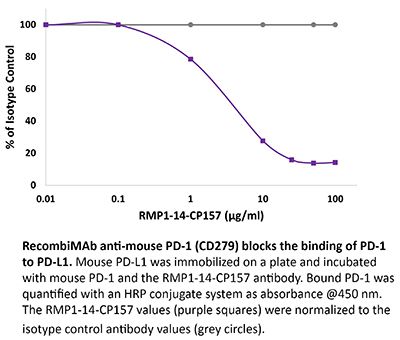
RecombiMAb anti-mouse PD-1 (CD279)
(switched from rat IgG2a)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| Reported Applications |
in vivo blocking of PD-1/PD-L signaling* *Reported for the original rat IgG2a RMP1-14 antibody |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL gel clotting assay |
| Aggregation |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from CHO cell supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2927526 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
Forced intracellular degradation of xenoantigens as a modality for cell-based cancer immunotherapy.
In iScience on 21 March 2025 by Bikorimana, J. P., Farah, R., et al.
PubMed
Given recent leverage of mesenchymal stromal cells (MSCs) as a potent vaccination platform, we investigated whether forced degradation of an expressed experimental antigen fused to small degron sequences could prime potent antitumoral responses. Retrovirally gene-engineered MSCs were evaluated for their in-vitro antigen presentation capacity, nature of generated peptide repertoire and therapeutic potency in syngeneic immunocompetent mice with pre-established solid T cell lymphoma. Despite lack of noticeable changes in gene expression, MSC-UBvR-OVA vaccination triggered potent T cell activation which can be attributable to the enriched cell surface presentation of OVA-derived peptides added to elevated mitochondrial reactive oxidative species (ROS) production, the latter being associated with efficient antigen processing. Where MSC-UBvR-OVA vaccination successfully controlled tumor growth in cancer-bearing mice, the effect is further enhanced using tranylcypromine-stimulated MSCs and anti-PD-1 combination. Such anti-tumoral response relies on efferocytosis by endogenous phagocytes. Altogether, UBvR facilitated forced antigen degradation represents a plausible modality for future development of tumor antigen-expressing MSC-based vaccine.
-
-
-
Mus musculus (Mouse)
A1-reprogrammed mesenchymal stromal cells prime potent antitumoral responses.
In iScience on 15 March 2024 by Gonçalves, M. P., Farah, R., et al.
PubMed
Mesenchymal stromal cells (MSCs) have been modified via genetic or pharmacological engineering into potent antigen-presenting cells-like capable of priming responding CD8 T cells. In this study, our screening of a variant library of Accum molecule revealed a molecule (A1) capable of eliciting antigen cross-presentation properties in MSCs. A1-reprogrammed MSCs (ARM) exhibited improved soluble antigen uptake and processing. Our comprehensive analysis, encompassing cross-presentation assays and molecular profiling, among other cellular investigations, elucidated A1's impact on endosomal escape, reactive oxygen species production, and cytokine secretion. By evaluating ARM-based cellular vaccine in mouse models of lymphoma and melanoma, we observe significant therapeutic potency, particularly in allogeneic setting and in combination with anti-PD-1 immune checkpoint inhibitor. Overall, this study introduces a strong target for developing an antigen-adaptable vaccination platform, capable of synergizing with immune checkpoint blockers to trigger tumor regression, supporting further investigation of ARMs as an effective and versatile anti-cancer vaccine.
-