InVivoMAb anti-mouse CD4
Product Description
Specifications
| Isotype | Rat IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
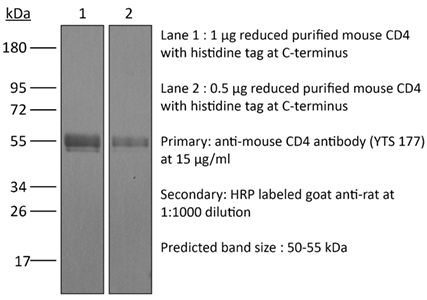
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse Spleen Cells |
| Reported Applications |
in vivo blockade of CD4+ T-cell responses Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107642 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blockade of CD4+ T-cell responses
Li, Z., et al (2015). "Pre-treatment of allogeneic bone marrow recipients with the CXCR4 antagonist AMD3100 transiently enhances hematopoietic chimerism without promoting donor-specific skin allograft tolerance" Transpl Immunol 33(2): 125-129.
PubMed
Hematopoietic chimerism established by allogeneic bone marrow transplantation is known to promote donor-specific organ allograft tolerance; however, clinical application is limited by the need for toxic host conditioning and “megadoses” of donor bone marrow cells. A potential solution to this problem has been suggested by the observation that recipient bone marrow mobilization by the CXCR4 antagonist AMD3100 promotes chimerism in congenic bone marrow transplantation experiments in mice. Here we report that a single subcutaneous dose of 10mg/kg AMD3100 in recipient C57BL/6 mice was able to enhance hematopoietic chimerism when complete MHC-mismatched BALB/c donor bone marrow cells were transplanted 1h after drug dosing. However, levels of chimerism measured 30days post-transplantation were not sustained when mice were reexamined on day 90 post-transplantation. Moreover, transient chimerism induced by this protocol did not support robust donor-specific skin allograft tolerance. Using the same transient immunosuppression protocol, we confirmed that “megadoses” of donor bone marrow cells could induce durable chimerism associated with donor-specific skin allograft tolerance without AMD3100 pre-treatment. We conclude that in this protocol AMD3100 pretreatment may empty bone marrow niches that become reoccupied by allogeneic donor hematopoietic progenitor cells but not by true long-lived donor hematopoietic stem cells, resulting in short-lived chimerism and failure to support durable donor-specific allograft tolerance.
in vivo blockade of CD4+ T-cell responses
Mayer, C. T., et al (2014). "Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals" J Autoimmun 50: 23-32.
PubMed
A major concept in autoimmunity is that disruption of Foxp3(+) regulatory T cells (Tregs) predisposes to breach of tolerance. This is exemplified by the Foxp3-linked disorder termed IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) which affects newborn children. There has been considerable clinical interest in the role of non-depleting anti-CD4 antibodies as a means of upregulating the function of Foxp3(+) Tregs in order to control detrimental inflammatory responses such as transplant rejection. However, according to the paradigm of a Treg-dependent mechanism of action, the effectiveness of anti-CD4 antibodies as a therapy for human autoimmune diseases is unclear considering that Treg function might be intrinsically impaired. Specifically, anti-CD4 therapy is expected to fail in patients suffering from the IPEX syndrome due to the lack of functional Foxp3(+) Tregs. Taking advantage of natural Foxp3 mutant scurfy (sf) mice closely resembling the IPEX syndrome, and genetically engineered mice depleted of Foxp3(+) Tregs, we report here that anti-CD4 treatment induces tolerance independent of Foxp3(+) Tregs. This so far undefined mechanism is dependent on the recessive non-infectious tolerization of autoreactive T cells. Treg-independent tolerance alone is powerful enough to suppress both the onset and severity of autoimmunity and reduces clinically relevant autoantibody levels and liver fibrosis. Mechanistically, tolerance induction requires the concomitant activation of autoreactive T cells and is associated with the down-regulation of the co-stimulatory TNF-receptor superfamily members OX40 and CD30 sustaining CD4(+) T cell survival. In the light of ongoing clinical trials, our results highlight an unexpected potency of anti-CD4 antibodies for the treatment of autoimmune diseases. Particularly, CD4 blockade might represent a novel therapeutic option for the human IPEX syndrome.
in vivo blockade of CD4+ T-cell responses
Rocca, C. J., et al (2014). "rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study" Gene Ther 21(6): 618-628.
PubMed
Effective gene therapy strategies for the treatment of kidney disorders remain elusive. We report an optimized kidney-targeted gene delivery strategy using recombinant adeno-associated virus (rAAV) administered via retrograde renal vein injection in mice. Renal vein injection of rAAV consistently resulted in superior kidney transduction compared with tail vein injection using as little as half the tail vein dose. We compared rAAV5, 6, 8 and 9, containing either green fluorescent protein (GFP) or luciferase reporter genes driven by the Cytomegalovirus promoter. We demonstrated that although rAAV6 and 8 injected via renal vein transduced the kidney, transgene expression was mainly restricted to the medulla. Transgene expression was systematically low after rAAV5 injection, attributed to T-cell immune response, which could be overcome by transient immunosuppression. However, rAAV9 was the only serotype that permitted high-transduction efficiency of both the cortex and medulla. Moreover, both the glomeruli and tubules were targeted, with a higher efficiency within the glomeruli. To improve the specificity of kidney-targeted gene delivery with rAAV9, we used the parathyroid hormone receptor ‘kidney-specific’ promoter. We obtained a more efficient transgene expression within the kidney, and a significant reduction in other tissues. Our work represents the first comprehensive and clinically relevant study for kidney gene delivery.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
CBD promotes antitumor activity by modulating tumor immune microenvironment in HPV associated head and neck squamous cell carcinoma.
In Front Immunol on 6 June 2025 by Sen, P., Sadat, S., et al.
PubMed
Marijuana use is associated with HPV-positive head and neck squamous cell carcinoma (HNSCC). However, cannabinoid use continues to increase in the US general population for recreational purposes as well as in cancer patients for palliative care. In this study, we explored the role of cannabidiol (CBD) in promoting anti-tumor activity by modulating immune response in HPV-positive HNSCC by using pre-clinical models.
-
-
-
Cell Biology
-
Immunology and Microbiology
Probiotics and their metabolite spermidine enhance IFN-γ+CD4+ T cell immunity to inhibit hepatitis B virus.
In Cell Rep Med on 19 November 2024 by Wang, T., Fan, Y., et al.
PubMed
The therapeutic potential of commensal microbes and their metabolites is promising in the functional cure of chronic hepatitis B virus (HBV) infection, which is defined as hepatitis B surface antigen (HBsAg) loss. Here, using both specific-pathogen-free and germ-free mice, we report that probiotics significantly promote the decline of HBsAg and inhibit HBV replication by enhancing intestinal homeostasis and provoking intrahepatic interferon (IFN)-γ+CD4+ T cell immune response. Depletion of CD4+ T cells or blockage of IFN-γ abolishes probiotics-mediated HBV inhibition. Specifically, probiotics-derived spermidine accumulates in the gut and transports to the liver, where it exhibits a similar anti-HBV effect. Mechanistically, spermidine enhances IFN-γ+CD4+ T cell immunity by autophagy. Strikingly, administration of probiotics in HBV patients reveals a preliminary trend to accelerate the decline of serum HBsAg. In conclusion, probiotics and their derived spermidine promote HBV clearance via autophagy-enhanced IFN-γ+CD4+ T cell immunity, highlighting the therapeutic potential of probiotics and spermidine for the functional cure of HBV patients.
-
-
-
Cancer Research
-
Immunology and Microbiology
A CXCR4 partial agonist improves immunotherapy by targeting polymorphonuclear myeloid-derived suppressor cells and cancer-driven granulopoiesis
In bioRxiv on 11 October 2024 by Qian, J., Ma, C., et al.
-
-
-
Cancer Research
Smyd3-mediated immuno-modulation in HPV-negative head and neck squamous cell carcinoma mouse models.
In iScience on 20 September 2024 by Tsai, D. E., Lovanov, A., et al.
PubMed
SET and MYND-domain containing protein 3 (SMYD3) mediates epigenetic repression of type I IFN response genes in human papillomavirus (HPV)-negative HNSCC cells, and Smyd3 depletion using anti-sense oligonucleotides (ASOs) increases the sensitivity of syngeneic mouse oral carcinoma (MOC1) models to anti-PD-1 therapy. In this study, we utilized single-cell RNA-seq of MOC1 tumors treated with Smyd3 ASOs and found enrichment of type I IFN response pathways in cancer cells, a shift of CD8+ T-cells toward an activated/memory phenotype, and a shift of neutrophils toward an anti-tumorigenic phenotype. Mechanisms of resistance to the Smyd3 ASO and anti-PD-1 combination were derived from cancer cells, macrophages, and CD8+ T-cells, including neutrophil enrichment through the upregulation of Cxcl2, repression of Cxcl9, and defective antigen presentation. This study sheds light on the immunomodulatory functions of Smyd3 in vivo and provides insight into actionable mechanisms of resistance to improve the efficacy of Smyd3 ASOs and anti-PD-1 combination.
-
-
-
Mus musculus (Mouse)
BCG as an Innovative Option for HCC Treatment: Repurposing and Mechanistic Insights.
In Adv Sci (Weinh) on 1 April 2024 by Vaziri, F., Setayesh, T., et al.
PubMed
This study investigates Bacillus Calmette-Guérin (BCG) as a potential treatment for hepatocellular carcinoma (HCC), a condition often associated with unfavorable treatment outcomes. Exploiting BCG's recognized immune-boosting properties, preclinical trials are conducted using HCC mice, with a single subcutaneous dose of BCG administered post-tumor formation. Results indicate that BCG treatment effectively diminishes tumor burden and extends survival in both male and female HCC mice. Positive influences on hepatic fibrosis and metabolism are observed, leading to a reduction in lipid levels. Spatial analysis underscores BCG's tumor-specific effects, inducing the enrichment of metabolic pathways and inhibiting various cancer-related pathways. Furthermore, BCG promotes immune cell infiltration, including CD4+, CD8+ T cells, and M1 macrophages, in both v-akt murine thymoma viral oncogene homolog 1(AKT)/neutoblastoma RAS viral oncogene homolog (RAS) and β-catenin positive HCC models. Interestingly, blocking T cells, trained immunity, and Interferon-γ (IFN-γ) function reverses BCG's anti-HCC effects. In conclusion, BCG emerges as a promising treatment option for HCC, characterized by a favorable safety profile and efficacy in inhibiting fibrosis, improving metabolism, and engaging both trained immunity and T cells in therapeutic mechanisms.
-
-
-
Mus musculus (Mouse)
High-titer AAV disrupts cerebrovascular integrity and induces lymphocyte infiltration in adult mouse brain.
In Mol Ther Methods Clin Dev on 14 December 2023 by Guo, Y., Chen, J., et al.
PubMed
The brain is often described as an "immune-privileged" organ due to the presence of the blood-brain-barrier (BBB), which limits the entry of immune cells. In general, intracranial injection of adeno-associated virus (AAV) is considered a relatively safe procedure. In this study, we discovered that AAV, a popular engineered viral vector for gene therapy, can disrupt the BBB and induce immune cell infiltration in a titer-dependent manner. First, our bulk RNA sequencing data revealed that injection of high-titer AAV significantly upregulated many genes involved in disrupting BBB integrity and antiviral adaptive immune responses. By using histologic analysis, we further demonstrated that the biological structure of the BBB was severely disrupted in the adult mouse brain. Meanwhile, we noticed abnormal leakage of blood components, including immune cells, within the brain parenchyma of high-titer AAV injected areas. Moreover, we identified that the majority of infiltrated immune cells were cytotoxic T lymphocytes (CTLs), which resulted in a massive loss of neurons at the site of AAV injection. In addition, antagonizing CTL function by administering antibodies significantly reduced neuronal toxicity induced by high-titer AAV. Collectively, our findings underscore potential severe side effects of intracranial injection of high-titer AAV, which might compromise proper data interpretation if unaware of.
-
-
-
Mus musculus (Mouse)
Skeletal phenotype amelioration in mucopolysaccharidosis VI requires intervention at the earliest stages of postnatal development.
In JCI Insight on 8 November 2023 by Hwang-Wong, E., Amar, G., et al.
PubMed
Mucopolysaccharidosis VI (MPS VI) is a rare lysosomal disease arising from impaired function of the enzyme arylsulfatase B (ARSB). This impairment causes aberrant accumulation of dermatan sulfate, a glycosaminoglycan (GAG) abundant in cartilage. While clinical severity varies along with age at first symptom manifestation, MPS VI usually presents early and strongly affects the skeleton. Current enzyme replacement therapy (ERT) does not provide effective treatment for the skeletal manifestations of MPS VI. This lack of efficacy may be due to an inability of ERT to reach affected cells or to the irreversibility of the disease. To address the question of reversibility of skeletal phenotypes, we generated a conditional by inversion (COIN) mouse model of MPS VI, ArsbCOIN/COIN, wherein Arsb is initially null and can be restored to WT using Cre. We restored Arsb at different times during postnatal development, using a tamoxifen-dependent global Cre driver. By restoring Arsb at P7, P21, and P56-P70, we determined that skeletal phenotypes can be fully rescued if Arsb restoration occurs at P7, while only achieving partial rescue at P21 and no significant rescue at P56-P70. This work has highlighted the importance of early intervention in patients with MPS VI to maximize therapeutic impact.
-
-
-
Cancer Research
-
Immunology and Microbiology
Anti-4-1BB immunotherapy enhances systemic immune effects of radiotherapy to induce B and T cell-dependent anti-tumor immune activation and improve tumor control at unirradiated sites.
In Cancer Immunol Immunother on 1 June 2023 by Martin, A. L., Powell, C., et al.
PubMed
Radiation therapy (RT) can prime and boost systemic anti-tumor effects via STING activation, resulting in enhanced tumor antigen presentation and antigen recognition by T cells. It is increasingly recognized that optimal anti-tumor immune responses benefit from coordinated cellular (T cell) and humoral (B cell) responses. However, the nature and functional relevance of the RT-induced immune response are controversial, beyond STING signaling, and agonistic interventions are lacking. Here, we show that B and CD4+ T cell accumulation at tumor beds in response to RT precedes the arrival of CD8+ T cells, and both cell types are absolutely required for abrogated tumor growth in non-irradiated tumors. Further, RT induces increased expression of 4-1BB (CD137) in both T and B cells; both in preclinical models and in a cohort of patients with small cell lung cancer treated with thoracic RT. Accordingly, the combination of RT and anti-41BB therapy leads to increased immune cell infiltration in the tumor microenvironment and significant abscopal effects. Thus, 4-1BB therapy enhances radiation-induced tumor-specific immune responses via coordinated B and T cell responses, thereby preventing malignant progression at unirradiated tumor sites. These findings provide a rationale for combining RT and 4-1bb therapy in future clinical trials.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes antitumor T-cell immunity.
In J Immunother Cancer on 1 November 2022 by Kalim, K. W., Yang, J. Q., et al.
PubMed
Cancer immunotherapy has taken center stage in cancer treatment. However, the current immunotherapies only benefit a small proportion of patients with cancer, necessitating better understanding of the mechanisms of tumor immune evasion and improved cancer immunotherapy strategies. Regulatory T (Treg) cells play an important role in maintaining immune tolerance through inhibiting effector T-cell function. In the tumor microenvironment, Treg cells are used by tumor cells to counteract effector T cell-mediated tumor suppression. Targeting Treg cells may thus unleash the antitumor activity of effector T cells. While systemic depletion of Treg cells can cause excessive effector T-cell responses and subsequent autoimmune diseases, controlled targeting of Treg cells may benefit patients with cancer.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes anti-tumor T cell immunity
In bioRxiv on 24 September 2021 by Kalim, K. W., Yang, J., et al.
-
-
-
Immunodepletion
-
Immunodepletion
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.
In Elife on 18 January 2021 by Angulo, G., Zeleznjak, J., et al.
PubMed
Viral infections are controlled, and very often cleared, by activated T lymphocytes. The inducible co-stimulator (ICOS) mediates its functions by binding to its ligand ICOSL, enhancing T-cell activation and optimal germinal center (GC) formation. Here, we show that ICOSL is heavily downmodulated during infection of antigen-presenting cells by different herpesviruses. We found that, in murine cytomegalovirus (MCMV), the immunoevasin m138/fcr-1 physically interacts with ICOSL, impeding its maturation and promoting its lysosomal degradation. This viral protein counteracts T-cell responses, in an ICOS-dependent manner, and limits virus control during the acute MCMV infection. Additionally, we report that blockade of ICOSL in MCMV-infected mice critically regulates the production of MCMV-specific antibodies due to a reduction of T follicular helper and GC B cells. Altogether, these findings reveal a novel mechanism evolved by MCMV to counteract adaptive immune surveillance, and demonstrates a role of the ICOS:ICOSL axis in the host defense against herpesviruses.
-