InVivoMAb anti-rat CD4
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Rat thymocyte glycoproteins |
| Reported Applications |
in vivo CD4+ T cell depletion Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
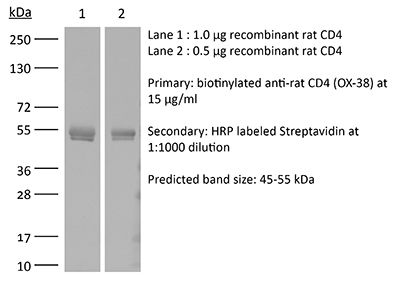
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2736988 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD4+ T cell depletion
Hartlage, A. S., et al (2019). "Vaccination to prevent T cell subversion can protect against persistent hepacivirus infection" Nat Commun 10(1): 1113.
PubMed
Efforts to develop an effective vaccine against the hepatitis C virus (HCV; human hepacivirus) have been stymied by a lack of small animal models. Here, we describe an experimental rat model of chronic HCV-related hepacivirus infection and its response to T cell immunization. Immune-competent rats challenged with a rodent hepacivirus (RHV) develop chronic viremia characterized by expansion of non-functional CD8(+) T cells. Single-dose vaccination with a recombinant adenovirus vector expressing hepacivirus non-structural proteins induces effective immunity in majority of rats. Resolution of infection coincides with a vigorous recall of intrahepatic cellular responses. Host selection of viral CD8 escape variants can subvert vaccine-conferred immunity. Transient depletion of CD8(+) cells from vaccinated rats prolongs infection, while CD4(+) cell depletion results in chronic viremia. These results provide direct evidence that co-operation between CD4(+) and CD8(+) T cells is important for hepacivirus immunity, and that subversion of responses can be prevented by prophylactic vaccination.
Flow Cytometry
Xiao, C. X., et al (2013). "Distribution of bone-marrow-derived endothelial and immune cells in a murine colitis-associated colorectal cancer model" PLoS One 8(9): e73666.
PubMed
Inflammatory bowel disease (IBD) can lead to an increased risk of developing colorectal cancer (CRC). The aim of this study was to establish a model for combined bone marrow transplantation (BMT) and colitis-associated colorectal cancer (CAC) and to define the contribution of BM-derived cells during the inflammation associated with carcinogenesis. We established a model for BMT using green fluorescent protein (GFP) transgenic mice, followed by AOM/DSS-induced CAC, and performed confocal microscopy analysis on in vivo living tissue and frozen tumor sections. Our imaging analyses showed that GFP-positive cells extensively infiltrated the tumor stroma and that some WGA and GFP or CD31 and GFP double-positive cells were observed in the lining of tumor vessels. Flow cytometry analysis of the tumor-infiltrating cells showed that the GFP-positive CD11c+ DCs cells were one-third of the GFP+/CD11C- cells, and that half of these DCs (0.96% vs 1.02%) were GFP-positive BM-derived cells. The majority of CD4(+) T cells were GFP-negative (12.02% vs 1.9%), and we discovered a novel CD4(+) CD11c(+) DC subset (0.34% vs 1.64%). In conclusion, we defined the distribution of BM-derived endothelial cells, CD11c(+) DCs and CD4(+) T cells in tumors. This model might be useful for elucidating the diverse BM-derived cell types and functions during the progression of colitis-associated colorectal cancer.
in vivo CD4+ T cell depletion
Siepert, A., et al (2012). "Permanent CNI treatment for prevention of renal allograft rejection in sensitized hosts can be replaced by regulatory T cells" Am J Transplant 12(9): 2384-2394.
PubMed
Recent data suggest that donor-specific memory T cells (T(mem)) are an independent risk factor for rejection and poor graft function in patients and a major challenge for immunosuppression minimizing strategies. Many tolerance induction protocols successfully proven in small animal models e.g. costimulatory blockade, T cell depletion failed in patients. Consequently, there is a need for more predictive transplant models to evaluate novel promising strategies, such as adoptive transfer of regulatory T cells (Treg). We established a clinically more relevant, life-supporting rat kidney transplant model using a high responder (DA to LEW) recipients that received donor-specific CD4(+)/ 8(+) GFP(+) T(mem) before transplantation to achieve similar pre-transplant frequencies of donor-specific T(mem) as seen in many patients. T cell depletion alone induced long-term graft survival in naive recipients but could not prevent acute rejection in T(mem)(+) rats, like in patients. Only if T cell depletion was combined with permanent CNI-treatment, the intragraft inflammation, and acute/chronic allograft rejection could be controlled long-term. Remarkably, combining 10 days CNI treatment and adoptive transfer of Tregs (day 3) but not Treg alone also induced long-term graft survival and an intragraft tolerance profile (e.g. high TOAG-1) in T(mem)(+) rats. Our model allows evaluation of novel therapies under clinically relevant conditions.
in vivo CD4+ T cell depletion
Mara-Koosham, G., et al (2011). "Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains" Infect Immun 79(4): 1770-1778.
PubMed
Pneumonic tularemia is a life-threatening disease caused by inhalation of the highly infectious intracellular bacterium Francisella tularensis. The most serious form of the disease associated with the type A strains can be prevented in experimental animals through vaccination with the attenuated live vaccine strain (LVS). The protection is largely cell mediated, but the contribution of antibodies remains controversial. We addressed this issue in a series of passive immunization studies in Fischer 344 (F344) rats. Subcutaneous LVS vaccination induced a robust serum antibody response dominated by IgM, IgG2a, and IgG2b antibodies. Prophylactic administration of LVS immune serum or purified immune IgG reduced the severity and duration of disease in naive rats challenged intratracheally with a lethal dose of the virulent type A strain SCHU S4. The level of resistance increased with the volume of immune serum given, but the maximum survivable SCHU S4 challenge dose was at least 100-fold lower than that shown for LVS-vaccinated rats. Protection correlated with reduced systemic bacterial growth, less severe histopathology in the liver and spleen during the early phase of infection, and bacterial clearance by a T cell-dependent mechanism. Our results suggest that treatment with immune serum limited the sequelae associated with infection, thereby enabling a sterilizing T cell response to develop and resolve the infection. Thus, antibodies induced by LVS vaccination may contribute to the defense of F344 rats against respiratory infection by type A strains of F. tularensis.
in vivo CD4+ T cell depletion
Goupille, C., et al (2000). "alpha1,2Fucosyltransferase increases resistance to apoptosis of rat colon carcinoma cells" Glycobiology 10(4): 375-382.
PubMed
Accumulation of histo-blood group antigens such as Lewis b, Lewis Y and H in colon cancer is indicative of poor prognosis. It is accompanied by increase in alpha1,2fucosyl-transferase activity, a key enzyme for synthesis of these antigens. Using a model of colon carcinoma, we previously showed that alpha1,2fucosylation increases tumorigenicity. We now show that tumorigenicity inversely correlates with the cells’ sensitivity to apoptosis. In addition, poorly tumorigenic REG cells independently transfected with three different alpha1,2fucosyltransferase cDNAs, the human FUT1, the rat FTA and FTB were more resistant than control cells to apoptosis induced in vitro by serum deprivation. Inversely, PRO cells, spontaneously tumorigenic in immunocompetent syngeneic animals and able to synthesize alpha1,2fucosylated glycans, became more sensitive to apoptosis after transfection with a fragment of the FTA cDNA in the antisense orientation. Expression of alpha1,2fucosyl-transferase in poorly tumorigenic REG cells dramatically enhanced their tumorigenicity in syngeneic rats. However, in immunodeficient animals, both control and alpha1,2fuco-syltransferase transfected REG cells were fully tumorigenic and metastatic, indicating that the presence of alpha1,2fucosylated antigens allowed REG tumor cells to escape immune control. Taken together, the results show that increased tumorigenicity mediated by alpha1,2fucosyl-ation is associated to increased resistance to apoptosis and to escape from immune control.
in vivo CD4+ T cell depletion
Westermann, W., et al (1999). "Th2 cells as effectors in postirradiation pulmonary damage preceding fibrosis in the rat" Int J Radiat Biol 75(5): 629-638.
PubMed
PURPOSE: Radiation-induced pneumonitis and subsequent pulmonary fibrosis are important dose-limiting complications of radiotherapy. Their pathogenesis is known only in part. T-lymphocytes comprise a significant part of the infiltrating cells but little is known about their role. The aim of this study was to define the function of T-lymphocytes during development of postirradiation pneumonitis and pulmonary fibrosis. MATERIALS AND METHODS: Rats received a unilateral lung irradiation of 20 Gy. Kinetics of T-lymphocytes isolated from irradiated and non-irradiated lungs were analysed. Subsequent CD4 depletion experiments were performed to affirm the importance of CD4+ T-cells in the development of lung fibrosis. Finally, the T helper-cell subtype of the T-lymphocytes was analysed by determining the cytokine mRNA by RT-PCR. RESULTS: A selective increase of CD4+ T-cells was observed peaking 4 weeks after irradiation in the irradiated lungs. When rats were depleted of these cells, the postirradiation thickening of parenchyma was significantly reduced as determined by morphometric analysis of lung tissue sections. In addition, it was found that IL-4 mRNA was selectively increased in the CD4+ T-cells isolated from irradiated lungs, which indicates a lymphocyte reactivity dominated by Th2 cells. CONCLUSION: The results suggest a critical role for Th2 CD4+ T-lymphocytes in the pathogenesis of radiation-induced pneumonitis preceding lung fibrosis.
in vivo CD4+ T cell depletion
Qi, Z., et al (1997). "Single dose anti-CD4 monoclonal antibody for induction of tolerance to cardiac allograft in high- and low-responder rat strain combinations" Transpl Immunol 5(3): 204-211.
PubMed
100 days). Linomide challenge affected CsA treatment in the high-responder combination but not tolerance induction in the low-responder combination, or the effect of OX-38. It was concluded that in rat heart transplantation a single-dose anti-CD4 mAb therapy may induce permanent donor-specific unresponsiveness in a low-responder strain combination, and that anti-CD4 mAb seems to be unique among immunosuppressive agents while being resistent to challenge by Linomide.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Repeated administration of monoclonal antibodies (mAb) directed against the CD4 lymphocyte receptor may induce specific, long-lasting unresponsiveness to fully MHC-mismatched cardiac allografts in rats without additional immunosuppression. We assessed the effect of a single dose of murine anti-rat depleting anti-CD4 mAb (OX-38) on allograft survival in high- and low-responder rat strain combinations. Isogenic strains of DA (RT1av1), PVG (RT1c), AUG (RT1c), and WF (RT1u) rats were used. Recipients in antibody treated groups were given one dose of 5 mg/kg OX-38 mAb on the day of transplant, a dose which was shown to effectively deplete (or block) circulating CD4+ T cells. Other groups were treated for 10 days with cyclosporin A (CsA) and/or Linomide, a novel immunomodulator, which is the first compound able to fully eliminate the effect of CsA in the rat cardiac allograft model. The DA strain was identified as a low-responder to the allogeneic haplotype RT1c (PVG or AUG), but not to RT1u (WF), and developed true tolerance following RT1c grafting and OX-38 or low-dose CsA (5 mg/kg) induction, as verified by the response to retransplantation of a graft from the same donor strain or a third-party challenge. PVG recipients of DA grafts were characterized by high response and only modest (OX-38; median 9.5 days) or moderate (CsA; 23.5 days) prolongation of graft survival. Contrasting graft survival results were obtained in the low-responder combination, either very early rejection (at 10 days) or permanent graft survival (> 100 days). Linomide challenge affected CsA treatment in the high-responder combination but not tolerance induction in the low-responder combination, or the effect of OX-38. It was concluded that in rat heart transplantation a single-dose anti-CD4 mAb therapy may induce permanent donor-specific unresponsiveness in a low-responder strain combination, and that anti-CD4 mAb seems to be unique among immunosuppressive agents while being resistent to challenge by Linomide.
Product Citations
-
Recombinant ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) decreases vascular calcification and prevents osteomalacia in a rat model of chronic kidney disease.
In JBMR Plus on 1 June 2025 by O'Brien, K., Laurion, L., et al.
PubMed
Chronic kidney disease (CKD) impacts a large percentage of the global population. Chronic kidney disease-mineral bone disorder (MBD) is the broad term describing alterations in key circulating factors involved in mineralization, ectopic calcification, and bone abnormalities. Cardiovascular complications, involving vascular calcification are one of the leading causes of death in this patient population. Plasma levels of pyrophosphate, a potent inhibitor of ectopic mineralization, are low in CKD and end-stage kidney disease patients. These data suggest that the correction of pyrophosphate levels could stand out as a crucial therapeutic goal to mitigate vascular calcifications and reduce cardiovascular mortality. The primary enzyme responsible for the generation of plasma pyrophosphate is ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1). We therefore evaluated INZ-701, a recombinant human ENPP1-Fc fusion protein, in an adenine-induced rat model of CKD. Our investigation revealed that INZ-701 administration resulted in significantly lower levels of calcification in the vasculature and soft tissues. Moreover, INZ-701 treatment significantly prevented the osteoid volume increase observed in vehicle-treated rats, addressing another critical clinical manifestation of CKD-MBD. These results underscore the potential of INZ-701 to reduce vascular calcification and bone mineralization abnormalities in CKD.
-
-
Cardiovascular biology
-
Neuroscience
Th1/Th2 Imbalance in Peripheral Blood Echoes Microglia State Dynamics in CNS During TLE Progression.
In Adv Sci (Weinh) on 1 October 2024 by Wang, J., Wu, Y., et al.
PubMed
Central and systemic inflammation play pivotal roles in epileptogenesis and proepileptogenesis in temporal lobe epilepsy (TLE). The interplay between peripheral CD4+ T cells and central microglia orchestrates the "systemic-central" immune response in TLE. However, the precise molecular mechanisms linking central and systemic inflammation in TLE remain unknown. This preliminary findings revealed an imbalance in Th1/Th2 subsets in the periphery,accompanied by related cytokines release in TLE patients. they proposed that this peripheral Th1/Th2 imbalance may influence central inflammation by mediating microglial state dynamics within epileptic foci and distant brain regions. In Li-pilocarpine-induced TLE rats, a peripheral Th1/Th2 imbalance and observed corresponding central and systemic responses is confirmed. Notably, CD4+ T cells infiltrated through the compromised blood-brain barrierand are spatially close to microglia around epileptic foci. Intravenous depletion and reinfusion of CD4+ T cells modulated microglia state dynamics and altered neuroinflammatory cytokines secretion. Moreover, mRNA sequencing of the human hippocampus identified Notch1 as a key regulator of Th1/Th2 differentiation, CD4+ T cell recruitment to brain infiltration sites, and the regulation of microglial responses, seizure frequency, and cognition. This study underscores the significance of Th1/Th2 imbalance in modulating the "systemic-central" response in TLE, highlighting Notch1 as a potential therapeutic target.
-
-
-
Immunodepletion
-
Immunodepletion
-
Immunology and Microbiology
Vaccination to prevent T cell subversion can protect against persistent hepacivirus infection.
In Nat Commun on 7 March 2019 by Hartlage, A. S., Murthy, S., et al.
PubMed
Efforts to develop an effective vaccine against the hepatitis C virus (HCV; human hepacivirus) have been stymied by a lack of small animal models. Here, we describe an experimental rat model of chronic HCV-related hepacivirus infection and its response to T cell immunization. Immune-competent rats challenged with a rodent hepacivirus (RHV) develop chronic viremia characterized by expansion of non-functional CD8+ T cells. Single-dose vaccination with a recombinant adenovirus vector expressing hepacivirus non-structural proteins induces effective immunity in majority of rats. Resolution of infection coincides with a vigorous recall of intrahepatic cellular responses. Host selection of viral CD8 escape variants can subvert vaccine-conferred immunity. Transient depletion of CD8+ cells from vaccinated rats prolongs infection, while CD4+ cell depletion results in chronic viremia. These results provide direct evidence that co-operation between CD4+ and CD8+ T cells is important for hepacivirus immunity, and that subversion of responses can be prevented by prophylactic vaccination.
-