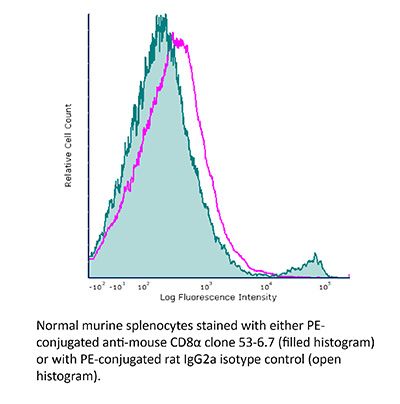
InVivoPlus anti-mouse CD8α
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse Spleen Cells or Thymocyte Membranes |
| Reported Applications |
in vivo CD8+ T cell depletion Immunofluorescence Flow cytometry Western blot |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107671 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD8+ T cell depletion
Flow Cytometry
Wang, W., et al (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo CD8+ T cell depletion
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo CD8+ T cell depletion
Yamada, D. H., et al (2015). "Suppression of Fcgamma-receptor-mediated antibody effector function during persistent viral infection" Immunity 42(2): 379-390.
PubMed
Understanding how viruses subvert host immunity and persist is essential for developing strategies to eliminate infection. T cell exhaustion during chronic viral infection is well described, but effects on antibody-mediated effector activity are unclear. Herein, we show that increased amounts of immune complexes generated in mice persistently infected with lymphocytic choriomeningitis virus (LCMV) suppressed multiple Fcgamma-receptor (FcgammaR) functions. The high amounts of immune complexes suppressed antibody-mediated cell depletion, therapeutic antibody-killing of LCMV infected cells and human CD20-expressing tumors, as well as reduced immune complex-mediated cross-presentation to T cells. Suppression of FcgammaR activity was not due to inhibitory FcgammaRs or high concentrations of free antibody, and proper FcgammaR functions were restored when persistently infected mice specifically lacked immune complexes. Thus, we identify a mechanism of immunosuppression during viral persistence with implications for understanding effective antibody activity aimed at pathogen control.
Immunofluorescence
Finisguerra, V., et al (2015). "MET is required for the recruitment of anti-tumoural neutrophils" Nature 522(7556): 349-353.
PubMed
Mutations or amplification of the MET proto-oncogene are involved in the pathogenesis of several tumours, which rely on the constitutive engagement of this pathway for their growth and survival. However, MET is expressed not only by cancer cells but also by tumour-associated stromal cells, although its precise role in this compartment is not well characterized. Here we show that MET is required for neutrophil chemoattraction and cytotoxicity in response to its ligand hepatocyte growth factor (HGF). Met deletion in mouse neutrophils enhances tumour growth and metastasis. This phenotype correlates with reduced neutrophil infiltration to both the primary tumour and metastatic sites. Similarly, Met is necessary for neutrophil transudation during colitis, skin rash or peritonitis. Mechanistically, Met is induced by tumour-derived tumour necrosis factor (TNF)-alpha or other inflammatory stimuli in both mouse and human neutrophils. This induction is instrumental for neutrophil transmigration across an activated endothelium and for inducible nitric oxide synthase production upon HGF stimulation. Consequently, HGF/MET-dependent nitric oxide release by neutrophils promotes cancer cell killing, which abates tumour growth and metastasis. After systemic administration of a MET kinase inhibitor, we prove that the therapeutic benefit of MET targeting in cancer cells is partly countered by the pro-tumoural effect arising from MET blockade in neutrophils. Our work identifies an unprecedented role of MET in neutrophils, suggests a potential ‘Achilles’ heel’ of MET-targeted therapies in cancer, and supports the rationale for evaluating anti-MET drugs in certain inflammatory diseases.
in vivo CD8+ T cell depletion
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo CD8+ T cell depletion
Flow Cytometry
Walsh, K. B., et al (2014). "Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy" J Virol 88(11): 6281-6293.
PubMed
The cytokine storm is an intensified, dysregulated, tissue-injurious inflammatory response driven by cytokine and immune cell components. The cytokine storm during influenza virus infection, whereby the amplified innate immune response is primarily responsible for pulmonary damage, has been well characterized. Now we describe a novel event where virus-specific T cells induce a cytokine storm. The paramyxovirus pneumonia virus of mice (PVM) is a model of human respiratory syncytial virus (hRSV). Unexpectedly, when C57BL/6 mice were infected with PVM, the innate inflammatory response was undetectable until day 5 postinfection, at which time CD8(+) T cells infiltrated into the lung, initiating a cytokine storm by their production of gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha). Administration of an immunomodulatory sphingosine-1-phosphate (S1P) receptor 1 (S1P1R) agonist significantly inhibited PVM-elicited cytokine storm by blunting the PVM-specific CD8(+) T cell response, resulting in diminished pulmonary disease and enhanced survival. IMPORTANCE: A dysregulated overly exuberant immune response, termed a “cytokine storm,” accompanies virus-induced acute respiratory diseases (VARV), is primarily responsible for the accompanying high morbidity and mortality, and can be controlled therapeutically in influenza virus infection of mice and ferrets by administration of sphingosine-1-phosphate 1 receptor (S1P1R) agonists. Here, two novel findings are recorded. First, in contrast to influenza infection, where the cytokine storm is initiated early by the innate immune system, for pneumonia virus of mice (PVM), a model of RSV, the cytokine storm is initiated late in infection by the adaptive immune response: specifically, by virus-specific CD8 T cells via their release of IFN-gamma and TNF-alpha. Blockading these cytokines with neutralizing antibodies blunts the cytokine storm and protects the host. Second, PVM infection is controlled by administration of an S1P1R agonist.
in vivo CD8+ T cell depletion
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
Immunofluorescence
Schwager, K., et al (2013). "The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF" J Invest Dermatol 133(3): 751-758.
PubMed
Systemic high-dose IL2 promotes long-term survival in a subset of metastatic melanoma patients, but this treatment is accompanied by severe toxicities. The immunocytokine L19-IL2, in which IL2 is fused to the human L19 antibody capable of selective accumulation on tumor neovasculature, has recently shown encouraging clinical activity in patients with metastatic melanoma. In this study, we have investigated the therapeutic performance of L19-IL2, administered systemically in combination with a murine anti-CTLA-4 antibody or with a second clinical-stage immunocytokine (L19-TNF) in two syngeneic immunocompetent mouse models of cancer. We observed complete tumor eradications when L19-IL2 was used in combination with CTLA-4 blockade. Interestingly, mice cured from F9 tumors developed new lesions when rechallenged with tumor cells after therapy, whereas mice cured from CT26 tumors were resistant to tumor rechallenge. Similarly, L19-IL2 induced complete remissions when administered in a single intratumoral injection in combination with L19-TNF, whereas the two components did not lead to cures when administered as single agents. These findings provide a rationale for combination trials in melanoma, as the individual therapeutic agents have been extensively studied in clinical trials, and the antigen recognized by the L19 antibody has an identical sequence in mouse and man.
in vivo CD8+ T cell depletion
Flow Cytometry
Cyktor, J. C., et al (2013). "Clonal expansions of CD8+ T cells with IL-10 secreting capacity occur during chronic Mycobacterium tuberculosis infection" PLoS One 8(3): e58612.
PubMed
The exact role of CD8(+) T cells during Mycobacterium tuberculosis (Mtb) infection has been heavily debated, yet it is generally accepted that CD8(+) T cells contribute to protection against Mtb. In this study, however, we show that the Mtb-susceptible CBA/J mouse strain accumulates large numbers of CD8(+) T cells in the lung as infection progresses, and that these cells display a dysfunctional and immunosuppressive phenotype (PD-1(+), Tim-3(+), CD122(+)). CD8(+) T cell expansions from the lungs of Mtb-infected CBA/J mice were also capable of secreting the immunosuppressive cytokine interleukin-10 (IL-10), although in vivo CD8(+) T cell depletion did not significantly alter Mtb burden. Further analysis revealed that pulmonary CD8(+) T cells from Mtb-infected CBA/J mice were clonally expanded, preferentially expressing T cell receptor (TcR) Vbeta chain 8 (8.2, 8.3) or Vbeta 14. Although Vbeta8(+) CD8(+) T cells were responsible for the majority of IL-10 production, in vivo depletion of Vbeta8(+) did not significantly change the outcome of Mtb infection, which we hypothesize was a consequence of their dual IL-10/IFN-gamma secreting profiles. Our data demonstrate that IL-10-secreting CD8(+) T cells can arise during chronic Mtb infection, although the significance of this T cell population in tuberculosis pathogenesis remains unclear.
in vivo CD8+ T cell depletion
Flow Cytometry
Hafalla, J. C., et al (2012). "The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology" PLoS Pathog 8(2): e1002504.
PubMed
The balance between pro-inflammatory and regulatory immune responses in determining optimal T cell activation is vital for the successful resolution of microbial infections. This balance is maintained in part by the negative regulators of T cell activation, CTLA-4 and PD-1/PD-L, which dampen effector responses during chronic infections. However, their role in acute infections, such as malaria, remains less clear. In this study, we determined the contribution of CTLA-4 and PD-1/PD-L to the regulation of T cell responses during Plasmodium berghei ANKA (PbA)-induced experimental cerebral malaria (ECM) in susceptible (C57BL/6) and resistant (BALB/c) mice. We found that the expression of CTLA-4 and PD-1 on T cells correlates with the extent of pro-inflammatory responses induced during PbA infection, being higher in C57BL/6 than in BALB/c mice. Thus, ECM develops despite high levels of expression of these inhibitory receptors. However, antibody-mediated blockade of either the CTLA-4 or PD-1/PD-L1, but not the PD-1/PD-L2, pathways during PbA-infection in ECM-resistant BALB/c mice resulted in higher levels of T cell activation, enhanced IFN-gamma production, increased intravascular arrest of both parasitised erythrocytes and CD8(+) T cells to the brain, and augmented incidence of ECM. Thus, in ECM-resistant BALB/c mice, CTLA-4 and PD-1/PD-L1 represent essential, independent and non-redundant pathways for maintaining T cell homeostasis during a virulent malaria infection. Moreover, neutralisation of IFN-gamma or depletion of CD8(+) T cells during PbA infection was shown to reverse the pathologic effects of regulatory pathway blockade, highlighting that the aetiology of ECM in the BALB/c mice is similar to that in C57BL/6 mice. In summary, our results underscore the differential and complex regulation that governs immune responses to malaria parasites.
in vivo CD8+ T cell depletion
Chyou, S., et al (2011). "Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells" J Immunol 187(11): 5558-5567.
PubMed
Lymph node blood vessels play important roles in the support and trafficking of immune cells. The blood vasculature is a component of the vascular-stromal compartment that also includes the lymphatic vasculature and fibroblastic reticular cells (FRCs). During immune responses as lymph nodes swell, the blood vasculature undergoes a rapid proliferative growth that is initially dependent on CD11c(+) cells and vascular endothelial growth factor (VEGF) but is independent of lymphocytes. The lymphatic vasculature grows with similar kinetics and VEGF dependence, suggesting coregulation of blood and lymphatic vascular growth, but lymphatic growth has been shown to be B cell dependent. In this article, we show that blood vascular, lymphatic, and FRC growth are coordinately regulated and identify two distinct phases of vascular-stromal growth–an initiation phase, characterized by upregulated vascular-stromal proliferation, and a subsequent expansion phase. The initiation phase is CD11c(+) cell dependent and T/B cell independent, whereas the expansion phase is dependent on B and T cells together. Using CCR7(-/-) mice and selective depletion of migratory skin dendritic cells, we show that endogenous skin-derived dendritic cells are not important during the initiation phase and uncover a modest regulatory role for CCR7. Finally, we show that FRC VEGF expression is upregulated during initiation and that dendritic cells can stimulate increased fibroblastic VEGF, suggesting the scenario that lymph node-resident CD11c(+) cells orchestrate the initiation of blood and lymphatic vascular growth in part by stimulating FRCs to upregulate VEGF. These results illustrate how the lymph node microenvironment is shaped by the cells it supports.
in vivo CD8+ T cell depletion
Kumar, D., et al (2011). "Intranasal administration of an inactivated Yersinia pestis vaccine with interleukin-12 generates protective immunity against pneumonic plague" Clin Vaccine Immunol 18(11): 1925-1935.
PubMed
Inhalation of Yersinia pestis causes pneumonic plague, which rapidly progresses to death. A previously licensed killed whole-cell vaccine is presently unavailable due to its reactogenicity and inconclusive evidence of efficacy. The present study now shows that vaccination intranasally (i.n.) with inactivated Y. pestis CO92 (iYp) adjuvanted with interleukin-12 (IL-12) followed by an i.n. challenge with a lethal dose of Y. pestis CO92 prevented bacterial colonization and protected 100% of mice from pneumonic plague. Survival of the vaccinated mice correlated with levels of systemic and lung antibodies, reduced pulmonary pathology and proinflammatory cytokines, and the presence of lung lymphoid cell aggregates. Protection against pneumonic plague was partially dependent upon Fc receptors and could be transferred to naive mice with immune mouse serum. On the other hand, protection was not dependent upon complement, and following vaccination, depletion of CD4 and/or CD8 T cells before challenge did not affect survival. In summary, the results demonstrate the safety, immunogenicity, and protective efficacy of i.n. administered iYp plus IL-12 in a mouse model of pneumonic plague.
in vivo CD8+ T cell depletion
Simma, O., et al (2009). "Identification of an indispensable role for tyrosine kinase 2 in CTL-mediated tumor surveillance" Cancer Res 69(1): 203-211.
PubMed
We showed previously that Tyk2(-/-) natural killer cells lack the ability to lyse leukemic cells. As a consequence, the animals are leukemia prone. Here, we show that the impaired tumor surveillance extends to T cells. Challenging Tyk2(-/-) mice with EL4 thymoma significantly decreased disease latency. The crucial role of Tyk2 for CTL function was further characterized using the ovalbumin-expressing EG7 cells. Tyk2(-/-) OT-1 mice developed EG7-induced tumors significantly faster compared with wild-type (wt) controls. In vivo assays confirmed the defect in CD8(+) cytotoxicity on Tyk2 deficiency and clearly linked it to type I IFN signaling. An impaired CTL activity was only observed in IFNAR1(-/-) animals but not on IFNgamma or IL12p35 deficiency. Accordingly, EG7-induced tumors grew faster in IFNAR1(-/-) and Tyk2(-/-) but not in IFNgamma(-/-) or IL12p35(-/-) mice. Adoptive transfer experiments defined a key role of Tyk2 in CTL-mediated tumor surveillance. In contrast to wt OT-1 cells, Tyk2(-/-) OT-1 T cells were incapable of controlling EG7-induced tumor growth.
Product Citations
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Inhibition of ENT1 relieves intracellular adenosine-mediated T cell suppression in cancer.
In Nat Immunol on 1 June 2025 by Sanders, T., Nabel, C. S., et al.
PubMed
The benefit of immune checkpoint blockade for cancer therapy is limited to subsets of patients because of factors including the accumulation of immunosuppressive metabolites, such as adenosine, within tumors. Pharmacological inhibition of adenosine generation and signaling is an active area of clinical investigation, but only limited clinical benefit has been reported. Here, we show that adenosine suppresses anti-cancer T cell responses following uptake into activated T cells by equilibrative nucleoside transporter 1 (ENT1) and inhibition of de novo pyrimidine nucleotide synthesis. We identify EOS301984 as a potent ENT1 antagonist that restores pyrimidine levels in activated T cells in adenosine-rich environments, resulting in enhanced tumor cell killing by memory T cells and increased ex vivo expansion of functional human tumor-infiltrating lymphocytes. A combination of EOS301984 with anti-PD-1 led to synergistic control of tumor growth in a humanized mouse model of triple-negative breast cancer. ENT1 inhibition, therefore, augments anti-cancer immune responses through the restoration of pyrimidine nucleotide synthesis in T cells suppressed by adenosine.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Replenishment of myeloid-derived suppressor cells (MDSCs) overrides CR-mediated protection against tumor growth in a murine model of triple-negative breast cancer.
In Geroscience on 1 October 2022 by Pomatto-Watson, L. C. D., Bodogai, M., et al.
PubMed
Caloric restriction (CR) is the leading non-pharmacological intervention to delay induced and spontaneous tumors in pre-clinical models. These effects of CR are largely attributed to canonical inhibition of pro-growth pathways. However, our recent data suggest that CR impairs primary tumor growth and cancer progression in the murine 4T1 model of triple negative breast cancer (TNBC), at least in part, through reduced frequency of the myeloid-derived suppressor cells (MDSC). In the present study, we sought to determine whether injection of excess MDSCs could block regression in 4T1 tumor growth and metastatic spread in BALB/cJ female mice undergoing daily CR. Our findings show that MDSC injection impeded CR-mediated protection against tumor growth without increasing lung metastatic burden. Overall, these results reveal that CR can slow cancer progression by affecting immune suppressive cells.Impact statement: Inoculation of MDSCs from donor mice effectively impedes the ability of calorie restriction to protect against primary tumor growth without impacting lung metastatic burden in recipient animals.
-
-
-
Cancer Research
-
Immunology and Microbiology
Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity.
In Cell Rep Med on 20 September 2022 by Mirlekar, B., Wang, Y., et al.
PubMed
Plasma cell responses are associated with anti-tumor immunity and favorable response to immunotherapy. B cells can amplify anti-tumor immune responses through antibody production; yet B cells in patients and tumor-bearing mice often fail to support this effector function. We identify dysregulated transcriptional program in B cells that disrupts differentiation of naive B cells into anti-tumor plasma cells. The signaling network contributing to this dysfunction is driven by interleukin (IL) 35 stimulation of a STAT3-PAX5 complex that upregulates the transcriptional regulator BCL6 in naive B cells. Transient inhibition of BCL6 in tumor-educated naive B cells is sufficient to reverse the dysfunction in B cell differentiation, stimulating the intra-tumoral accumulation of plasma cells and effector T cells and rendering pancreatic tumors sensitive to anti-programmed cell death protein 1 (PD-1) blockade. Our findings argue that B cell effector dysfunction in cancer can be due to an active systemic suppression program that can be targeted to synergize with T cell-directed immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Antigens Expressed by Breast Cancer Cells Undergoing EMT Stimulate Cytotoxic CD8+ T Cell Immunity.
In Cancers (Basel) on 9 September 2022 by Camp, F. A., Brunetti, T. M., et al.
PubMed
Antigenic differences formed by alterations in gene expression and alternative splicing are predicted in breast cancer cells undergoing epithelial to mesenchymal transition (EMT) and the reverse plasticity known as MET. How these antigenic differences impact immune interactions and the degree to which they can be exploited to enhance immune responses against mesenchymal cells is not fully understood. We utilized a master microRNA regulator of EMT to alter mesenchymal-like EO771 mammary carcinoma cells to a more epithelial phenotype. A computational approach was used to identify neoantigens derived from the resultant differentially expressed somatic variants (SNV) and alternative splicing events (neojunctions). Using whole cell vaccines and peptide-based vaccines, we find superior cytotoxicity against the more-epithelial cells and explore the potential of neojunction-derived antigens to elicit T cell responses through experiments designed to validate the computationally predicted neoantigens. Overall, results identify EMT-associated splicing factors common to both mouse and human breast cancer cells as well as immunogenic SNV- and neojunction-derived neoantigens in mammary carcinoma cells.
-
-
-
Immunology and Microbiology
-
Cancer Research
Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity.
In Nat Commun on 6 September 2022 by Feng, Q., Liu, Z., et al.
PubMed
Lactate is a key metabolite produced from glycolytic metabolism of glucose molecules, yet it also serves as a primary carbon fuel source for many cell types. In the tumor-immune microenvironment, effect of lactate on cancer and immune cells can be highly complex and hard to decipher, which is further confounded by acidic protons, a co-product of glycolysis. Here we show that lactate is able to increase stemness of CD8+ T cells and augments anti-tumor immunity. Subcutaneous administration of sodium lactate but not glucose to mice bearing transplanted MC38 tumors results in CD8+ T cell-dependent tumor growth inhibition. Single cell transcriptomics analysis reveals increased proportion of stem-like TCF-1-expressing CD8+ T cells among intra-tumoral CD3+ cells, a phenotype validated by in vitro lactate treatment of T cells. Mechanistically, lactate inhibits histone deacetylase activity, which results in increased acetylation at H3K27 of the Tcf7 super enhancer locus, leading to increased Tcf7 gene expression. CD8+ T cells in vitro pre-treated with lactate efficiently inhibit tumor growth upon adoptive transfer to tumor-bearing mice. Our results provide evidence for an intrinsic role of lactate in anti-tumor immunity independent of the pH-dependent effect of lactic acid, and might advance cancer immune therapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy.
In Nat Nanotechnol on 1 May 2022 by Liu, C., Liu, X., et al.
PubMed
The strategy of combining a vaccine with immune checkpoint inhibitors has been widely investigated in cancer management, but the complete response rate for this strategy is still unresolved. We describe a genetically engineered cell membrane nanovesicle that integrates antigen self-presentation and immunosuppression reversal (ASPIRE) for cancer immunotherapy. The ASPIRE nanovaccine is derived from recombinant adenovirus-infected dendritic cells in which specific peptide-major histocompatibility complex class I (pMHC-I), anti-PD1 antibody and B7 co-stimulatory molecules are simultaneously anchored by a programmed process. ASPIRE can markedly improve antigen delivery to lymphoid organs and generate broad-spectrum T-cell responses that eliminate established tumours. This work presents a powerful vaccine formula that can directly activate both native T cells and exhausted T cells, and suggests a general strategy for personalized cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Expression of the mono-ADP-ribosyltransferase ART1 by tumor cells mediates immune resistance in non-small cell lung cancer.
In Sci Transl Med on 16 March 2022 by Wennerberg, E., Mukherjee, S., et al.
PubMed
Most patients with non-small cell lung cancer (NSCLC) do not achieve durable clinical responses from immune checkpoint inhibitors, suggesting the existence of additional resistance mechanisms. Nicotinamide adenine dinucleotide (NAD)-induced cell death (NICD) of P2X7 receptor (P2X7R)-expressing T cells regulates immune homeostasis in inflamed tissues. This process is mediated by mono-adenosine 5'-diphosphate (ADP)-ribosyltransferases (ARTs). We found an association between membranous expression of ART1 on tumor cells and reduced CD8 T cell infiltration. Specifically, we observed a reduction in the P2X7R+ CD8 T cell subset in human lung adenocarcinomas. In vitro, P2X7R+ CD8 T cells were susceptible to ART1-mediated ADP-ribosylation and NICD, which was exacerbated upon blockade of the NAD+-degrading ADP-ribosyl cyclase CD38. Last, in murine NSCLC and melanoma models, we demonstrate that genetic and antibody-mediated ART1 inhibition slowed tumor growth in a CD8 T cell-dependent manner. This was associated with increased infiltration of activated P2X7R+CD8 T cells into tumors. In conclusion, we describe ART1-mediated NICD as a mechanism of immune resistance in NSCLC and provide preclinical evidence that antibody-mediated targeting of ART1 can improve tumor control, supporting pursuit of this approach in clinical studies.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Antagonistic Antibody Targeting TNFR2 Inhibits Regulatory T Cell Function to Promote Anti-Tumor Activity.
In Front Immunol on 8 March 2022 by Chen, Y., Jia, M., et al.
PubMed
Infiltration of regulatory T cells (Tregs) in the tumor microenvironment suppresses anti-tumor immune response, and promotes tumor progression. Tumor necrosis factor receptor-2 (TNFR2), which is highly expressed on Tregs, activates Tregs through nuclear factor kappa B (NF-κB) pathway. Moreover, TNFR2+ Tregs have been shown to be most suppressive among all Tregs populations in tumor. Due to the unique expression pattern and function of TNFR2 on Tregs, a TNFR2 blocking antibody is expected to compromise Tregs function, relieve Tregs-mediated immunosuppression, and hence to enhance anti-tumor immune response. AN3025 is an antagonistic anti-human TNFR2 (hTNFR2) antibody that is currently under preclinical development. This study investigates the immunomodulatory and anti-tumor activity of AN3025. AN3025 was generated through rabbit immunization with extracellular domain of human TNFR2 and subsequent humanization by complementarity-determining regions (CDRs) grafting. AN3025 binds to the extracellular domain of both human and cynomolgus with sub-nanomolar affinity and specificity, but not mouse or rat TNFR2. AN3025 inhibited tumor necrosis factor alpha (TNFα) induced cell death of hTNFR2-overexpressing Jurkat cells by competing with TNFα for binding to hTNFR2. In the Tregs/T effector co-culture assay, AN3025 increased T effector proliferation and enhanced interferon gamma (IFNγ) production. As a monotherapy, AN3025 significantly inhibited MC38 tumor growth in TNFR2 humanized mouse model. Subsequent flow cytometry (FACS) and immunohistochemistry (IHC) analysis revealed that administration of AN3025 led to decreased Tregs population, increased CD4+ and CD8+ T cell numbers in the tumor. The anti-tumor activity of AN3025 was dependent on the existence of CD4+ and CD8+ T cells, as depletion of CD4+ and CD8+ T cells abolished the anti-tumor activity of AN3025. In addition, AN3025 in combination with anti-PD-1 antibody demonstrated stronger in-vivo anti-tumor activity. The potent anti-tumor efficacy of AN3025, either as a monotherapy or in combination with anti-PD-1 antibody, supports its further clinical development for the treatment of various human tumors.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
B Cell Receptor Signaling and Protein Kinase D2 Support Regulatory B Cell Function in Pancreatic Cancer.
In Front Immunol on 21 January 2022 by Michaud, D., Mirlekar, B., et al.
PubMed
B cells can act as potent suppressors of anti-tumor T cell immunity, presenting a mechanism of resistance to immunotherapy. In pancreatic ductal adenocarcinoma, B cells can display a T cell-suppressive or regulatory phenotype centered on the expression of the cytokine Interleukin 35 (IL-35). While B cell-mediated immunosuppression presents a barrier to anti-tumorigenic T cell function, it is not clear how regulatory B cell function could be targeted, and the signals that promote this suppressive phenotype in B cells are not well understood. Here we use a novel IL-35 reporter model to understand which signaling pathways are important for immunosuppressive properties in B cells. In vitro analysis of IL-35 reporter B cells revealed a synergy between the BCR and TLR4 signaling pathways is sufficient to induce IL-35 expression. However, in vivo, B cell receptor activation, as opposed to MyD88 signaling in B cells, is central to B cell-mediated suppression and promotion of pancreatic cancer growth. Further analysis identified protein kinase D2 (PKD2) as being a key downstream regulator of IL-35 expression in B cells. Regulatory B cells with an inactivating mutation in PKD2 failed to produce IL-35 or fully suppress effector T cell function in vitro. Furthermore, inhibition of PKD in B cells decreased tumor growth and promoted effector T cell function upon adoptive transfer into B cell-deficient mice. Collectively, these data provide insight into how regulatory B cell function is promoted in pancreatic cancer and identify potential therapeutic targets to restrain this function.
-
-
p21 produces a bioactive secretome that places stressed cells under immunosurveillance.
In Science on 29 October 2021 by Sturmlechner, I., Zhang, C., et al.
PubMed
Immune cells identify and destroy damaged cells to prevent them from causing cancer or other pathologies by mechanisms that remain poorly understood. Here, we report that the cell-cycle inhibitor p21 places cells under immunosurveillance to establish a biological timer mechanism that controls cell fate. p21 activates retinoblastoma protein (Rb)–dependent transcription at select gene promoters to generate a complex bioactive secretome, termed p21-activated secretory phenotype (PASP). The PASP includes the chemokine CXCL14, which promptly attracts macrophages. These macrophages disengage if cells normalize p21 within 4 days, but if p21 induction persists, they polarize toward an M1 phenotype and lymphocytes mount a cytotoxic T cell response to eliminate target cells, including preneoplastic cells. Thus, p21 concurrently induces proliferative arrest and immunosurveillance of cells under duress.
-
-
Immunology and Microbiology
Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral immune modulation.
In Mol Ther Oncolytics on 24 September 2021 by Sugawara, K., Iwai, M., et al.
PubMed
Oncolytic virus therapy can increase the immunogenicity of tumors and remodel the immunosuppressive tumor microenvironment, leading to an increased antitumor response to immune-checkpoint inhibitors. Here, we investigated the therapeutic potential of G47Δ, a third-generation oncolytic herpes simplex virus type 1, in combination with immune-checkpoint inhibitors using various syngeneic murine subcutaneous tumor models. Intratumoral inoculations with G47Δ and systemic anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody administration caused an enhanced antitumor activity when combined and worked synergistically. Conversely, the efficacy of G47Δ in combination with anti-programmed cell death protein-1 (PD-1) antibody was equivalent to that of the anti-PD-1 antibody alone in all murine models examined. The combination of intratumoral G47Δ and systemic anti-CTLA-4 antibody was shown to recruit effector T cells into the tumor efficiently while decreasing regulatory T cells. Furthermore, a wide range of gene signatures related to inflammation, lymphoid lineage, and T cell activation was highly upregulated with the combination therapy, suggesting the conversion of immune-insusceptible tumors to immune susceptible. The therapeutic effect proved tumor specific and long lasting. Immune cell subset depletion studies demonstrated that CD4+ T cells were required for synergistic curative activity. The results depict the dynamics of immune modulation of the tumor microenvironment and provide a clinical rationale for using G47Δ with immune checkpoint inhibitors.
-
-
-
Immunology and Microbiology
The PD-1 checkpoint receptor maintains tolerance of self-reactive CD8 T cell in skin
In bioRxiv on 10 July 2021 by Damo, M., Cui, C., et al.
-
-
-
Cancer Research
Radiotherapy-Activated Hafnium Oxide Nanoparticles Produce Abscopal Effect in a Mouse Colorectal Cancer Model.
In Int J Nanomedicine on 26 June 2020 by Zhang, P., Darmon, A., et al.
PubMed
Despite tremendous results achieved by immune checkpoint inhibitors, most patients are not responders, mainly because of the lack of a pre-existing anti-tumor immune response. Thus, solutions to efficiently prime this immune response are currently under intensive investigations. Radiotherapy elicits cancer cell death, generating an antitumor-specific T cell response, turning tumors in personalized in situ vaccines, with potentially systemic effects (abscopal effect). Nonetheless, clinical evidence of sustained anti-tumor immunity as abscopal effect are rare.
-
-
-
Cancer Research
4-1BB Agonism Averts TIL Exhaustion and Licenses PD-1 Blockade in Glioblastoma and Other Intracranial Cancers.
In Clin Cancer Res on 15 March 2020 by Woroniecka, K. I., Rhodin, K. E., et al.
PubMed
The success of checkpoint blockade against glioblastoma (GBM) has been disappointing. Anti-PD-1 strategies may be hampered by severe T-cell exhaustion. We sought to develop a strategy that might license new efficacy for checkpoint blockade in GBM.
-
-
-
-
-
Cancer Research
-
Immunology and Microbiology
B cell-Derived IL35 Drives STAT3-Dependent CD8+ T-cell Exclusion in Pancreatic Cancer.
In Cancer Immunol Res on 1 March 2020 by Mirlekar, B., Michaud, D., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignancy characterized by a paucity of tumor-proximal CD8+ T cells and resistance to immunotherapeutic interventions. Cancer-associated mechanisms that elicit CD8+ T-cell exclusion and resistance to immunotherapy are not well-known. Here, using a Kras- and p53-driven model of PDA, we describe a mechanism of action for the protumorigenic cytokine IL35 through STAT3 activation in CD8+ T cells. Distinct from its action on CD4+ T cells, IL35 signaling in gp130+CD8+ T cells activated the transcription factor STAT3, which antagonized intratumoral infiltration and effector function of CD8+ T cells via suppression of CXCR3, CCR5, and IFNγ expression. Inhibition of STAT3 signaling in tumor-educated CD8+ T cells improved PDA growth control upon adoptive transfer to tumor-bearing mice. We showed that activation of STAT3 in CD8+ T cells was driven by B cell- but not regulatory T cell-specific production of IL35. We also demonstrated that B cell-specific deletion of IL35 facilitated CD8+ T-cell activation independently of effector or regulatory CD4+ T cells and was sufficient to phenocopy therapeutic anti-IL35 blockade in overcoming resistance to anti-PD-1 immunotherapy. Finally, we identified a circulating IL35+ B-cell subset in patients with PDA and demonstrated that the presence of IL35+ cells predicted increased occurrence of phosphorylated (p)Stat3+CXCR3-CD8+ T cells in tumors and inversely correlated with a cytotoxic T-cell signature in patients. Together, these data identified B cell-mediated IL35/gp130/STAT3 signaling as an important direct link to CD8+ T-cell exclusion and immunotherapy resistance in PDA.
-
-
-
Cancer Research
-
Immunology and Microbiology
Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP.
In Immunity on 18 February 2020 by Zhou, Y., Fei, M., et al.
PubMed
Clearance of apoptotic cells by macrophages prevents excessive inflammation and supports immune tolerance. Here, we examined the effect of blocking apoptotic cell clearance on anti-tumor immune response. We generated an antibody that selectively inhibited efferocytosis by phagocytic receptor MerTK. Blockade of MerTK resulted in accumulation of apoptotic cells within tumors and triggered a type I interferon response. Treatment of tumor-bearing mice with anti-MerTK antibody stimulated T cell activation and synergized with anti-PD-1 or anti-PD-L1 therapy. The anti-tumor effect induced by anti-MerTK treatment was lost in Stinggt/gt mice, but not in Cgas-/- mice. Abolishing cGAMP production in Cgas-/- tumor cells, depletion of extracellular ATP, or inactivation of the ATP-gated P2X7R channel also compromised the effects of MerTK blockade. Mechanistically, extracellular ATP acted via P2X7R to enhance the transport of extracellular cGAMP into macrophages and subsequent STING activation. Thus, MerTK blockade increases tumor immunogenicity and potentiates anti-tumor immunity, which has implications for cancer immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer.
In Int J Hyperthermia on 1 November 2019 by Oei, A. L., Korangath, P., et al.
PubMed
Purpose: Enhancing immune responses in triple negative breast cancers (TNBCs) remains a challenge. Our study aimed to determine whether magnetic iron oxide nanoparticle (MION) hyperthermia (HT) can enhance abscopal effects with radiotherapy (RT) and immune checkpoint inhibitors (IT) in a metastatic TNBC model.Methods: One week after implanting 4T1-luc cells into the mammary glands of BALB/c mice, tumors were treated with RT (3 × 8 Gy)±local HT, mild (HTM, 43 °C/20 min) or partially ablative (HTAbl, 45 °C/5 min plus 43 °C/15 min),±IT with anti-PD-1 and anti-CTLA-4 antibodies (both 4 × 10 mg/kg, i.p.). Tumor growth was measured daily. Two weeks after treatment, lungs and livers were harvested for histopathology evaluation of metastases.Results: Compared to untreated controls, all treatment groups demonstrated a decreased tumor volume; however, when compared against surgical resection, only RT + HTM+IT, RT + HTAbl+IT and RT + HTAbl had similar or smaller tumors. These cohorts showed more infiltration of CD3+ T-lymphocytes into the primary tumor. Tumor growth effects were partially reversed with T-cell depletion. Combinations that proved most effective for primary tumors generated modest reductions in numbers of lung metastases. Conversely, numbers of lung metastases showed potential to increase following HT + IT treatment, particularly when compared to RT. Compared to untreated controls, there was no improvement in survival with any treatment.Conclusions: Single-fraction MION HT added to RT + IT improved local tumor control and recruitment of CD3+ T-lymphocytes, with only a modest effect to reduce lung metastases and no improvement in overall survival. HT + IT showed potential to increase metastatic dissemination to lungs.
-