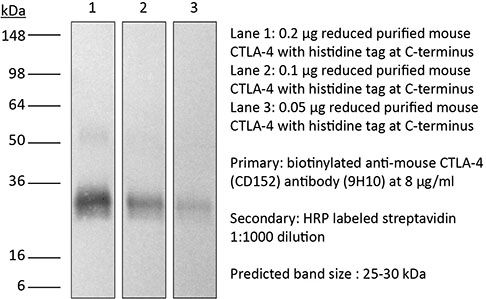
InVivoMAb anti-mouse CTLA-4 (CD152)
Product Description
Specifications
| Isotype | Syrian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Syrian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CTLA-4-human IgG1 fusion protein |
| Reported Applications |
in vivo CTLA-4 neutralization in vitro CTLA-4 neutralization Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950184 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CTLA-4 neutralization
Ariyan, C. E., et al (2018). "Robust Antitumor Responses Result from Local Chemotherapy and CTLA-4 Blockade" Cancer Immunol Res 6(2): 189-200.
PubMed
Clinical responses to immunotherapy have been associated with augmentation of preexisting immune responses, manifested by heightened inflammation in the tumor microenvironment. However, many tumors have a noninflamed microenvironment, and response rates to immunotherapy in melanoma have been <50%. We approached this problem by utilizing immunotherapy (CTLA-4 blockade) combined with chemotherapy to induce local inflammation. In murine models of melanoma and prostate cancer, the combination of chemotherapy and CTLA-4 blockade induced a shift in the cellular composition of the tumor microenvironment, with infiltrating CD8(+) and CD4(+) T cells increasing the CD8/Foxp3 T-cell ratio. These changes were associated with improved survival of the mice. To translate these findings into a clinical setting, 26 patients with advanced melanoma were treated locally by isolated limb infusion with the nitrogen mustard alkylating agent melphalan followed by systemic administration of CTLA-4 blocking antibody (ipilimumab) in a phase II trial. This combination of local chemotherapy with systemic checkpoint blockade inhibitor resulted in a response rate of 85% at 3 months (62% complete and 23% partial response rate) and a 58% progression-free survival at 1 year. The clinical response was associated with increased T-cell infiltration, similar to that seen in the murine models. Together, our findings suggest that local chemotherapy combined with checkpoint blockade-based immunotherapy results in a durable response to cancer therapy.
in vivo CTLA-4 neutralization
Gao, J., et al (2016). "Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy" Cell 167(2): 397-404 e399.
PubMed
Antibody blockade of the inhibitory CTLA-4 pathway has led to clinical benefit in a subset of patients with metastatic melanoma. Anti-CTLA-4 enhances T cell responses, including production of IFN-gamma, which is a critical cytokine for host immune responses. However, the role of IFN-gamma signaling in tumor cells in the setting of anti-CTLA-4 therapy remains unknown. Here, we demonstrate that patients identified as non-responders to anti-CTLA-4 (ipilimumab) have tumors with genomic defects in IFN-gamma pathway genes. Furthermore, mice bearing melanoma tumors with knockdown of IFN-gamma receptor 1 (IFNGR1) have impaired tumor rejection upon anti-CTLA-4 therapy. These data highlight that loss of the IFN-gamma signaling pathway is associated with primary resistance to anti-CTLA-4 therapy. Our findings demonstrate the importance of tumor genomic data, especially IFN-gamma related genes, as prognostic information for patients selected to receive treatment with immune checkpoint therapy.
in vivo CTLA-4 neutralization
Bartkowiak, T., et al (2015). "Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine" Proc Natl Acad Sci U S A 112(38): E5290-5299.
PubMed
Antibody modulation of T-cell coinhibitory (e.g., CTLA-4) or costimulatory (e.g., 4-1BB) receptors promotes clinical responses to a variety of cancers. Therapeutic cancer vaccination, in contrast, has produced limited clinical benefit and no curative therapies. The E6 and E7 oncoproteins of human papilloma virus (HPV) drive the majority of genital cancers, and many oropharyngeal tumors. We discovered 15-19 amino acid peptides from HPV-16 E6/E7 for which induction of T-cell immunity correlates with disease-free survival in patients treated for high-grade cervical neoplasia. We report here that intranasal vaccination with these peptides and the adjuvant alpha-galactosylceramide elicits systemic and mucosal T-cell responses leading to reduced HPV(+) TC-1 tumor growth and prolonged survival in mice. We hypothesized that the inability of these T cells to fully reject established tumors resulted from suppression in the tumor microenvironment which could be ameliorated through checkpoint modulation. Combining this E6/E7 peptide vaccine with checkpoint blockade produced only modest benefit; however, coadministration with a 4-1BB agonist antibody promoted durable regression of established genital TC-1 tumors. Relative to other therapies tested, this combination of vaccine and alpha4-1BB promoted the highest CD8(+) versus regulatory FoxP3(+) T-cell ratios, elicited 2- to 5-fold higher infiltration by E7-specific CTL, and evoked higher densities of highly cytotoxic TcEO (T cytotoxic Eomesodermin) CD8 (>70-fold) and ThEO (T helper Eomesodermin) CD4 (>17-fold) T cells. These findings have immediate clinical relevance both in terms of the direct clinical utility of the vaccine studied and in illustrating the potential of 4-1BB antibody to convert therapeutic E6/E7 vaccines already in clinical trials into curative therapies.
in vivo CTLA-4 neutralization
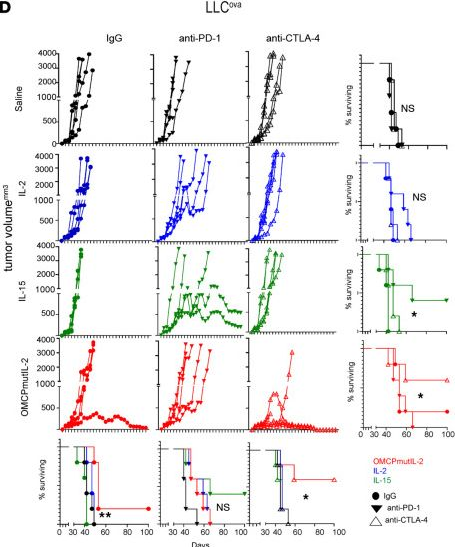
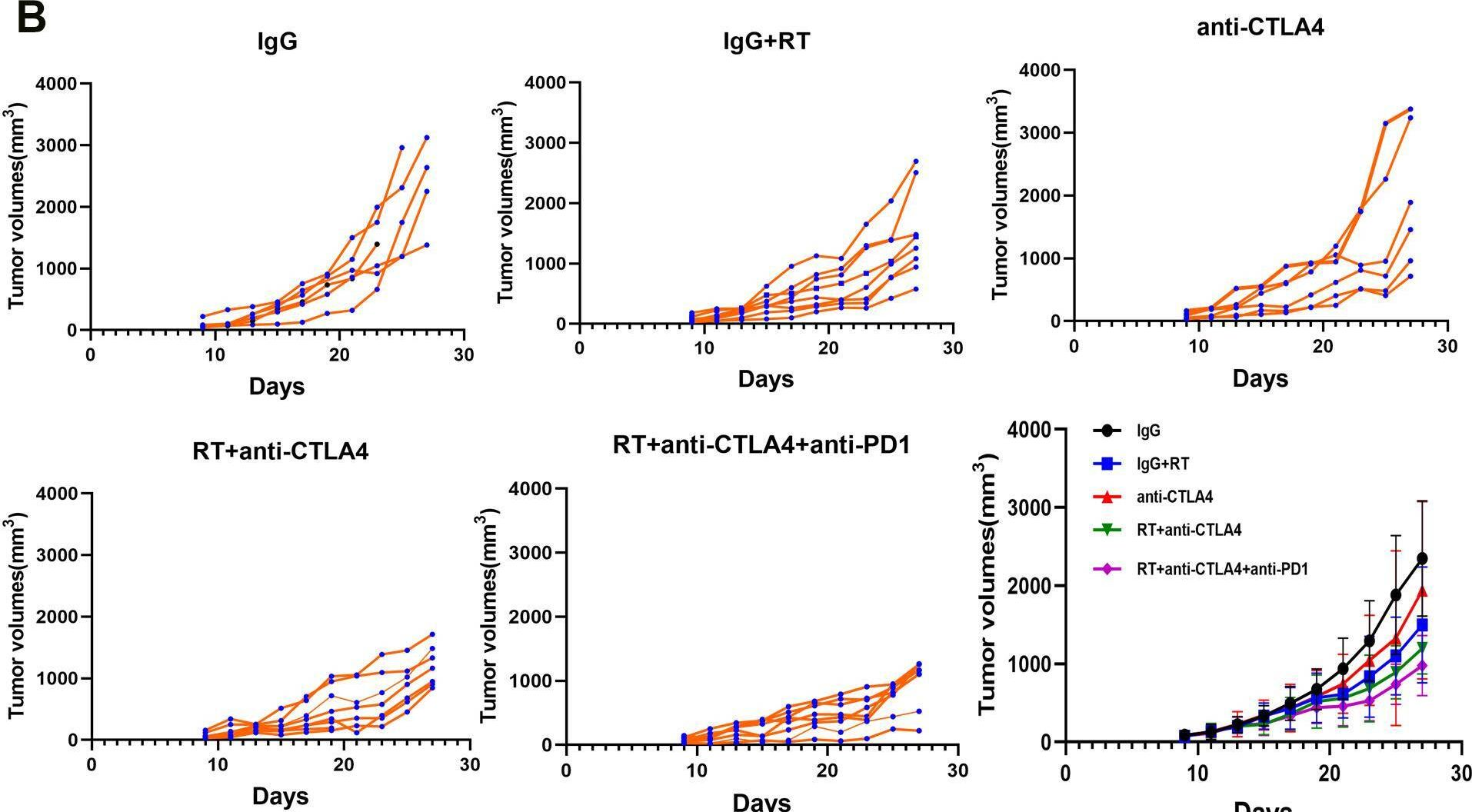
Twyman-Saint Victor, C., et al (2015). "Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer" Nature 520(7547): 373-377.
PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
in vivo CTLA-4 neutralization
Spranger, S., et al (2015). "Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity" Nature 523(7559): 231-235.
PubMed
Melanoma treatment is being revolutionized by the development of effective immunotherapeutic approaches. These strategies include blockade of immune-inhibitory receptors on activated T cells; for example, using monoclonal antibodies against CTLA-4, PD-1, and PD-L1 (refs 3-5). However, only a subset of patients responds to these treatments, and data suggest that therapeutic benefit is preferentially achieved in patients with a pre-existing T-cell response against their tumour, as evidenced by a baseline CD8(+) T-cell infiltration within the tumour microenvironment. Understanding the molecular mechanisms that underlie the presence or absence of a spontaneous anti-tumour T-cell response in subsets of cases, therefore, should enable the development of therapeutic solutions for patients lacking a T-cell infiltrate. Here we identify a melanoma-cell-intrinsic oncogenic pathway that contributes to a lack of T-cell infiltration in melanoma. Molecular analysis of human metastatic melanoma samples revealed a correlation between activation of the WNT/beta-catenin signalling pathway and absence of a T-cell gene expression signature. Using autochthonous mouse melanoma models we identified the mechanism by which tumour-intrinsic active beta-catenin signalling results in T-cell exclusion and resistance to anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy. Specific oncogenic signals, therefore, can mediate cancer immune evasion and resistance to immunotherapies, pointing to new candidate targets for immune potentiation.
in vivo CTLA-4 neutralization
Stephan, S. B., et al (2015). "Biopolymer implants enhance the efficacy of adoptive T-cell therapy" Nat Biotechnol 33(1): 97-101.
PubMed
Although adoptive T-cell therapy holds promise for the treatment of many cancers, its clinical utility has been limited by problems in delivering targeted lymphocytes to tumor sites, and the cells’ inefficient expansion in the immunosuppressive tumor microenvironment. Here we describe a bioactive polymer implant capable of delivering, expanding and dispersing tumor-reactive T cells. The approach can be used to treat inoperable or incompletely removed tumors by situating implants near them or at resection sites. Using a mouse breast cancer resection model, we show that the implants effectively support tumor-targeting T cells throughout resection beds and associated lymph nodes, and reduce tumor relapse compared to conventional delivery modalities. In a multifocal ovarian cancer model, we demonstrate that polymer-delivered T cells trigger regression, whereas injected tumor-reactive lymphocytes have little curative effect. Scaffold-based T-cell delivery may provide a viable treatment option for inoperable tumors and reduce the rate of metastatic relapse after surgery.
in vitro CTLA-4 neutralization
Krummey, S. M., et al (2014). "Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade" J Immunol 192(5): 2495-2504.
PubMed
Effector and memory T cells may cross-react with allogeneic Ags to mediate graft rejection. Whereas the costimulation properties of Th1 cells are well studied, relatively little is known about the costimulation requirements of microbe-elicited Th17 cells. The costimulation blocker CTLA-4 Ig has been ineffective in the treatment of several Th17-driven autoimmune diseases and is associated with severe acute rejection following renal transplantation, leading us to investigate whether Th17 cells play a role in CD28/CTLA-4 blockade-resistant alloreactivity. We established an Ag-specific model in which Th1 and Th17 cells were elicited via Mycobacterium tuberculosis and Candida albicans immunization, respectively. C. albicans immunization elicited a higher frequency of Th17 cells and conferred resistance to costimulation blockade following transplantation. Compared with the M. tuberculosis group, C. albicans-elicited Th17 cells contained a higher frequency of IL-17(+)IFN-gamma(+) producers and a lower frequency of IL-10(+) and IL-10(+)IL-17(+) cells. Importantly, Th17 cells differentially regulated the CD28/CTLA-4 pathway, expressing similarly high CD28 but significantly greater amounts of CTLA-4 compared with Th1 cells. Ex vivo blockade experiments demonstrated that Th17 cells are more sensitive to CTLA-4 coinhibition and therefore less susceptible to CTLA-4 Ig. These novel insights into the differential regulation of CTLA-4 coinhibition on CD4(+) T cells have implications for the immunomodulation of pathologic T cell responses during transplantation and autoimmunity.
in vivo CTLA-4 neutralization
Ozdemir, B. C., et al (2014). "Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival" Cancer Cell 25(6): 719-734.
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is associated with marked fibrosis and stromal myofibroblasts, but their functional contribution remains unknown. Transgenic mice with the ability to delete alphaSMA(+) myofibroblasts in pancreatic cancer were generated. Depletion starting at either noninvasive precursor (pancreatic intraepithelial neoplasia) or the PDAC stage led to invasive, undifferentiated tumors with enhanced hypoxia, epithelial-to-mesenchymal transition, and cancer stem cells, with diminished animal survival. In PDAC patients, fewer myofibroblasts in their tumors also correlated with reduced survival. Suppressed immune surveillance with increased CD4(+)Foxp3(+) Tregs was observed in myofibroblast-depleted mouse tumors. Although myofibroblast-depleted tumors did not respond to gemcitabine, anti-CTLA4 immunotherapy reversed disease acceleration and prolonged animal survival. This study underscores the need for caution in targeting carcinoma-associated fibroblasts in PDAC.
in vivo CTLA-4 neutralization
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
in vivo CTLA-4 neutralization
Goding, S. R., et al (2013). "Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma" J Immunol 190(9): 4899-4909.
PubMed
Recurrent solid malignancies are often refractory to standard therapies. Although adoptive T cell transfer may benefit select individuals, the majority of patients succumb to their disease. To address this important clinical dilemma, we developed a mouse melanoma model in which initial regression of advanced disease was followed by tumor recurrence. During recurrence, Foxp3(+) tumor-specific CD4(+) T cells became PD-1(+) and represented >60% of the tumor-specific CD4(+) T cells in the host. Concomitantly, tumor-specific CD4(+) T effector cells showed traits of chronic exhaustion, as evidenced by their high expression of the PD-1, TIM-3, 2B4, TIGIT, and LAG-3 inhibitory molecules. Although blockade of the PD-1/PD-L1 pathway with anti-PD-L1 Abs or depletion of tumor-specific regulatory T cells (Tregs) alone failed to reverse tumor recurrence, the combination of PD-L1 blockade with tumor-specific Treg depletion effectively mediated disease regression. Furthermore, blockade with a combination of anti-PD-L1 and anti-LAG-3 Abs overcame the requirement to deplete tumor-specific Tregs. In contrast, successful treatment of primary melanoma with adoptive cell therapy required only Treg depletion or Ab therapy, underscoring the differences in the characteristics of treatment between primary and relapsing cancer. These data highlight the need for preclinical development of combined immunotherapy approaches specifically targeting recurrent disease.
in vivo CTLA-4 neutralization
Waitz, R., et al (2012). "Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy" Cancer Res 72(2): 430-439.
PubMed
Thermal ablation to destroy tumor tissue may help activate tumor-specific T cells by elevating the presentation of tumor antigens to the immune system. However, the antitumor activity of these T cells may be restrained by their expression of the inhibitory T-cell coreceptor CTLA-4, the target of the recently U.S. Food and Drug Administration-approved antibody drug ipilumimab. By relieving this restraint, CTLA-4-blocking antibodies such as ipilumimab can promote tumor rejection, but the full scope of their most suitable applications has yet to be fully determined. In this study, we offer a preclinical proof-of-concept in the TRAMP C2 mouse model of prostate cancer that CTLA-4 blockade cooperates with cryoablation of a primary tumor to prevent the outgrowth of secondary tumors seeded by challenge at a distant site. Although growth of secondary tumors was unaffected by cryoablation alone, the combination treatment was sufficient to slow growth or trigger rejection. In addition, secondary tumors were highly infiltrated by CD4(+) T cells and CD8(+) T cells, and there was a significant increase in the ratio of intratumoral T effector cells to CD4(+)FoxP3(+) T regulatory cells, compared with monotherapy. These findings documented for the first time an effect of this immunotherapeutic intervention on the intratumoral accumulation and systemic expansion of CD8(+) T cells specific for the TRAMP C2-specific antigen SPAS-1. Although cryoablation is currently used to treat a targeted tumor nodule, our results suggest that combination therapy with CTLA-4 blockade will augment antitumor immunity and rejection of tumor metastases in this setting.
in vivo CTLA-4 neutralization
Balachandran, V. P., et al (2011). "Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido" Nat Med 17(9): 1094-1100.
PubMed
Imatinib mesylate targets mutated KIT oncoproteins in gastrointestinal stromal tumor (GIST) and produces a clinical response in 80% of patients. The mechanism is believed to depend predominantly on the inhibition of KIT-driven signals for tumor-cell survival and proliferation. Using a mouse model of spontaneous GIST, we found that the immune system contributes substantially to the antitumor effects of imatinib. Imatinib therapy activated CD8(+) T cells and induced regulatory T cell (T(reg) cell) apoptosis within the tumor by reducing tumor-cell expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (Ido). Concurrent immunotherapy augmented the efficacy of imatinib in mouse GIST. In freshly obtained human GIST specimens, the T cell profile correlated with imatinib sensitivity and IDO expression. Thus, T cells are crucial to the antitumor effects of imatinib in GIST, and concomitant immunotherapy may further improve outcomes in human cancers treated with targeted agents.
in vivo CTLA-4 neutralization
Pedicord, V. A., et al (2011). "Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance" Proc Natl Acad Sci U S A 108(1): 266-271.
PubMed
CTLA-4, an Ig superfamily molecule with homology to CD28, is one of the most potent negative regulators of T-cell responses. In vivo blockade of CTLA-4 exacerbates autoimmunity, enhances tumor-specific T-cell responses, and may inhibit the induction of T-cell anergy. Clinical trials of CTLA-4-blocking antibodies to augment T-cell responses to malignant melanoma are at an advanced stage; however, little is known about the effects of CTLA-4 blockade on memory CD8(+) T-cell responses and the formation and maintenance of long-term CD8(+) T-cell memory. In our studies, we show that during in vivo memory CD8(+) T-cell responses to Listeria monocytogenes infection, CTLA-4 blockade enhances bacterial clearance and increases memory CD8(+) T-cell expansion. This is followed by an accumulation of memory cells that are capable of producing the effector cytokines IFN-gamma and TNF-alpha. We also demonstrate that in a vaccination setting, blocking CTLA-4 during CD8(+) T-cell priming leads to increased expansion and maintenance of antigen-specific memory CD8(+) T cells without adversely affecting the overall T-cell repertoire. This leads to an increase in memory cell effector function and improved protective immunity against further bacterial challenges. These results indicate that transient blockade of CTLA-4 enhances memory CD8(+) T-cell responses and support the possible use of CTLA-4-blocking antibodies during vaccination to augment memory formation and maintenance.
in vivo CTLA-4 neutralization
Quezada, S. A., et al (2010). "Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts" J Exp Med 207(3): 637-650.
PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Tumor-intrinsic MHC-II activation in pancreatic ductal adenocarcinoma enhances immune response and treatment efficacy
In bioRxiv on 22 October 2025 by Chen, C., Gribbin, K. P., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Combination LIGHT overexpression and checkpoint blockade disrupts the tumor immune environment impacting colorectal liver metastases.
In Sci Adv on 10 October 2025 by Keenan, B. P., Qiao, G., et al.
PubMed
Colorectal cancer and liver metastases are a leading cause of cancer-related mortality. Overexpression of the immunostimulatory cytokine TNFSF14/LIGHT associates with improved survival and correlates with increased tumor-infiltrating lymphocytes in patients and a clinically relevant model of colorectal liver metastases. We demonstrate that LIGHT monotherapy activates T cells, but also induces T cell exhaustion and the recruitment of immunosuppressive elements. As colorectal liver metastases exhibit high levels of CTLA-4 expression, we combined LIGHT overexpression with anti-CTLA-4, leading to complete tumor control. The combination functions by homing tumor-infiltrating lymphocytes, inducing tumor antigen-specific T cells, and reversing T cell exhaustion. Whereas both LIGHT overexpression and anti-CTLA-4 increase tumor-promoting macrophages, the combination eliminates this population. The ability of LIGHT overexpression combined with CTLA-4 inhibition to reverse T cell exhaustion and myeloid cell suppression is supported by analysis of complementary patient cohorts and has strong clinical relevance, especially given that liver metastases contribute to immunotherapy resistance across various cancer types.
-
-
-
Immunology and Microbiology
BRD9 inhibition overcomes oncolytic virus therapy resistance in glioblastoma.
In Cell Rep Med on 19 August 2025 by Guo, C., Long, Z., et al.
PubMed
Long-term survival of glioblastoma multiforme (GBM) remains challenging, spurring the development of novel therapies such as oncolytic virus therapy. While oncolytic virus shows promise in clinical trials, many patients do not respond to this therapy. Here, we perform a CRISPR screening and identify the non-canonical BRG1/BRM-associated factor (ncBAF) complex as a pivotal tumor-intrinsic factor for oncolytic virotherapy resistance. Knocking out the ncBAF-specific subunit bromodomain-containing protein 9 (BRD9) markedly augments the oncolytic efficacy of oncolytic herpes simplex virus type 1 (oHSV1) and enhances antitumor immunity. Mechanistically, BRD9 binds to RELA and potentiates the expression of downstream antiviral genes. Notably, the application of BRD9 inhibitor (IBRD9) significantly enhances the oncolytic activity of oHSV1 in various GBM models. Moreover, reduced BRD9 levels strongly correlate with improved outcomes in clinical trials of oHSV1. These findings suggest that BRD9 is an attractive target for overcoming the resistance to oHSV1 in glioblastoma treatment.
-
-
-
Immunology and Microbiology
-
Cancer Research
Different tumour-resident memory T-cell subsets regulate responses to anti-PD-1 and anti-CTLA-4 cancer immunotherapies.
In Nat Commun on 1 July 2025 by Damei, I., Caidi, A., et al.
PubMed
The involvement of tumour-resident memory T (TRM) cells in responses to immune checkpoint inhibitors remains unclear. Here, we show that while CD103+CD8 TRM cells are involved in response to PD-1 blockade, CD49a+CD4 TRM cells are required for the response to anti-CTLA-4. Using preclinical mouse models, we demonstrate that the benefits of anti-PD-1 treatment are compromised in animals challenged with anti-CD8 and anti-CD103 blocking antibodies. By contrast, the benefits of anti-CTLA-4 are decreased by anti-CD4 and anti-CD49a neutralizing antibodies. Single-cell RNA sequencing on tumour-infiltrating T-lymphocytes (TIL) reveals a CD49a+CD4 TRM signature, enriched in Ctla-4 transcripts, exacerbated upon anti-CTLA-4. CTLA-4 blockade expands CD49a+CD4 TRM cells and increases tumour-specific CD4-TIL-mediated cytotoxicity. A CD49a+CD4 TRM signature enriched in CTLA-4 and cytotoxicity-linked transcripts is also identified in human TILs. Multiplex immunohistochemistry in a cohort of anti-CTLA-4-plus-anti-PD-1-treated melanomas reveals an increase in CD49a+CD4 T-cell density in pre-treatment tumours, which correlates with higher rates of patient progression-free survival. Thus, CD49a+CD4 TRM cells may correspond to a predictive biomarker of response to combined immunotherapy.
-
-
-
Cancer Research
Defining CDK12 as a tumor suppressor and therapeutic target in mouse models of tubo-ovarian high-grade serous carcinoma.
In Proc Natl Acad Sci U S A on 17 June 2025 by Tien, J. C., Zhai, Y., et al.
PubMed
Ovarian cancer is the sixth leading cause of cancer death among American women, with most fatalities attributable to tubo-ovarian high-grade serous carcinoma (HGSC). This malignancy usually develops resistance to conventional chemotherapy, underscoring the need for robust preclinical models to guide the development of novel therapies. Here, we introduce an HGSC mouse model generated via Ovgp1-driven Cre recombinase effecting CRISPR/Cas9-mediated deletion of Trp53, Rb1, and Nf1 tumor suppressors in mouse oviductal epithelium (m-sgPRN model). Cyclin-dependent kinase 12 (CDK12) inactivation-frequently observed in human HGSC-is associated with poorer outcomes, DNA damage accumulation (including tandem duplications), and increased tumor immunogenicity. In our system, coablation of Cdk12 (m-sgPRN;Cdk12KO) recapitulated hallmark features of HGSC, while accelerating tumor progression and reducing survival. In a conventional (Cre-lox-mediated) Trp53/Nf1/Rb1 triple knockout model with concurrent Cdk12 ablation (PRN;Cdk12KO mice), we observed T cell-rich immune infiltrates mirroring those seen clinically. We established both models as subcutaneous or intraperitoneal syngeneic allografts of CDK12-inactivated HGSC that exhibited sensitivity to immune checkpoint blockade. Furthermore, a CRISPR/Cas9 synthetic lethality screen in PRN;Cdk12KO-derived cell lines identified CDK13-an essential paralog of CDK12-as the most depleted candidate, confirming a previously reported synthetic lethal interaction. Pharmacologic CDK13/12 degradation (employing YJ1206) demonstrated enhanced efficacy in cell lines derived from both m-sgPRN;Cdk12KO and PRN;Cdk12KO models. Our results define CDK12 as a key tumor suppressor in tubo-ovarian HGSC and highlight CDK13 targeting as a promising therapeutic approach in CDK12-inactive disease. Additionally, we have established valuable in vivo resources to facilitate further investigation and drug development in this challenging malignancy.
-
-
-
Cancer Research
Fc-optimized anti-CTLA-4 antibodies increase tumor-associated high endothelial venules and sensitize refractory tumors to PD-1 blockade.
In Cell Rep Med on 17 June 2025 by Blanchard, L., Vina, E., et al.
PubMed
The lack of T cells in tumors is a major hurdle to successful immune checkpoint therapy (ICT). Therefore, therapeutic strategies promoting T cell recruitment into tumors are warranted to improve the treatment efficacy. Here, we report that Fc-optimized anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies are potent remodelers of tumor vasculature that increase tumor-associated high endothelial venules (TA-HEVs), specialized blood vessels supporting lymphocyte entry into tumors. Mechanistically, this effect is dependent on the Fc domain of anti-CTLA-4 antibodies and CD4+ T cells and involves interferon gamma (IFNγ). Unexpectedly, we find that the human anti-CTLA-4 antibody ipilimumab fails to increase TA-HEVs in a humanized mouse model. However, increasing its Fc effector function rescues the modulation of TA-HEVs, promotes CD4+ and CD8+ T cell infiltration into tumors, and sensitizes recalcitrant tumors to programmed cell death protein 1 (PD-1) blockade. Our findings suggest that Fc-optimized anti-CTLA-4 antibodies could be used to reprogram tumor vasculature in poorly immunogenic cold tumors and improve the efficacy of ICT.
-
-
-
Immunology and Microbiology
Characterisation of an autochthonous mouse ccRCC model of immune checkpoint inhibitor therapy resistance.
In Sci Rep on 5 June 2025 by Peighambari, A., Huang, H., et al.
PubMed
Many metastatic clear cell renal cell carcinomas (ccRCC) are resistant to immune checkpoint inhibitor therapies, however the mechanisms underlying sensitivity or resistance remain incompletely characterised. We demonstrate that ccRCCs in the Vhl/Trp53/Rb1 mutant mouse model are resistant to combined anti-PD-1/anti-CTLA-4 therapy alone and in combination with additional therapeutic agents that reflect current ccRCC clinical trials. However, in some animals in vivo checkpoint therapy allowed isolated splenic T cells to recognise cultured ccRCC cells from the same animal, implicating the tumour microenvironment in suppression of T cell activation. We identified putative immunosuppressive myeloid cell populations with features similar to myeloid cells in the microenvironment of human ccRCC. The expression patterns of immune checkpoint ligands in both the mouse model and in human ccRCC suggests that several checkpoint systems other than PD-1 and CTLA-4 are likely to represent the dominant T cell suppressive forces in ccRCC. Our findings characterise an autochthonous mouse ccRCC model of immune checkpoint inhibitor therapy resistance and pave the way for a systematic functional dissection of the identified potential molecular barriers to effective immune therapy of ccRCC.
-
-
-
Immunology and Microbiology
Deubiquitinase USP24 activated by IL-6/STAT3 enhances PD-1 protein stability and suppresses T cell antitumor response.
In Sci Adv on 18 April 2025 by Hsieh, H. C., Young, M. J., et al.
PubMed
Persisting programmed cell death-1 (PD-1) signaling impairs T cell effector function, which is highly associated with T cell exhaustion and immunotherapy failure. However, the mechanism responsible for PD-1 deubiquitination and T cell dysfunction remains unclear. Here, we show that ubiquitin-specific peptidase 24 (USP24) promotes PD-1 protein stability by removing K48-linked polyubiquitin. Increased interleukin-6 level transcriptionally activates the USP24 expression, which leads to PD-1 stabilization. Furthermore, USP24 deficiency reduces PD-1 levels in CD8+ T cells and attenuates EgfrL858R-driven lung tumorigenesis in Usp24C1695A catalytic deficient mice. Targeting PD-1 stability with the USP24-specific inhibitor USP24-i-101 boosts cytotoxic T cell activity, restrains lung tumor growth, and achieves superior therapeutic effects when combined with anti-CTLA4 immunotherapy. Clinically, patients with lung cancer exhibiting high USP24 expression in tumor-infiltrating CD8+ T cells display exhausted features and show unfavorable responses to immunotherapy. Our findings dissect the mechanism for regulating enhanced PD-1 stability in tumor-infiltrating CD8+ T cells and reveal USP24 as a potential target of antitumor immunotherapy.
-
-
-
Cancer Research
ACVR2A attenuation impacts lactate production and hyperglycolytic conditions attracting regulatory T cells in hepatocellular carcinoma.
In Cell Rep Med on 15 April 2025 by Yasukawa, K., Shimada, S., et al.
PubMed
Although ACVR2A mutations are prevalent in non-viral hepatocellular carcinomas (HCCs), the underlying mechanism remains unelucidated. Our molecular investigation reveals that ACVR2A impairment induces hyperglycolysis through the inactivation of the SMAD signaling pathway. Using syngeneic transplantation models and human clinical samples, we clarify that ACVR2A-deficient HCC cells produce and secrete lactate via the upregulation of lactate dehydrogenase A (LDHA) and monocarboxylate transporter 4 (MCT4) expression levels, which promotes regulatory T (Treg) cell accumulation and then acquires resistance to immune checkpoint inhibitors. Remarkably, genetic knockdown and pharmacological inhibition of MCT4 ameliorate the high-lactate milieu in ACVR2A-deficient HCC, resulting in the suppression of intratumoral Treg cell recruitment and the restoration of the sensitivity to PD-1 blockade. These findings furnish compelling evidence that lactate attenuates anti-tumor immunity and that therapeutics targeting this pathway present a promising strategy for mitigating immunotherapy resistance in ACVR2A-deficient HCC.
-
-
-
Cancer Research
CIT tumor lines: A novel series of immunogenic squamous cell skin carcinoma cell lines derived from chemical carcinogenesis
In bioRxiv on 8 April 2025 by Tanaka, M., Letchworth, R., et al.
-
-
-
Cardiovascular biology
-
Immunology and Microbiology
MLKL-Mediated Necroptosis Predominantly Contributes to Immune-Associated Myocardial Damage.
In Inflammation on 7 April 2025 by Sun, J., Wu, W., et al.
PubMed
Activated T cells and macrophages play a critical role in immune-associated myocarditis. However, the molecular and cellular mechanisms driving cardiomyocyte damage by immune cells remain poorly understood. In this study, we co-cultured human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) with activated human peripheral blood mononuclear cells (aPBMCs) to recapitulate myocardial infiltration of immune cells. Our results demonstrated that aPBMCs induced hiPSC-CMs death in a dose- and time-dependent manner. Transcriptome analysis revealed the activation of several death pathways, including pyroptosis, apoptosis and necroptosis. The time course of immunofluorescence staining of key proteins related to different death pathways demonstrated that necroptosis was the earliest activated pathway. Pharmacological blockade of necroptosis by targeting mixed lineage kinase domain-like protein (MLKL), receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein RIPK1 kinase 3 (RIPK3) protected hiPSC-CMs against injury induced by aPBMCs, while inhibitors of pyroptosis and apoptosis showed no protective effect. Moreover, MLKL knockdown in hiPSC-CMs prevented cell death due to aPBMCs challenge. Additionally, we validated the cardioprotective effects of blocking necroptosis in a mouse model of immune checkpoint inhibitors (ICIs)-related myocarditis using a combination of long-term anti-programmed cell death 1 (PD- 1) and anti-cytotoxic T-lymphocyte antigen- 4 (CTLA- 4) antibodies. ICIs led to elevation of myocardial injury markers in serum and activated immune cells infiltration. Furthermore, in vivo administration of a MLKL inhibitor prevented ICIs-induced myocardial injury. In conclusion, our findings suggested that MLKL-mediated necroptosis predominantly contributed to cardiomyocyte death resulting from activated immune cells. Suppressing necroptosis may be an effective therapeutic approach against myocardial damage in myocarditis.
-
-
-
Cancer Research
-
Immunology and Microbiology
Antigen-presenting cancer-associated fibroblasts in murine pancreatic tumors differentially control regulatory T cell phenotype and function via CXCL9 and CCL22
In bioRxiv on 1 April 2025 by Maru, S. Y., Wetzel, M., et al.
-
-
-
Cancer Research
Oncolytic adenovirus inhibits TNBC tumor growth/metastasis in mice by targeting TGF-β and overexpressing GM-CSF.
In Mol Ther Oncol on 20 March 2025 by Nhan, N. T. T., Shin, S. C., et al.
PubMed
Despite therapeutic advancements, metastatic triple-negative breast cancer (TNBC) remains mostly incurable and is a frequent cause of cancer-related deaths. We tested the hypothesis that inhibiting suppressive signals sustained by transforming growth factor (TGF)-β and concurrently stimulating recruitment of inflammatory cells with granulocyte-macrophage colony-stimulating factor (GM-CSF) by oncolytic viruses would result in improved anti-tumor responses. Thus, we developed a new oncolytic adenovirus rAd.sT.GM (AMUN-003) that expresses both sTGFβRIIFc (a TGF-β decoy), and GM-CSF and tested it in a mouse TNBC (4T1) subcutaneous model. rAd.sT.GM was safe to use and more effective in controlling tumor progression and lung metastasis following intratumoral injections when compared with control adenoviruses without modifications. In the same model, combinations of immune checkpoint inhibitor (ICI) therapy with rAd.sT.GM resulted in better inhibition of tumor growth and metastasis. Furthermore, we examined key immune response and prognosis biomarkers in sera, lungs, spleens, and tumors to evaluate the treatment efficacy. We found several key anti-tumor Th1 cytokines such as interleukin (IL)-2, IL-4, and interferon-γ, were stimulated by the combination therapy either systemically or in tumors or both, as well as anti-tumor biomarkers such as Granzyme B and perforin. These results support advancement to clinical testing with the combination therapy of rAd.sT.GM and ICIs for TNBC patients.
-
-
-
Cancer Research
Inhibitors of oncogenic Kras specifically prime CTLA4 blockade to transcriptionally reprogram Tregs and overcome resistance to suppress pancreas cancer
In bioRxiv on 4 March 2025 by Mahadevan, K. K., Maldonado, A. S., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Glutathione peroxidase 4 (GPX4) and obesity interact to impact tumor progression and treatment response in triple negative breast cancer.
In Cancer Metab on 25 February 2025 by Devericks, E. N., Brosnan, B. H., et al.
PubMed
Triple-negative breast cancer (TNBC), which tends to be more advanced when diagnosed and more aggressive than other breast cancer subtypes, is accelerated by obesity. Hypertrophic adipocytes and cancer cells exhibit increased oxidative stress and altered redox homeostasis, influencing therapeutic outcomes. Enzymes implicated in both redox regulation and TNBC include glutathione peroxidase 4 (GPX4; reduces lipid peroxides) and pyruvate carboxylase (PC; essential in oxidative stress protection). Using preclinical models, we characterized interactions between GPX4, PC, and oxidative stress in TNBC cells, and established effects of GPX4 suppression on TNBC progression. In TNBC cells, PC knockdown increased GPX4 expression, while GPX4 knockdown increased PC expression. GPX4 inhibition by erastin or RSL3 enhanced TNBC cell death in vitro, and antioxidants mitigated the cytotoxicity. In obese mice, GPX4 knockdown, versus scramble control: (i) reduced tumor burden following orthotopic transplantation of TNBC cells; and (ii) reduced lung metastasis following tail vein injection of TNBC cells in combination with chemotherapy (carboplatin) but not immunotherapy (anti-CTLA4 plus anti-PD1). We conclude that GPX4 and PC expression are inversely related in TNBC cells, and GPX4 and obesity interact to impact TNBC progression and treatment responses. Moreover, GPX4-mediated redox defense, alone or in combination with chemotherapy, is a targetable vulnerability for treating TNBC, including obesity-related TNBC.
-
-
-
Immunology and Microbiology
-
Cancer Research
CD4 T cell depletion increases memory differentiation of endogenous and CAR T cells and enhances the efficacy of Super2 and IL-33-armored CAR T cells against solid tumors.
In J Immunother Cancer on 11 February 2025 by Mohamed, A. O., Boone, D. T., et al.
PubMed
Responsiveness to chimeric antigen receptor (CAR) T cell therapy correlates with CAR T cell expansion and persistence in vivo. Multiple strategies improve persistence by increasing stem-like properties or sustaining CAR T cell activity with combination therapies. Here, we describe the intrinsic ability of CAR T cells to differentiate into memory T cells, the effect of cytokine armoring, and neoadjuvant CD4 depletion therapy on CAR and tumor-specific endogenous memory T cells.
-
-
-
Immunology and Microbiology
-
Cancer Research
T Cells Instruct Immune Checkpoint Inhibitor Therapy Resistance in Tumors Responsive to IL1 and TNFα Inflammation.
In Cancer Immunol Res on 3 February 2025 by Cho, N. W., Guldberg, S. M., et al.
PubMed
Resistance to immune checkpoint inhibitors (ICI) is common, even in tumors with T-cell infiltration. We thus investigated consequences of ICI-induced T-cell infiltration in the microenvironment of resistant tumors. T cells and neutrophil numbers increased in ICI-resistant tumors following treatment, in contrast to ICI-responsive tumors. Resistant tumors were distinguished by high expression of IL1 receptor 1, enabling a synergistic response to IL1 and TNFα to induce G-CSF, CXCL1, and CXCL2 via NF-κB signaling, supporting immunosuppressive neutrophil accumulation in tumor. Perturbation of this inflammatory resistance circuit sensitized tumors to ICIs. Paradoxically, T cells drove this resistance circuit via TNFα both in vitro and in vivo. Evidence of this inflammatory resistance circuit and its impact also translated to human cancers. These data support a mechanism of ICI resistance, wherein treatment-induced T-cell activity can drive resistance in tumors responsive to IL1 and TNFα, with important therapeutic implications.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Lactobacillus rhamnosus GG induces STING-dependent IL-10 in intestinal monocytes and alleviates inflammatory colitis in mice.
In J Clin Invest on 3 February 2025 by Si, W., Zhao, X., et al.
PubMed
Preclinical and clinical observations indicate that the probiotic Lactobacillus rhamnosus GG (LGG) can modulate colonic inflammation. However, the underlying mechanisms have not been explored in depth. Here, we demonstrate that oral administration of live LGG alleviated inflammatory colitis by increasing IL-10 expression in intestinal Ly6C+ monocytes. Mechanistically, LGG induced IL-10 production via the stimulator of IFN genes (STING)/TBK1/NF-κB (RELA) signaling pathway in intestinal Ly6C+ monocytes, enhancing their immune-suppressive function. Elevated IL-10 subsequently activated IL-10 signaling in Ly6C+ monocytes, resulting in an IL-10-based autocrine regulatory loop and inhibition of proinflammatory cytokine production. Furthermore, LGG shifted the gut microbial community and its metabolic functions, leading to intestinal immune responses against colitis. Fecal microbiota transplantation from LGG-colonized mice alleviated immune checkpoint blockade-associated colitis. Our findings highlight the importance of STING signaling in IL-10-dependent antiinflammatory immunity and establish an empirical basis for developing oral administration of live LGG as an efficient and safe therapeutic strategy against inflammatory colitis.
-
-
-
Cancer Research
CDK8 remodels the tumor microenvironment to resist the therapeutic efficacy of targeted KRASG12Dinhibition in pancreatic ductal adenocarcinoma
In bioRxiv on 2 February 2025 by McAndrews, K. M., Mahadevan, K. K., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Developing an Effective Therapeutic HPV Vaccine to Eradicate Large Tumors by Genetically Fusing Xcl1 and Incorporating IL-9 as Molecular Adjuvants.
In Vaccines (Basel) on 9 January 2025 by Sun, Z., Wu, Z., et al.
PubMed
Human papillomavirus (HPV) is a prevalent infection affecting both men and women, leading to various cytological lesions. Therapeutic vaccines mount a HPV-specific CD8+ cytotoxic T lymphocyte response, thus clearing HPV-infected cells. However, no therapeutic vaccines targeting HPV are currently approved for clinical treatment due to limited efficacy. Our goal is to develop a vaccine that can effectively eliminate tumors caused by HPV.
-