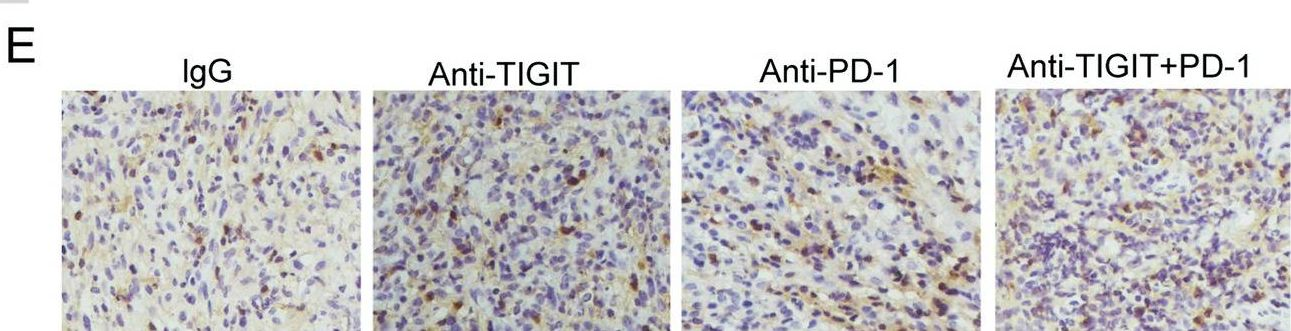
Blocking TIGIT/CD155 signalling reverses CD8+ T cell exhaustion and enhances the antitumor activity in cervical cancer.
In J Transl Med on 21 June 2022 by Liu, L., Wang, A., et al.
PubMed
TIGIT/CD155 has attracted widespread attention as a new immune checkpoint and a potential target for cancer immunotherapy. In our study, we evaluated the role of TIGIT/CD155 checkpoints in the progression of cervical cancer.
-