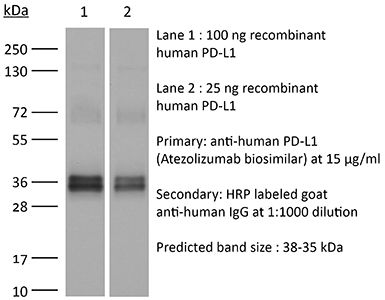
InVivoSIM anti-human PD-L1 (Atezolizumab Biosimilar)
Product Description
Specifications
| Isotype | Human IgG1 |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG1 (N297A) isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Mutations | N297A |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo PD-L1 blockade Flow Cytometry Western Blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894730 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo PD-L1 blockade
Deng R, Tian R, Li X, Xu Y, Li Y, Wang X, Li H, Wang L, Xu B, Yang D, Tang S, Xue B, Zuo C, Zhu H (2024). "ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis" iScience 27(4):10953
PubMed
Hepatitis B virus (HBV) infection generally elicits weak type-I interferon (IFN) immune response in hepatocytes, covering the regulatory effect of IFN-stimulated genes. In this study, low level of IFN-stimulated gene 12a (ISG12a) predicted malignant transformation and poor prognosis of HBV-associated hepatocellular carcinoma (HCC), whereas high level of ISG12a indicated active NK cell phenotypes. ISG12a interacts with TRIM21 to inhibit the phosphorylation activation of protein kinase B (PKB, also known as AKT) and β-catenin, suppressing PD-L1 expression to block PD-1/PD-L1 signaling, thereby enhancing the anticancer effect of NK cells. The suppression of PD-1-deficient NK-92 cells on HBV-associated tumors was independent of ISG12a expression, whereas the anticancer effect of PD-1-expressed NK-92 cells on HBV-associated tumors was enhanced by ISG12a and treatments of atezolizumab and nivolumab. Thus, tumor intrinsic ISG12a promotes the anticancer effect of NK cells by regulating PD-1/PD-L1 signaling, presenting the significant role of innate immunity in defending against HBV-associated HCC.
in vitro PD-L1 blockade
Sun R, Meng Z, Lee H, Offringa R, Niehrs C (2023). "ROTACs leverage signaling-incompetent R-spondin for targeted protein degradation" Cell Chem Biol 30(7):739-752.e8.
PubMed
Proteolysis-targeting chimeras (PROTACs) are an emerging technology for therapeutic intervention, but options to target cell surface proteins and receptors remain limited. Here we introduce ROTACs, bispecific WNT- and BMP-signaling-disabled R-spondin (RSPO) chimeras, which leverage the specificity of these stem cell growth factors for ZNRF3/RNF43 E3 transmembrane ligases, to target degradation of transmembrane proteins. As a proof-of-concept, we targeted the immune checkpoint protein, programmed death ligand 1 (PD-L1), a prominent cancer therapeutic target, with a bispecific RSPO2 chimera, R2PD1. The R2PD1 chimeric protein binds to PD-L1 and at picomolar concentration induces its lysosomal degradation. In three melanoma cell lines, R2PD1 induced between 50 and 90% PD-L1 protein degradation. PD-L1 degradation was strictly dependent on ZNRF3/RNF43. Moreover, R2PD1 reactivates cytotoxic T cells and inhibits tumor cell proliferation more potently than Atezolizumab. We suggest that signaling-disabled ROTACs represent a paradigm to target cell surface proteins for degradation in a range of applications.
in vitro PD-L1 blockade
Zhao Y, Caron C, Chan YY, Lee CK, Xu X, Zhang J, Masubuchi T, Wu C, Bui JD, Hui E (2023). "cis-B7:CD28 interactions at invaginated synaptic membranes provide CD28 co-stimulation and promote CD8+ T cell function and anti-tumor immunity" Immunity 56(6)
PubMed
B7 ligands (CD80 and CD86), expressed by professional antigen-presenting cells (APCs), activate the main co-stimulatory receptor CD28 on T cells in trans. However, in peripheral tissues, APCs expressing B7 ligands are relatively scarce. This raises the questions of whether and how CD28 co-stimulation occurs in peripheral tissues. Here, we report that CD8+ T cells displayed B7 ligands that interacted with CD28 in cis at membrane invaginations of the immunological synapse as a result of membrane remodeling driven by phosphoinositide-3-kinase (PI3K) and sorting-nexin-9 (SNX9). cis-B7:CD28 interactions triggered CD28 signaling through protein kinase C theta (PKCθ) and promoted CD8+ T cell survival, migration, and cytokine production. In mouse tumor models, loss of T cell-intrinsic cis-B7:CD28 interactions decreased intratumoral T cells and accelerated tumor growth. Thus, B7 ligands on CD8+ T cells can evoke cell-autonomous CD28 co-stimulation in cis in peripheral tissues, suggesting cis-signaling as a general mechanism for boosting T cell functionality.
in vivo PD-L1 blockade
Lee DH, Ahn H, Sim HI, Choi E, Choi S, Jo Y, Yun B, Song HK, Oh SJ, Denda-Nagai K, Park CS, Irimura T, Park Y, Jin HS (2023). "A CRISPR activation screen identifies MUC-21 as critical for resistance to NK and T cell-mediated cytotoxicity" J Exp Clin
PubMed
Background: Immunotherapy has significantly advanced cancer treatments, but many patients do not respond to it, partly due to immunosuppressive mechanisms used by tumor cells. These cells employ immunosuppressive ligands to evade detection and elimination by the immune system. Therefore, the discovery and characterization of novel immunosuppressive ligands that facilitate immune evasion are crucial for developing more potent anti-cancer therapies. Methods: We conducted gain-of-function screens using a CRISPRa (CRISPR activation) library that covered the entire human transmembrane sub-genome to identify surface molecules capable of hindering NK-mediated cytotoxicity. The immunosuppressive role and mechanism of MUC21 were validated using NK and T cell mediated cytotoxicity assays. Bioinformatics tools were employed to assess the clinical implications of mucin-21 (MUC21) in cancer cell immunity. Results: Our genetic screens revealed that MUC21 expression on cancer cell surfaces inhibits both the cytotoxic activity of NK cells and antibody-dependent cellular cytotoxicity, but not affecting complement-dependent cytotoxicity. Additionally, MUC21 expression hinders T cell activation by impeding antigen recognition, thereby diminishing the effectiveness of the immune checkpoint inhibitor, anti-PD-L1. Moreover, MUC21 expression suppress the antitumor function of both CAR-T cells and CAR-NK cells. Mechanistically, MUC21 facilitates immune evasion by creating steric hindrance, preventing interactions between cancer and immune cells. Bioinformatics analysis revealed elevated MUC21 expression in lung cancer, which correlated with reduced infiltration and activation of cytotoxic immune cells. Intriguingly, MUC21 expression was higher in non-small cell lung cancer (NSCLC) tumors that were non-responsive to anti-PD-(L)1 treatment compared to responsive tumors. Conclusions: These findings indicate that surface MUC21 serves as a potent immunosuppressive ligand, shielding cancer cells from NK and CD8+T cell attacks. This suggests that inhibiting MUC21 could be a promising strategy to improve cancer immunotherapy.
Product Citations
-
-
Cancer Research
Functional Role of NOXA in Hypoxia-Mediated PD-L1 Inhibitor Response in Hepatocellular Carcinoma.
In Int J Mol Sci on 16 May 2025 by Huang, M., Lan, T., et al.
PubMed
Hypoxia is a crucial characteristic of hepatocellular carcinoma (HCC) and contributes to immune resistance by upregulating PD-L1 and recruiting immunosuppressive cells. However, the molecular mechanisms of hypoxia-induced immunotherapy resistance are still unclear. The hypoxia-related immunotherapy response (IRH) genes were identified and used to develop a hypoxia risk score model to predict patient survival. The model was validated using GSE233802 and EGAD00001008128 datasets. The hypoxia risk score model including NOXA effectively stratified patients based on risk and demonstrated excellent survival predictive ability (p = 0.0236). A hypoxia-induced drug-resistant (HepG2-R) cell line was established by co-culturing HepG2 cells with Jurkat T cells under CoCl2-induced hypoxia and PD-L1 inhibitor administration. Prolonged exposure to hypoxia (48 h) in HepG2 cells significantly led to the increased hypoxia risk score (p < 0.02). The establishment of the HepG2-R cell line showed that prolonged hypoxia reduced cancer cell apoptosis, which implies potential treatment resistance. The effect of NOXA knockdown on the apoptosis of HepG2-R cells under the same co-culture conditions was examined. Under hypoxia and PD-L1 inhibitor treatment, NOXA knockdown increased the survival rate of HepG2-R cells and reduced early and late apoptosis. This indicates that NOXA plays a crucial role in apoptosis regulation and immune response in hypoxic tumors. NOXA knockdown significantly reduces apoptosis in immunotherapy-resistant cells induced by hypoxia. These findings provide important evidence that targeting NOXA may enhance immunotherapy efficacy and help overcome treatment resistance in HCC, highlighting its potential as a therapeutic target.
-
-
-
Cancer Research
-
Immunology and Microbiology
Kynurenine promotes the immune escape of colorectal cancer cells via NAT10-mediated ac4C acetylation of PD-L1.
In Clinics (Sao Paulo) on 18 April 2025 by Wang, Z., Yin, M., et al.
PubMed
This study aimed to investigate the role of kynurenine in Colorectal Cancer (CRC) and the underlying mechanism.
-
-
Development and first-in-human CAR T therapy against the pathognomonic MiT-fusion driven protein GPNMB
In medRxiv on 27 February 2025 by Zemp, F. J., Breckenridge, Z., et al.
-
-
Cancer Research
-
Immunology and Microbiology
ISG12a promotes immunotherapy of HBV-associated hepatocellular carcinoma through blocking TRIM21/AKT/β-catenin/PD-L1 axis.
In iScience on 19 April 2024 by Deng, R., Tian, R., et al.
PubMed
Hepatitis B virus (HBV) infection generally elicits weak type-I interferon (IFN) immune response in hepatocytes, covering the regulatory effect of IFN-stimulated genes. In this study, low level of IFN-stimulated gene 12a (ISG12a) predicted malignant transformation and poor prognosis of HBV-associated hepatocellular carcinoma (HCC), whereas high level of ISG12a indicated active NK cell phenotypes. ISG12a interacts with TRIM21 to inhibit the phosphorylation activation of protein kinase B (PKB, also known as AKT) and β-catenin, suppressing PD-L1 expression to block PD-1/PD-L1 signaling, thereby enhancing the anticancer effect of NK cells. The suppression of PD-1-deficient NK-92 cells on HBV-associated tumors was independent of ISG12a expression, whereas the anticancer effect of PD-1-expressed NK-92 cells on HBV-associated tumors was enhanced by ISG12a and treatments of atezolizumab and nivolumab. Thus, tumor intrinsic ISG12a promotes the anticancer effect of NK cells by regulating PD-1/PD-L1 signaling, presenting the significant role of innate immunity in defending against HBV-associated HCC.
-