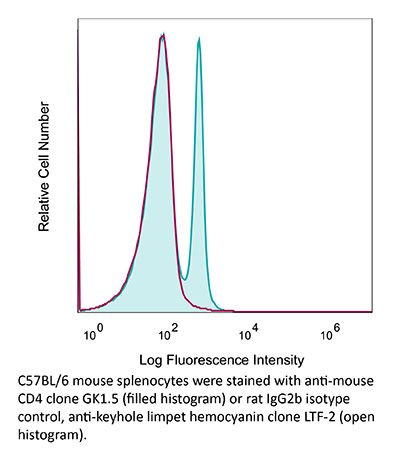
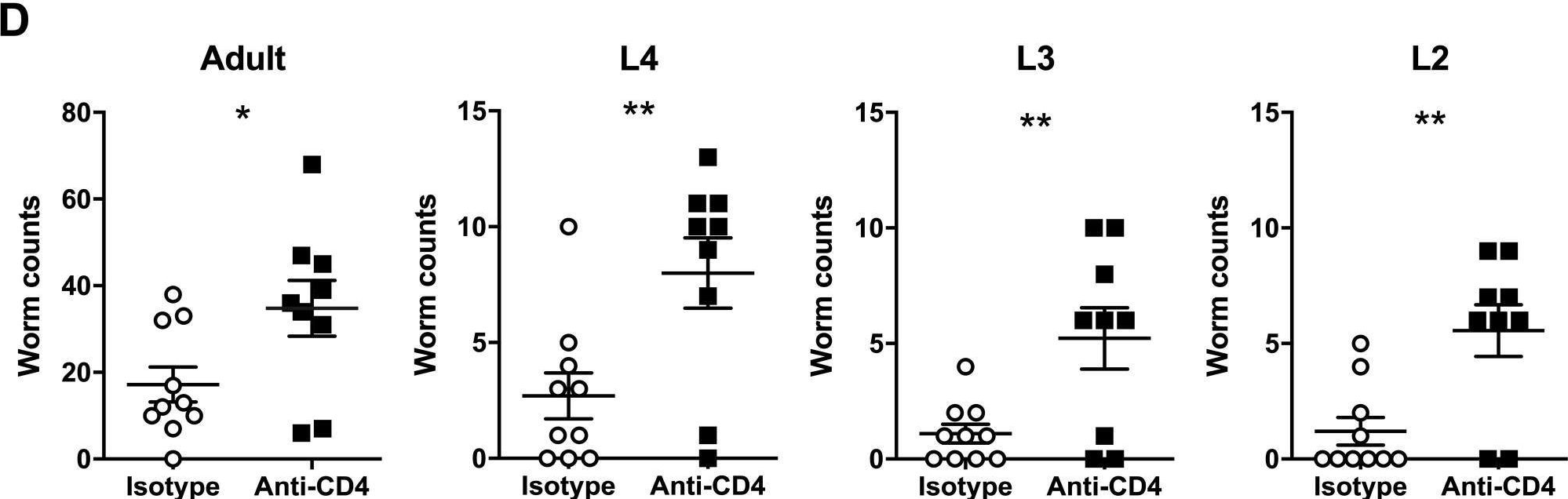
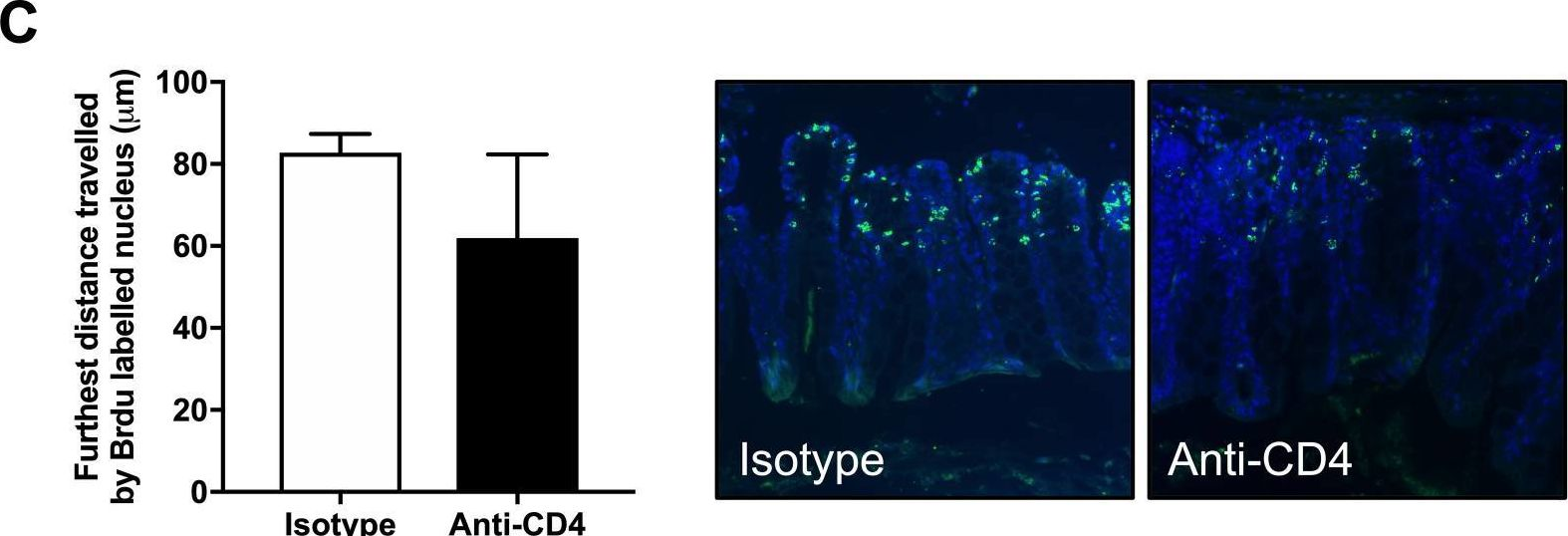
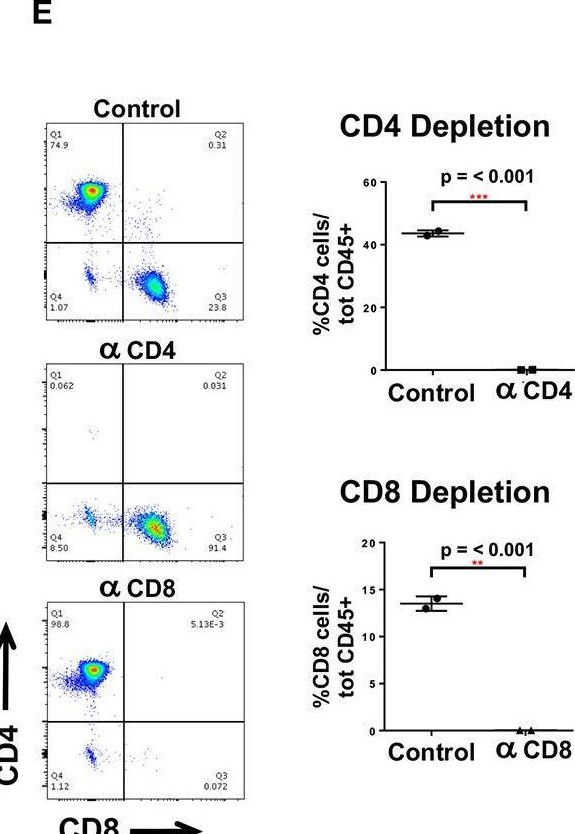
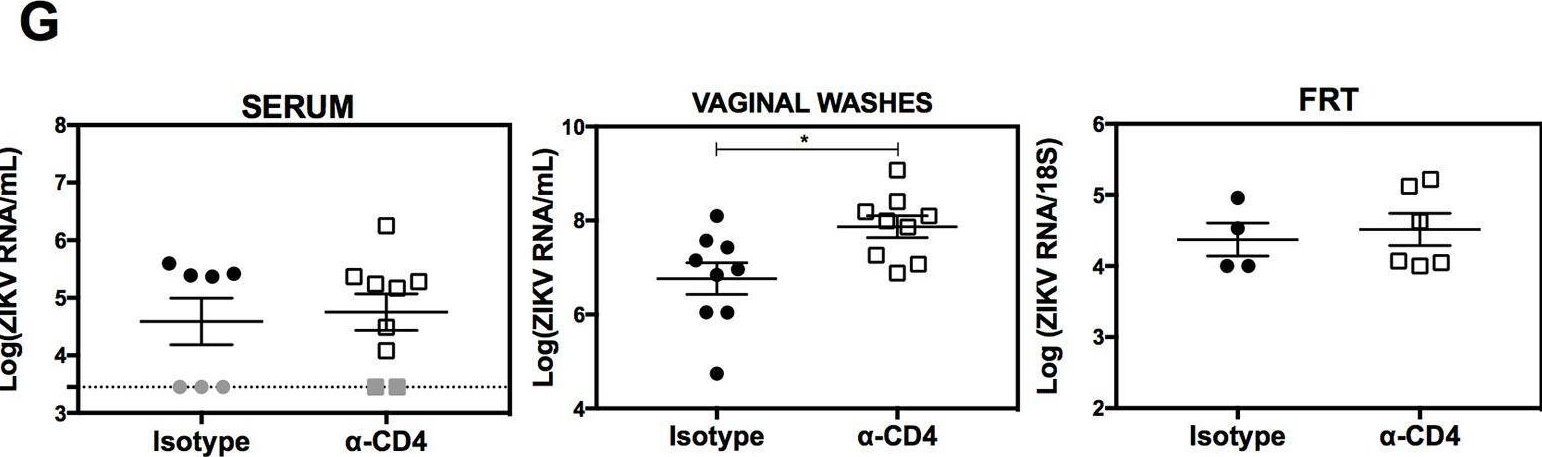
InVivoMAb anti-mouse CD4
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CTL clone V4 |
| Reported Applications |
in vivo CD4+ T cell depletion Flow cytometry Western blot |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107636 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD4+ T cell depletion
Budda, S. A. and L. A. Zenewicz (2018). "IL-22 deficiency increases CD4 T cell responses to mucosal immunization" Vaccine 36(25): 3694-3700.
PubMed
Mucosal vaccines are a promising platform for combatting infectious diseases for which we still lack effective preventative measures. Optimizing these vaccines to generate the best protective immune responses with the least complicated immunization regimen is imperative. Mucosal barriers are the first line of defense against many pathogens and, as such, we looked to their biology for strategies to improve vaccine delivery. Interleukin-22 (IL-22) is a key cytokine in both healthy and inflamed mucosal tissues. IL-22 promotes epithelial cell proliferation and inhibits apoptosis, upregulates mucin and antimicrobial peptides, all of which promote mucosal barrier integrity. In this study, we find that IL-22 impairs the development of a T cell response during mucosal immunization. Compared to wild-type control mice, IL-22 deficient mice had increased antigen-specific CD4 T cell responses to intrarectal immunization using a protein and cholera toxin adjuvant vaccine. When immunized systemically with the same protein antigen adsorbed to alum, no differences in the CD4 T cell response between wild-type and IL-22 deficient mice were detected. This suggests that transiently inhibiting IL-22 during mucosal vaccination could enhance T cell responses. The broad-applicability of this proposed approach would allow for improvement of many existing mucosal vaccine regimens and have positive implications in the development of more efficacious mucosal vaccines.
in vivo CD4+ T cell depletion
Flow Cytometry
Balogh, K. N., et al (2018). "Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses" PLoS One 13(6): e0197702.
PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo CD4+ T cell depletion
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo CD4+ T cell depletion
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo CD4+ T cell depletion
Flow Cytometry
Liu, G., et al (2015). "IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4+ T Cells and IFN-gamma" PLoS Pathog 11(7): e1005065.
PubMed
African trypanosomes are extracellular protozoan parasites causing a chronic debilitating disease associated with a persistent inflammatory response. Maintaining the balance of the inflammatory response via downregulation of activation of M1-type myeloid cells was previously shown to be crucial to allow prolonged survival. Here we demonstrate that infection with African trypanosomes of IL-27 receptor-deficient (IL-27R-/-) mice results in severe liver immunopathology and dramatically reduced survival as compared to wild-type mice. This coincides with the development of an exacerbated Th1-mediated immune response with overactivation of CD4+ T cells and strongly enhanced production of inflammatory cytokines including IFN-gamma. What is important is that IL-10 production was not impaired in infected IL-27R-/- mice. Depletion of CD4+ T cells in infected IL-27R-/- mice resulted in a dramatically reduced production of IFN-gamma, preventing the early mortality of infected IL-27R-/- mice. This was accompanied by a significantly reduced inflammatory response and a major amelioration of liver pathology. These results could be mimicked by treating IL-27R-/- mice with a neutralizing anti-IFN-gamma antibody. Thus, our data identify IL-27 signaling as a novel pathway to prevent early mortality via inhibiting hyperactivation of CD4+ Th1 cells and their excessive secretion of IFN-gamma during infection with African trypanosomes. These data are the first to demonstrate the essential role of IL-27 signaling in regulating immune responses to extracellular protozoan infections.
in vivo CD4+ T cell depletion
Kim, J., et al (2015). "Memory programming in CD8(+) T-cell differentiation is intrinsic and is not determined by CD4 help" Nat Commun 6: 7994.
PubMed
CD8(+) T cells activated without CD4(+) T-cell help are impaired in memory expansion. To understand the underlying cellular mechanism, here we track the dynamics of helper-deficient CD8(+) T-cell response to a minor histocompatibility antigen by phenotypic and in vivo imaging analyses. Helper-deficient CD8(+) T cells show reduced burst expansion, rapid peripheral egress, delayed antigen clearance and continuous activation, and are eventually exhausted. Contrary to the general consensus that CD4 help encodes memory programmes in CD8(+) T cells and helper-deficient CD8(+) T cells are abortive, these cells can differentiate into effectors and memory precursors. Importantly, accelerating antigen clearance or simply increasing the burst effector size enables generation of memory cells by CD8(+) T cells, regardless of CD4 help. These results suggest that the memory programme is CD8(+) T-cell-intrinsic, and provide insight into the role of CD4 help in CD8(+) T-cell responses.
in vivo CD4+ T cell depletion
Guo, L., et al (2015). "Innate immunological function of TH2 cells in vivo" Nat Immunol 16(10): 1051-1059.
PubMed
Type 2 helper T cells (TH2 cells) produce interleukin 13 (IL-13) when stimulated by papain or house dust mite extract (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2 cells) are the dominant innate producers of IL-13 in naive mice, we found here that helminth-infected mice had more TH2 cells compared to uninfected mice, and thes e cells became major mediators of innate type 2 responses. TH2 cells made important contributions to HDM-induced antigen-nonspecific eosinophilic inflammation and protected mice recovering from infection with Ascaris suum against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role for effector TH2 cells during TCR-independent innate-like immune responses.
in vivo CD4+ T cell depletion
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
Evans, E. E., et al (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo CD4+ T cell depletion
Vanpouille-Box, C., et al (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo CD4+ T cell depletion
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo CD4+ T cell depletion
Church, S. E., et al (2014). "Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells" Eur J Immunol 44(1): 69-79.
PubMed
Immunotherapies that augment antitumor T cells have had recent success for treating patients with cancer. Here we examined whether tumor-specific CD4(+) T cells enhance CD8(+) T-cell adoptive immunotherapy in a lymphopenic environment. Our model employed physiological doses of tyrosinase-related protein 1-specific CD4(+) transgenic T cells-CD4(+) T cells and pmel-CD8(+) T cells that when transferred individually were subtherapeutic; however, when transferred together provided significant (p
in vivo CD4+ T cell depletion
Krupnick, A. S., et al (2014). "Central memory CD8+ T lymphocytes mediate lung allograft acceptance" J Clin Invest 124(3): 1130-1143.
PubMed
Memory T lymphocytes are commonly viewed as a major barrier for long-term survival of organ allografts and are thought to accelerate rejection responses due to their rapid infiltration into allografts, low threshold for activation, and ability to produce inflammatory mediators. Because memory T cells are usually associated with rejection, preclinical protocols have been developed to target this population in transplant recipients. Here, using a murine model, we found that costimulatory blockade-mediated lung allograft acceptance depended on the rapid infiltration of the graft by central memory CD8+ T cells (CD44(hi)CD62L(hi)CCR7+). Chemokine receptor signaling and alloantigen recognition were required for trafficking of these memory T cells to lung allografts. Intravital 2-photon imaging revealed that CCR7 expression on CD8+ T cells was critical for formation of stable synapses with antigen-presenting cells, resulting in IFN-gamma production, which induced NO and downregulated alloimmune responses. Thus, we describe a critical role for CD8+ central memory T cells in lung allograft acceptance and highlight the need for tailored approaches for tolerance induction in the lung.
in vivo CD4+ T cell depletion
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo CD4+ T cell depletion
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
in vivo CD4+ T cell depletion
Flow Cytometry
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo CD4+ T cell depletion
Butler, N. S., et al (2012). "Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection" Nat Immunol 13(2): 188-195.
PubMed
Infection of erythrocytes with Plasmodium species induces clinical malaria. Parasite-specific CD4(+) T cells correlate with lower parasite burdens and severity of human malaria and are needed to control blood-stage infection in mice. However, the characteristics of CD4(+) T cells that determine protection or parasite persistence remain unknown. Here we show that infection of humans with Plasmodium falciparum resulted in higher expression of the inhibitory receptor PD-1 associated with T cell dysfunction. In vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4(+) T cell function, amplified the number of follicular helper T cells and germinal-center B cells and plasmablasts, enhanced protective antibodies and rapidly cleared blood-stage malaria in mice. Thus, chronic malaria drives specific T cell dysfunction, and proper function can be restored by inhibitory therapies to enhance parasite control.
in vivo CD4+ T cell depletion
Flow Cytometry
Krieg, C., et al (2010). "Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells" Proc Natl Acad Sci U S A 107(26): 11906-11911.
PubMed
IL-2 immunotherapy is an attractive treatment option for certain metastatic cancers. However, administration of IL-2 to patients can lead, by ill-defined mechanisms, to toxic adverse effects including severe pulmonary edema. Here, we show that IL-2-induced pulmonary edema is caused by direct interaction of IL-2 with functional IL-2 receptors (IL-2R) on lung endothelial cells in vivo. Treatment of mice with high-dose IL-2 led to efficient expansion of effector immune cells expressing high levels of IL-2Rbetagamma, including CD8(+) T cells and natural killer cells, which resulted in a considerable antitumor response against s.c. and pulmonary B16 melanoma nodules. However, high-dose IL-2 treatment also affected immune cell lineage marker-negative CD31(+) pulmonary endothelial cells via binding to functional alphabetagamma IL-2Rs, expressed at low to intermediate levels on these cells, thus causing pulmonary edema. Notably, IL-2-mediated pulmonary edema was abrogated by a blocking antibody to IL-2Ralpha (CD25), genetic disruption of CD25, or the use of IL-2Rbetagamma-directed IL-2/anti-IL-2 antibody complexes, thereby interfering with IL-2 binding to IL-2Ralphabetagamma(+) pulmonary endothelial cells. Moreover, IL-2/anti-IL-2 antibody complexes led to vigorous activation of IL-2Rbetagamma(+) effector immune cells, which generated a dramatic antitumor response. Thus, IL-2/anti-IL-2 antibody complexes might improve current strategies of IL-2-based tumor immunotherapy.
Product Citations
-
-
Genetics
-
Immunology and Microbiology
mRNA vaccine expressing enterovirus D68 virus-like particles induces potent neutralizing antibodies and protects against infection.
In Mol Ther Nucleic Acids on 9 December 2025 by Kunishima, Y., Senpuku, K., et al.
PubMed
Enterovirus D68 (EV-D68) causes respiratory illness in children. It also causes severe paralysis called acute flaccid myelitis (AFM), which has become a global health threat. Here, we generated an mRNA vaccine expressing virus-like particles (VLPs) of EV-D68. We found that the mRNA vaccine elicited potent neutralizing antibodies against EV-D68 in the blood, and the neutralizing titer was superior to that of the inactivated whole virion (IWV) vaccine. The mRNA vaccine showed protective effects against intranasal challenge with EV-D68, and antisera from the vaccinated mice prevented the paralysis caused by EV-D68 infection in neonatal mice. Moreover, the mRNA vaccine induced neutralizing antibodies in the respiratory tract, which is the entry site for EV-D68. Additionally, it attenuated infection with coxsackievirus B3 (CVB3), which belongs to another enterovirus group, via CD8+ T cell responses. In conclusion, our results suggest that this mRNA vaccine is a promising candidate for EV-D68 prevention.
-
-
-
Immunology and Microbiology
Salmonella-superspreader hosts require gut regulatory T cells to maintain a disease-tolerant state.
In J Exp Med on 3 November 2025 by Di Luccia, B., Massis, L. M., et al.
PubMed
Host-pathogen interactions involve two critical strategies: resistance, whereby hosts clear invading microbes, and tolerance, whereby hosts carry high pathogen burden asymptomatically. Here, we investigate mechanisms by which Salmonella-superspreader (SSP) hosts maintain an asymptomatic state during chronic infection. We found that regulatory T cells (Tregs) are essential for this disease-tolerant state, limiting intestinal immunopathology and enabling SSP hosts to thrive, while facilitating Salmonella transmission. Treg depletion in SSP mice resulted in decreased survival, heightened gut inflammation, and impairment of the intestinal barrier, without affecting Salmonella persistence. Colonic Tregs from SSP mice exhibited a unique transcriptomic profile characterized by the upregulation of type 1 inflammatory genes, including the transcription factor T-bet. In the absence of Tregs, we observed robust expansion of cytotoxic CD4+ T cells, with CD4+ T cell depletion restoring homeostasis. These results uncover a critical host strategy to establish disease tolerance during chronic enteric infection, providing novel insights into mucosal responses to persistent pathogens and chronic intestinal inflammation.
-
-
-
Pathology
-
Neuroscience
-
Immunology and Microbiology
Stage-specific roles of clonally expanded CD8+ T cells in regulating amyloid pathology in Alzheimer's disease models.
In Nat Commun on 27 October 2025 by Ohyagi, M., Ito, M., et al.
PubMed
Clonally expanded CD8+ T cells may contribute to Alzheimer's disease (AD) pathology through interactions with brain-resident cells. However, the functional impact of AD-specific T cell receptor (TCR) clonotypes remains unclear. Here, we demonstrate that CD8+ T cells undergo clonal expansion in early-stage AD mouse models, AppNL-G-F and 5xFAD, and that their depletion reduces amyloid plaque accumulation. Expanded TCR-expressing CD8+ T cells preferentially infiltrate the brain, exacerbating plaque deposition. Moreover, brain-infiltrating CD8+ T cells impair microglial transition into disease-associated states, suppressing amyloid clearance via CCL5-CCR5 signaling. Pharmacological blockade of CCL5 attenuates amyloid deposition, whereas CCL5 administration aggravates pathology. Notably, T cell depletion at later disease stages exacerbates amyloid pathology, suggesting a temporal shift in their function. Early-stage CD8+ T cells exhibit cytotoxic and effector profiles, whereas late-stage cells acquire tissue-resident and exhausted phenotypes. This temporal switch-from pathogenic to protective roles-highlights the stage-specific contribution of CD8+ T cells to AD and their potential as therapeutic targets.
-
-
-
Immunology and Microbiology
Limited nasal IFN production contributes to delayed respiratory virus clearance and suboptimal vaccine responses.
In JCI Insight on 22 October 2025 by Sojati, J., Parks, O. B., et al.
PubMed
Acute lower respiratory infections are the primary cause of global mortality in postneonatal children. Most respiratory viruses primarily involve upper airway infection and inflammation, yet nasal responses are poorly characterized. Using a mouse model of human metapneumovirus (HMPV), we found viral burden was higher in nasal airways and exhibited delayed clearance. Despite high burden, there was low nasal expression of type I and III interferon (IFN). Single-cell RNA-sequencing (scRNA-Seq) from HMPV-infected mice showed lower nasal IFN-stimulated gene (ISG) expression and nasal enrichment of genes negatively regulating IFN. scRNA-Seq of patients with COVID-19 verified lower ISG expression in upper airways. HMPV infection downregulated nasal expression of IFN regulatory factor 3, suggesting a mechanism for limited response. To rescue the quiescent environment, we administered type I or III IFN to upper airways early postinfection, leading to lower nasal HMPV titer and virus-specific CD8+ T cell upregulation. Intranasal immunization adjuvanted with type I or III IFN improved immune response, reduced clinical disease, and enhanced viral clearance in HMPV and influenza infection. IFN adjuvant increased recruitment of dendritic cells, recruitment of resident memory T cells, and neutralizing antibodies. These findings reveal locally suppressed IFN production contributes to a quiescent nasal immune landscape that delays viral clearance and impairs mucosal vaccine responses.
-
-
-
Immunology and Microbiology
-
Cancer Research
αTIGIT-IL2 achieves tumor regression by promoting tumor-infiltrating regulatory T cell fragility in mouse models.
In Nat Commun on 17 October 2025 by Wang, T., Xu, Y., et al.
PubMed
Administration of IL-2 may promote the suppressive function and proliferation of Treg cells that cause immune tolerance in patients with cancer, which causes low-dose IL-2 to fail in achieving an optimal anti-tumor effect. Here, we designed an immunocytokine by fusing IL-2 and an anti-TIGIT monoclonal antibody, named αTIGIT-IL2, that targets Treg cells and promotes their fragility in the tumor milieu. These fragile-like Treg cells show impaired suppressive function and high IFN-γ production, triggering an immune-reactive tumor microenvironment. Such inflammation leads to the recruitment and functional reprogramming of intratumoral neutrophils, improving cross-talk between neutrophils and CD8+ T cells and enhancing the antitumor ability of CD8+ T cells. Combination therapy with αTIGIT-IL2 and PD-1 blocker could eliminate triple-negative breast cancer (TNBC) tumors resistant to immune checkpoint blockade (ICB) therapy. These findings provide the basis for developing a new generation of immunocytokines that target Treg cells and promote their fragility in the tumor milieu, resulting in robust antitumor immunity.
-
-
-
Immunology and Microbiology
-
Cancer Research
A next-generation anti-CTLA-4 probody mitigates toxicity and enhances anti-tumor immunity in mice.
In Nat Commun on 10 October 2025 by Cao, W., Chen, J., et al.
PubMed
CTLA-4 is a promising target for immune checkpoint inhibition in cancer therapy, with CTLA-4 blockade achieving prolonged overall survival for responding patients. However, the progressively elevated doses of anti-CTLA-4 agents, aimed at achieving better efficacy, result in increased toxicities, limiting their clinical applications. Here, we generate a prodrug design of the anti-CTLA-4 antibody, named ProCTLA-4, by folding the Fab fragment of the antibody in a tumor-associated protease-based manner. In preclinical mouse models, ProCTLA-4 effectively depletes suppressive regulatory T cells within the tumor microenvironment and enhances tumor-associated antigen-specific CD8+ T cell responses, while exhibiting reduced toxicity compared to currently available CTLA-4 blockade approaches. Furthermore, compared to the currently used Probody therapeutics for anti-CTLA-4 (BMS986288), ProCTLA-4 has more advantages in efficacy amplification, such as in poor immunogenic melanoma. Our design establishes an alternative paradigm for antibody agents that limits the emergence of immune-related adverse events (irAE) while increasing therapeutic efficacy.
-
-
-
Immunology and Microbiology
-
Cancer Research
Elevated Transglutaminase-2 in SOX10-Deficient Melanoma Promotes Tumor Onset and Decreases Intratumoral CD4+ T Cells.
In Cancer Res on 1 October 2025 by Caksa, S., Purwin, T. J., et al.
PubMed
Melanoma heterogeneity contributes to therapy resistance and immune evasion. The loss of SOX10, a neural crest lineage-specific transcription factor, leads to phenotypic switching from a proliferative cell state to an invasive, drug-tolerant cell state. SOX10-deficient cells are able to persist during immunotherapy treatment, highlighting the need to characterize the factors that regulate immune evasion downstream of SOX10 loss. In this study, we found that SOX10-deficient melanoma cell lines and patient samples express elevated levels of TGM2, a transglutaminase family member. TGM2 upregulation in SOX10 knockout cells was reversed by inhibition of epigenetic reader BET proteins. Knockdown of TGM2 did not affect the SOX10-deficient invasive cell state; however, overexpression of TGM2 in syngeneic melanomas promoted tumor onset in immunocompetent mice, but not in immunodeficient mice, suggesting an immune-mediated effect. TGM2 overexpression in melanoma was associated with decreased intratumoral CD4+ T cells, and depletion of CD4+ T cells abolished the tumor-promoting effect of TGM2. These data indicate that TGM2 is negatively regulated by SOX10 in melanoma and can promote an immunosuppressive tumor microenvironment.
-
-
-
Immunology and Microbiology
Simultaneous STING and lymphotoxin-β receptor activation induces B cell responses in tertiary lymphoid structures to potentiate antitumor immunity.
In Nat Immunol on 1 October 2025 by Sawada, J., Kikuchi, Y., et al.
PubMed
B cell-rich tertiary lymphoid structures (TLS) are associated with favorable prognosis and positive response to immunotherapy in cancer. Here we show that simultaneous activation of innate immune effectors, STING and lymphotoxin-β receptor (LTβR), results in CD8+ T cell-dependent tumor suppression while inducing high endothelial venule development and germinal center-like B cell responses in tumors to generate functional TLS in a T cell-dependent manner. In a neoadjuvant setting, activation of STING and LTβR by their agonists effectively immunized mice against tumor recurrence, leading to long-term survival. STING activation alone was insufficient for inducing B cell-containing TLS or eliciting long-term therapeutic effects. However, when combined with LTβR activation, it improved the fitness of TLS with B cell expansion and maturation to IgG-producing long-lived plasma cells and memory cells, increased CD4+ T cell recruitment and memory CD8+ T cell expansion, and shifted the TH2/TH17 balance, resulting in the potentiation of humoral and cellular immunity against tumors. These findings suggest a therapeutic approach of simultaneously activating STING and lymphotoxin pathways.
-
-
-
Immunology and Microbiology
-
Cell Biology
Single-Cell Sequencing Reveals That CD4+ T Cells Eliminate Senescent Prostate Epithelium to Delay Progression of Benign Prostatic Hyperplasia.
In Aging Cell on 1 October 2025 by Li, Z., Wang, X., et al.
PubMed
Benign prostatic hyperplasia (BPH) is an age-related condition characterized by progressive prostate enlargement driven in part by the accumulation of senescent epithelial cells and their pro-inflammatory secretome. Using human single-cell RNA sequencing and laser capture microdissection, we identified C-X-C Motif Chemokine Ligand 13 (CXCL13) as a key chemokine secreted by senescent prostate epithelial cells. CXCL13 recruits CD4+ T cells via the C-X-C Chemokine Receptor Type 5 (CXCR5) receptor, facilitating immune recognition through human leukocyte antigen-DR isotype (HLA-DR) and promoting senescent cell clearance. Functional assays revealed that CD4+ cytotoxic T lymphocytes (CTLs) mediate this clearance, while regulatory T cells (Tregs) suppress it, forming a functional dichotomy. Immunohistochemistry, transwell migration, and co-culture assays confirmed this CXCL13-CXCR5-HLA-DR axis. In a testosterone-induced BPH mouse model, CXCL13 treatment enhanced CD4+ T cell infiltration and reduced epithelial senescence, while CD4+ T cell depletion reversed these effects. Single-cell transcriptomics in mice further validated increased CXCL13 expression and CD4+ T cell engagement. These findings uncover a critical immune surveillance mechanism in BPH and suggest that targeting the CXCL13-CD4+ T cell axis may offer a novel therapeutic strategy for age-related prostate enlargement.
-
-
-
Cancer Research
-
Immunology and Microbiology
NOTCH1 reverses immune suppression in small cell lung cancer through reactivation of STING.
In J Clin Invest on 16 September 2025 by Kim, Y. S., Nabet, B. Y., et al.
PubMed
Downregulation of antigen presentation and lack of immune infiltration are defining features of small cell lung cancer (SCLC), limiting response to immune checkpoint blockade (ICB). While a high-MHC class I, immune-inflamed subset benefits from ICB, underlying mechanisms of immune response in SCLC have yet to be elucidated. Here we show that in the IMpower133 clinical trial, high, but not low, NOTCH1 expression was significantly associated with longer survival with the addition of ICB to chemotherapy among approximately 80% of SCLC patients with NE-enriched tumors (ASCL1-enriched, HR 0.39, P = 0.0012; NEUROD1-enriched, HR 0.44, P = 0.024). Overexpression or pharmacologic activation of NOTCH1 in ASCL1 and NEUROD1 SCLC cell lines dramatically upregulated MHC class I through epigenetic reactivation of STING. In syngeneic mouse models, Notch1 activation reprogrammed SCLC tumors from immune-excluded to immune-inflamed, facilitating durable, complete responses with ICB combined with a STING agonist. STING1 expression was significantly enriched in high- compared with low-NOTCH1-expressing tumors in IMpower133, validating our proposed mechanism. Our data reveal a previously undiscovered role for NOTCH1 as a critical driver of SCLC immunogenicity and a potential predictive biomarker for ICB in SCLC. NOTCH1 activation may be a therapeutic strategy to unleash antitumor immune responses in SCLC and other neuroendocrine cancers in which NOTCH1 is typically suppressed.
-
-
-
Immunology and Microbiology
Next-generation Candida albicans recombinant Als3p and Hyr1p dual antigen vaccine for invasive Candida infections
In Research Square on 5 September 2025 by Singh, S., Youssef, E. G., et al.
-
-
-
Cancer Research
Intrinsic Properties of the Lymph Node Render It Immunologically Susceptible to Metastasis.
In Cancer Discov on 4 September 2025 by Kahn, B., Ng, R. W. S., et al.
PubMed
Lymph nodes (LN) are the staging grounds for antitumor immunity; therefore, their high susceptibility to metastatic colonization is a paradox. Previous studies have suggested that extrinsic tumor-derived factors precondition the draining LN to enable tumor cell survival by promoting a state of immune suppression. In this study, we investigate whether properties of the LN itself may impede its ability to clear metastasizing tumor cells. Using multiple immunocompetent transplant models, we show that LNs possess intrinsic features, independent of preconditioning, which make them an advantageous site for tumor cells to evade T-cell control. Tumor growth in the LN is facilitated by regulatory T cells, which locally suppress the cytolytic capacity of tumor-specific CD8+ T cells by restricting IL2. These findings identify an intrinsic mechanism that contributes to the high rate of LN metastasis in solid tumors.
-
-
-
Immunology and Microbiology
Pre-existing YFV-17D immunity mediates T cell cross-protection against dengue virus serotype 2 infection in mice.
In Commun Biol on 2 September 2025 by Tonto, P. B., Gallon, S., et al.
PubMed
Widespread yellow fever virus (YFV) immunity in Sub-Saharan Africa may mitigate orthoflavivirus outbreaks. Here, we investigate whether pre-existing YFV-17D immunity confers cross-protection against dengue virus serotype 2 (DENV-2) infection in a murine model. IFNAR1-/- mice immunized with YFV-17D exhibited significantly reduced DENV-2 viremia, weight loss, and disease severity, with improved survival compared to naïve controls. Mechanistic studies revealed that cross-protection was mediated by heterologous T cell responses rather than cross-binding antibodies. Depletion of T cells in YFV-17D-immune mice prior to DENV-2 challenge resulted in increased viremia, weight loss, and disease severity, underscoring the protective role of YFV-17D-elicited T cell immunity. Furthermore, YFV-17D-specific T cells displayed cytotoxicity against DENV NS3- and NS5-pulsed cells, demonstrating their functional role in viral control. These findings highlight the critical contribution of heterologous T cell immunity in YFV-17D-mediated protection against DENV-2 and suggest that vaccines designed to elicit T cell responses could enhance cross-protection against orthoflavivirus infections.
-
-
-
Pathology
-
Immunology and Microbiology
Interleukin-10 limits immune-mediated pathology in chronic subclinical plasmodial infection.
In PLoS Negl Trop Dis on 1 September 2025 by Silva, L. S., Monks, B. G., et al.
PubMed
Subclinical parasitemia constitutes the predominant proportion of Plasmodium spp. infections in hyperendemic regions of the world. Elevated levels of serum interleukin-10 (IL-10) are observed in both acute symptomatic and chronic subclinical Plasmodium spp. infections. The role of IL-10 in acute infection has been extensively studied; however, the role of sustained elevated levels of IL-10 in chronic subclinical plasmodial infections remains to be determined. We investigated the role of IL-10 in a long-term subclinical and patent Plasmodium chabaudi chabaudi-AS (Pc) infection using mice lacking humoral immunity (µMT-/- mice). Pc-infected µMT-/- mice exhibit a long-term (99 days) chronic infection, with microscopic levels of parasitemia and without any outward signs of disease. We found that chronically infected mice have slightly elevated levels of tumor necrosis factor α (TNFα) and interferon-γ (IFNγ), and high levels of IL-10 in the circulation. The source of IL-10 was CD4+ T cells. We found that elevated IL-10 levels were mechanistically linked to subclinical Plasmodium infection by blocking IL-10 signaling. Anti-IL-10R resulted in a marked, albeit transient, reduction of the parasitemia that was accompanied by a robust pro-inflammatory response and death of chronically infected µMT-/- mice. A similar outcome was observed in infected µMT-/- mice after CD4+ T cell depletion with anti-CD4 antibody. CD4-depleted infected µMT-/- mice exhibited reduced IL-10 and rapid weight loss, succumbing to infection by day 6 after CD4 neutralization. Our results showed that IL-10 from CD4+ T cells limits immune-mediated pathology in chronic subclinical Pc infection in µMT-/- mice by protecting against excessive inflammatory responses to blood-stage parasites.
-
-
-
Immunology and Microbiology
Epicutaneous Staphylococcus aureus initiates cross-tissue IL-36R signaling for neutrophilic lung inflammation in a model of the atopic march.
In Cell Rep on 26 August 2025 by Kline, S. N., Feller, L. E., et al.
PubMed
Patients with atopic dermatitis exhibit abundant Staphylococcus aureus skin colonization and an increased risk of atopic march diseases, including allergic rhinitis, food allergies, and asthma. We have previously shown that S. aureus skin exposure exacerbates allergic lung inflammation in an interleukin-36 receptor (IL-36R)-dependent manner. However, the cellular and molecular mechanisms by which S. aureus skin exposure and IL-36R signaling orchestrate the progression from skin to lung inflammation are unclear. Using a preclinical model of the atopic march, we found that S. aureus skin exposure promoted robust neutrophilic lung inflammation via keratinocyte- and lung epithelia-specific IL-36R signaling. Unexpectedly, neutrophil IL-36R signaling triggered neutrophil extracellular trap (NET) formation and augmented lung pathology. Importantly, anti-IL-36R monoclonal antibody (mAb) treatment prevented the development of neutrophilic lung inflammation. Collectively, our findings suggested that S. aureus skin exposure exacerbates lung inflammation via distinct IL-36R signaling mechanisms on epithelia and neutrophils, which has therapeutic potential in halting the progression of the atopic march.
-
-
-
Immunology and Microbiology
Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity.
In Cell Rep Med on 19 August 2025 by Ahn, J. J., Dudics, S., et al.
PubMed
Wild-type interleukin (IL)-2 induces anti-tumor immunity and toxicity, predominated by vascular leak syndrome (VLS) leading to edema, hypotension, organ toxicity, and regulatory T cell (Treg) expansion. Efforts to uncouple IL-2 toxicity from its potency have failed in the clinic. We hypothesize that IL-2 toxicity is driven by cytokine release syndrome (CRS) followed by VLS and that coupling IL-2 with IL-10 will ameliorate toxicity. Our data, generated using human primary cells, mouse models, and non-human primates, suggest that coupling of these cytokines prevents toxicity while retaining cytotoxic T cell activation and limiting Treg expansion. In syngeneic murine tumor models, DK210 epidermal growth factor receptor (EGFR), an IL-2/IL-10 fusion molecule targeted to EGFR via an anti-EGFR single-chain variable fragment (scFV), potently activates T cells and natural killer (NK) cells and elicits interferon (IFN)γ-dependent anti-tumor function without peripheral inflammatory toxicity or Treg accumulation. Therefore, combining IL-2 with IL-10 uncouples toxicity from immune activation, leading to a balanced and pleiotropic anti-tumor immune response.
-
-
-
Cancer Research
-
Immunology and Microbiology
Identification of anti-TIM-3 based checkpoint inhibitor combinations with activity in immunotherapy refractory melanoma models.
In J Immunother Cancer on 18 August 2025 by Phadke, M. S., Li, J., et al.
PubMed
A significant percentage of melanomas are refractory to immune checkpoint inhibitor (ICI) monotherapies and combinations. As there are currently no effective second-line therapies available for ICI-resistant patients, we sought to identify novel checkpoint inhibitor combinations for future clinical evaluation.
-
-
-
Cancer Research
-
Immunology and Microbiology
TIM3+ breast cancer cells license immune evasion during micrometastasis outbreak.
In Cancer Cell on 11 August 2025 by Rozalén, C., Sangrador, I., et al.
PubMed
In metastasis, the dynamics of tumor-immune interactions during micrometastasis remain unclear. Identifying the vulnerabilities of micrometastases before outbreaking into macrometastases can reveal therapeutic opportunities for metastasis. Here, we report a function of T cell immunoglobulin and mucin domain 3 (TIM3) in tumor cells during micrometastasis using breast cancer (BC) metastasis mouse models. TIM3 is highly upregulated in micrometastases, promoting survival, stemness, and immune escape. TIM3+ tumor cells are specifically selected during early seeding of micrometastasis. Mechanistically, TIM3 increases β-catenin/interleukin-1β (IL-1β) signaling, leading to stemness and immune-evasion by inducing immunosuppressive γδ T cells and reducing CD8 T cells during micrometastasis. Clinical data confirm increased TIM3+ tumor cells in BC metastasis and TIM3+ tumor cells as a biomarker of poor outcome in BC patients. (Neo)adjuvant TIM3 blockade reduces the metastatic seeding and incidence in preclinical models. These findings unveil a specific mechanism of micrometastasis immune-evasion and the potential use of TIM3 blockade for subclinical metastasis.
-
-
-
Immunology and Microbiology
Immune cells promote paralytic disease in mice infected with enterovirus D68.
In J Clin Invest on 1 August 2025 by Woods Acevedo, M. A., Lan, J., et al.
PubMed
Enterovirus D68 (EV-D68) is associated with acute flaccid myelitis (AFM), a poliomyelitis-like illness causing paralysis in young children. However, the mechanisms of paralysis are unclear, and antiviral therapies are lacking. To better understand EV-D68 disease, we inoculated newborn mice intracranially to assess viral tropism, virulence, and immune responses. WT mice inoculated intracranially with a neurovirulent strain of EV-D68 showed infection of spinal cord neurons and developed paralysis. Spinal tissue from infected mice revealed increased levels of chemokines, inflammatory monocytes, macrophages, and T cells relative to those in controls, suggesting that immune cell infiltration influences pathogenesis. To define the contribution of cytokine-mediated immune cell recruitment to disease, we inoculated mice lacking CCR2, a receptor for several EV-D68-upregulated cytokines, or RAG1, which is required for lymphocyte maturation. WT, Ccr2-/-, and Rag1-/- mice had comparable viral titers in spinal tissue. However, Ccr2-/- and Rag1-/- mice were significantly less likely to be paralyzed relative to WT mice. Consistent with impaired T cell recruitment to sites of infection in Ccr2-/- and Rag1-/- mice, antibody-mediated depletion of CD4+ or CD8+ T cells from WT mice diminished paralysis. These results indicate that immune cell recruitment to the spinal cord promotes EV-D68-associated paralysis and illuminate potential new targets for therapeutic intervention.
-
-
-
Immunology and Microbiology
Photoconverted cells allow rapid assessment of vaccine adjuvant potency in mice.
In iScience on 18 July 2025 by Zhong, Y., Chen, M., et al.
PubMed
Identifying vaccine adjuvants that optimally enhance CD8+ T cell responses remain a challenge, often requiring time-consuming and resource-intensive methods. Here, we introduce a photoconversion-based approach using KikGR mice to track migratory dendritic cells (Mig DCs) within 48 h post-vaccination. This method enables real-time visualization of skin-derived Mig DCs migration to draining lymph nodes (dLNs), providing an early and reliable predictor of CD8+ T cell priming and anti-tumor efficacy. Unlike traditional techniques that demand extensive experimentation, this system allows for faster, cost-effective screening of adjuvants. We further demonstrate that blocking CCR7 significantly reduces Mig DCs migration, impairing CD8+ T cell responses. Our approach revolutionizes adjuvant evaluation, enabling swift and accurate immune assessments, particularly in therapeutic vaccine development.
-