InVivoSIM anti-human PD-L1 (Avelumab Biosimilar)
Product Description
Specifications
| Isotype | Human IgG1, λ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG1 isotype control, anti-respiratory syncytial virus |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
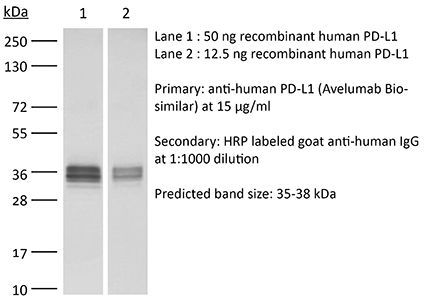
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Full length human PD-L1 |
| Reported Applications |
in vitro functional assay in vitro PD-L1 blockade ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro functional assay
Müller T, Tasser C, Tesar M, Fucek I, Schniegler-Mattox U, Koch J, Ellwanger K (2023). "Selection of bispecific antibodies with optimal developability using FcRn‑Ph‑HPLC as an optimized FcRn affinity chromatography method" MAbs 15(1):2245519.
PubMed
A challenge when developing therapeutic antibodies is the identification of candidates with favorable pharmacokinetics (PK) early in development. A key determinant of immunoglobulin (IgG) serum half‑life in vivo is the efficiency of pH-dependent binding to the neonatal Fc receptor (FcRn). Numerous studies have proposed techniques to assess FcRn binding of IgG-based therapeutics in vitro, enabling prediction of serum half-life prior to clinical assessment. FcRn high-performance liquid chromatography (HPLC) assays FcRn binding of therapeutic IgGs across a pH gradient, allowing the correlation of IgG column retention time to the half‑life of a therapeutic IgG in vivo. However, as FcRn retention time cannot be directly compared to an in vivo parameter, modifications to FcRn-HPLC are required to enable interpretation of the data within a physiological context, to provide more accurate estimations of serum half-life. This study presents an important modification to this method, FcRn-pH-HPLC, which reproducibly measures FcRn dissociation pH, allowing correlation with previously established half-lives of therapeutic antibodies. Furthermore, the influence of incorporating various antibody modifications, binding modules, and their orientations within IgGs and bispecifics on FcRn dissociation pH was evaluated using antibodies from the redirected optimized cell killing (ROCK®) platform. Target and effector antigen-binding domain sequences, their presentation format and orientation within a bispecific antibody alter FcRn retention; tested Fc domain modifications and incorporating stabilizing disulfide bonds had minimal effect. This study may inform the generation of mono-, bi- and multi-specific antibodies with tailored half-lives based on FcRn binding properties in vitro, to differentiate antibody-based therapeutic candidates with optimal developability.