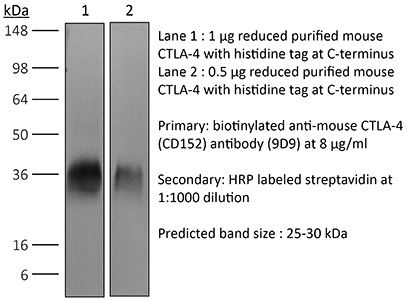
InVivoPlus anti-mouse CTLA-4 (CD152)
Product Description
Specifications
| Isotype | Mouse IgG2b |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus mouse IgG2b isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo CTLA-4 neutralization Western blot in vivo intra-tumoral regulatory T cell depletion in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_10949609 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro Organoids/Organ-on-Chip
Sivakumar R, Chan M, Shin JS, Nishida-Aoki N, Kenerson HL, Elemento O, Beltran H, Yeung R, Gujral TS (2019). "Organotypic tumor slice cultures provide a versatile platform for immuno-oncology and drug discovery" Oncoimmunology 8(12):e1670019.
PubMed
Organotypic tumor slices represent a physiologically-relevant culture system for studying the tumor microenvironment. Systematic characterization of the tumor slice culture system will enable its effective application for translational research. Here, using flow cytometry-based immunophenotyping, we performed a comprehensive characterization of the immune cell composition in organotypic tumor slices prepared from four syngeneic mouse tumor models and a human liver tumor. We found that the immune cell compositions of organotypic tumor slices prepared on the same day as the tumor cores were harvested are similar. Differences were primarily observed in the lymphocyte population of a clinical hepatocellular carcinoma case. Viable populations of immune cells persisted in the tumor slices for 7 days. Despite some changes in the immune cell populations, we showed the utility of mouse tumor slices for assessing responses to immune-modulatory agents. Further, we demonstrated the ability to use patient-derived xenograft tumor slices for assessing responses to targeted and cytotoxic drugs. Overall, tumor slices provide a broadly useful platform for studying the tumor microenvironment and evaluating the preclinical efficacy of cancer therapeutics.
in vivo CTLA-4 neutralization
Dai, M., et al (2015). "Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies" Clin Cancer Res 21(5): 1127-1138.
PubMed
PURPOSE: Immunomodulatory mAbs can treat cancer, but cures are rare except for small tumors. Our objective was to explore whether the therapeutic window increases by combining mAbs with different modes of action and injecting them into tumors. EXPERIMENTAL DESIGN: Combinations of mAbs to CD137/PD-1/CTLA-4 or CD137/PD-1/CTLA-4/CD19 were administrated intratumorally to mice with syngeneic tumors (B16 and SW1 melanoma, TC1 lung carcinoma), including tumors with a mean surface of approximately 80 mm(2). Survival and tumor growth were assessed. Immunologic responses were evaluated using flow cytometry and qRT-PCR. RESULTS: More than 50% of tumor-bearing mice had complete regression and long-term survival after tumor injection with mAbs recognizing CD137/PD-1/CTLA-4/CD19 with similar responses in three models. Intratumoral injection was more efficacious than intraperitoneal injection in causing rejection also of untreated tumors in the same mice. The three-mAb combination could also induce regression, but was less efficacious. There were few side effects, and therapy-resistant tumors were not observed. Transplanted tumor cells rapidly caused a Th2 response with increased CD19 cells. Successful therapy shifted this response to the Th1 phenotype with decreased CD19 cells and increased numbers of long-term memory CD8 effector cells and T cells making IFNgamma and TNFalpha. CONCLUSIONS: Intratumoral injection of mAbs recognizing CD137/PD-1/CTLA-4/CD19 can eradicate established tumors and reverse a Th2 response with tumor-associated CD19 cells to Th1 immunity, whereas a combination lacking anti-CD19 is less effective. There are several human cancers for which a similar approach may provide clinical benefit.
in vivo CTLA-4 neutralization
Zippelius, A., et al (2015). "Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment" Cancer Immunol Res 3(3): 236-244.
PubMed
CD40 stimulation on antigen-presenting cells (APC) allows direct activation of CD8(+) cytotoxic T cells, independent of CD4(+) T-cell help. Agonistic anti-CD40 antibodies have been demonstrated to induce beneficial antitumor T-cell responses in mouse models of cancer and early clinical trials. We report here that anti-CD40 treatment induces programmed death ligand-1 (PD-L1) upregulation on tumor-infiltrating monocytes and macrophages, which was strictly dependent on T cells and IFNgamma. PD-L1 expression could be counteracted by coadministration of antibodies blocking the PD-1 (programmed death-1)/PD-L1 axis as shown for T cells from tumor models and human donors. The combined treatment was highly synergistic and induced complete tumor rejection in about 50% of mice bearing MC-38 colon and EMT-6 breast tumors. Mechanistically, this was reflected by a strong increase of IFNgamma and granzyme-B production in intratumoral CD8(+) T cells. Concomitant CTLA-4 blockade further improved rejection of established tumors in mice. This study uncovers a novel mechanism of acquired resistance upon agonistic CD40 stimulation and proposes that the concomitant blockade of the PD-1/PD-L1 axis is a viable therapeutic strategy to optimize clinical outcomes.
in vivo CTLA-4 neutralization
Redmond, W. L., et al (2014). "Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity" Cancer Immunol Res 2(2): 142-153.
PubMed
Ligation of the TNF receptor family costimulatory molecule OX40 (CD134) with an agonist anti-OX40 monoclonal antibody (mAb) enhances antitumor immunity by augmenting T-cell differentiation as well as turning off the suppressive activity of the FoxP3(+)CD4(+) regulatory T cells (Treg). In addition, antibody-mediated blockade of the checkpoint inhibitor CTLA-4 releases the “brakes” on T cells to augment tumor immunotherapy. However, monotherapy with these agents has limited therapeutic benefit particularly against poorly immunogenic murine tumors. Therefore, we examined whether the administration of agonist anti-OX40 therapy in the presence of CTLA-4 blockade would enhance tumor immunotherapy. Combined anti-OX40/anti-CTLA-4 immunotherapy significantly enhanced tumor regression and the survival of tumor-bearing hosts in a CD4 and CD8 T cell-dependent manner. Mechanistic studies revealed that the combination immunotherapy directed the expansion of effector T-bet(high)/Eomes(high) granzyme B(+) CD8 T cells. Dual immunotherapy also induced distinct populations of Th1 [interleukin (IL)-2, IFN-gamma], and, surprisingly, Th2 (IL-4, IL-5, and IL-13) CD4 T cells exhibiting increased T-bet and Gata-3 expression. Furthermore, IL-4 blockade inhibited the Th2 response, while maintaining the Th1 CD4 and effector CD8 T cells that enhanced tumor-free survival. These data demonstrate that refining the global T-cell response during combination immunotherapy can further enhance the therapeutic efficacy of these agents.
in vivo CTLA-4 neutralization
Condamine, T., et al (2014). "ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis" J Clin Invest 124(6): 2626-2639.
PubMed
Myeloid-derived suppressor cells (MDSCs) dampen the immune response thorough inhibition of T cell activation and proliferation and often are expanded in pathological conditions. Here, we studied the fate of MDSCs in cancer. Unexpectedly, MDSCs had lower viability and a shorter half-life in tumor-bearing mice compared with neutrophils and monocytes. The reduction of MDSC viability was due to increased apoptosis, which was mediated by increased expression of TNF-related apoptosis-induced ligand receptors (TRAIL-Rs) in these cells. Targeting TRAIL-Rs in naive mice did not affect myeloid cell populations, but it dramatically reduced the presence of MDSCs and improved immune responses in tumor-bearing mice. Treatment of myeloid cells with proinflammatory cytokines did not affect TRAIL-R expression; however, induction of ER stress in myeloid cells recapitulated changes in TRAIL-R expression observed in tumor-bearing hosts. The ER stress response was detected in MDSCs isolated from cancer patients and tumor-bearing mice, but not in control neutrophils or monocytes, and blockade of ER stress abrogated tumor-associated changes in TRAIL-Rs. Together, these data indicate that MDSC pathophysiology is linked to ER stress, which shortens the lifespan of these cells in the periphery and promotes expansion in BM. Furthermore, TRAIL-Rs can be considered as potential targets for selectively inhibiting MDSCs.
in vivo CTLA-4 neutralization
Muller, P., et al (2014). "Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells" Cancer Immunol Res 2(8): 741-755.
PubMed
Antibody-drug conjugates (ADC) are emerging as powerful treatment strategies with outstanding target-specificity and high therapeutic activity in patients with cancer. Brentuximab vedotin represents a first-in-class ADC directed against CD30(+) malignancies. We hypothesized that its sustained clinical responses could be related to the stimulation of an anticancer immune response. In this study, we demonstrate that the dolastatin family of microtubule inhibitors, from which the cytotoxic component of brentuximab vedotin is derived, comprises potent inducers of phenotypic and functional dendritic cell (DC) maturation. In addition to the direct cytotoxic effect on tumor cells, dolastatins efficiently promoted antigen uptake and migration of tumor-resident DCs to the tumor-draining lymph nodes. Exposure of murine and human DCs to dolastatins significantly increased their capacity to prime T cells. Underlining the requirement of an intact host immune system for the full therapeutic benefit of dolastatins, the antitumor effect was far less pronounced in immunocompromised mice. We observed substantial therapeutic synergies when combining dolastatins with tumor antigen-specific vaccination or blockade of the PD-1-PD-L1 and CTLA-4 coinhibitory pathways. Ultimately, treatment with ADCs using dolastatins induces DC homing and activates cellular antitumor immune responses in patients. Our data reveal a novel mechanism of action for dolastatins and provide a strong rationale for clinical treatment regimens combining dolastatin-based therapies, such as brentuximab vedotin, with immune-based therapies.
in vivo CTLA-4 neutralization
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo CTLA-4 neutralization
Wei, H., et al (2013). "Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin" PLoS One 8(12): e84927.
PubMed
90 days (and was probably curative) by a mechanism which included a systemic CD8(+) T cell response with tumor specificity and immunological memory. Strikingly, combined treatment of cisplatin and CD137/PD-1 mAb also gave rise to the long-term survival of mice with established TC1 lung tumors. A similar combination of the 2 mAbs and cisplatin should be considered for clinical ‘translation’.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>There is an urgent need for improved therapy for advanced ovarian carcinoma, which may be met by administering immune-modulatory monoclonal antibodies (mAbs) to generate a tumor-destructive immune response. Using the ID8 mouse ovarian cancer model, we investigated the therapeutic efficacy of various mAb combinations in mice with intraperitoneal (i.p.) tumor established by transplanting 3 x 10(6) ID8 cells 10 days previously. While most of the tested mAbs were ineffective when given individually or together, the data confirm our previous finding that 2 i.p. injections of a combination of anti-CD137 with anti-PD-1 mAbs doubles overall survival. Mice treated with this mAb combination have a significantly increased frequency and total number of CD8(+) T cells both in the peritoneal lavage and spleens, and these cells are functional as demonstrated by antigen-specific cytolytic activity and IFN-gamma production. While administration of anti-CD137 mAb as a single agent similarly increases CD8(+) T cells, these have no functional activity, which may be attributed to up-regulation of co-inhibitory PD-1 and TIM-3 molecules induced by CD137. Addition of the anti-cancer drug cisplatin to the 2 mAb combination increased overall survival >90 days (and was probably curative) by a mechanism which included a systemic CD8(+) T cell response with tumor specificity and immunological memory. Strikingly, combined treatment of cisplatin and CD137/PD-1 mAb also gave rise to the long-term survival of mice with established TC1 lung tumors. A similar combination of the 2 mAbs and cisplatin should be considered for clinical ‘translation’.
in vivo CTLA-4 neutralization
Bulliard, Y., et al (2013). "Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies" J Exp Med 210(9): 1685-1693.
PubMed
Fc gamma receptor (FcgammaR) coengagement can facilitate antibody-mediated receptor activation in target cells. In particular, agonistic antibodies that target tumor necrosis factor receptor (TNFR) family members have shown dependence on expression of the inhibitory FcgammaR, FcgammaRIIB. It remains unclear if engagement of FcgammaRIIB also extends to the activities of antibodies targeting immunoregulatory TNFRs expressed by T cells. We have explored the requirement for activating and inhibitory FcgammaRs for the antitumor effects of antibodies targeting the TNFR glucocorticoid-induced TNFR-related protein (GITR; TNFRSF18; CD357) expressed on activated and regulatory T cells (T reg cells). We found that although FcgammaRIIB was dispensable for the in vivo efficacy of anti-GITR antibodies, in contrast, activating FcgammaRs were essential. Surprisingly, the dependence on activating FcgammaRs extended to an antibody targeting the non-TNFR receptor CTLA-4 (CD152) that acts as a negative regulator of T cell immunity. We define a common mechanism that correlated with tumor efficacy, whereby antibodies that coengaged activating FcgammaRs expressed by tumor-associated leukocytes facilitated the selective elimination of intratumoral T cell populations, particularly T reg cells. These findings may have broad implications for antibody engineering efforts aimed at enhancing the therapeutic activity of immunomodulatory antibodies.
in vivo CTLA-4 neutralization
Hooijkaas, A., et al (2012). "Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma" Oncoimmunology 1(5): 609-617.
PubMed
The development of targeted therapies and immunotherapies has markedly advanced the treatment of metastasized melanoma. While treatment with selective BRAF(V600E) inhibitors (like vemurafenib or dabrafenib) leads to high response rates but short response duration, CTLA-4 blocking therapies induce sustained responses, but only in a limited number of patients. The combination of these diametric treatment approaches may further improve survival, but pre-clinical data concerning this approach is limited. We investigated, using Tyr::CreER(T2)PTEN(F-/-)BRAF(F-V600E/+) inducible melanoma mice, whether BRAF(V600E) inhibition can synergize with anti-CTLA-4 mAb treatment, focusing on the interaction between the BRAF(V600E) inhibitor PLX4720 and the immune system. While PLX4720 treatment strongly decreased tumor growth, it did not induce cell death in BRAF(V600E)/PTEN(-/-) melanomas. More strikingly, PLX4720 treatment led to a decreased frequency of tumor-resident T cells, NK-cells, MDSCs and macrophages, which could not be restored by the addition of anti-CTLA-4 mAb. As this effect was not observed upon treatment of BRAF wild-type B16F10 tumors, we conclude that the decreased frequency of immune cells correlates to BRAF(V600E) inhibition in tumor cells and is not due to an off-target effect of PLX4720 on immune cells. Furthermore, anti-CTLA-4 mAb treatment of inducible melanoma mice treated with PLX4720 did not result in enhanced tumor control, while anti-CTLA-4 mAb treatment did improve the effect of tumor-vaccination in B16F10-inoculated mice. Our data suggest that vemurafenib may negatively affect the immune activity within the tumor. Therefore, the potential effect of targeted therapy on the tumor-microenvironment should be taken into consideration in the design of clinical trials combining targeted and immunotherapy.
in vivo CTLA-4 neutralization
Curran, M. A., et al (2011). "Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production" PLoS One 6(4): e19499.
PubMed
BACKGROUND: The co-inhibitory receptor Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) attenuates immune responses and prevent autoimmunity, however, tumors exploit this pathway to evade the host T-cell response. The T-cell co-stimulatory receptor 4-1BB is transiently upregulated on T-cells following activation and increases their proliferation and inflammatory cytokine production when engaged. Antibodies which block CTLA-4 or which activate 4-1BB can promote the rejection of some murine tumors, but fail to cure poorly immunogenic tumors like B16 melanoma as single agents. METHODOLOGY/PRINCIPAL FINDINGS: We find that combining alphaCTLA-4 and alpha4-1BB antibodies in the context of a Flt3-ligand, but not a GM-CSF, based B16 melanoma vaccine promoted synergistic levels of tumor rejection. 4-1BB activation elicited strong infiltration of CD8+ T-cells into the tumor and drove the proliferation of these cells, while CTLA-4 blockade did the same for CD4+ effector T-cells. Anti-4-1BB also depressed regulatory T-cell infiltration of tumors. 4-1BB activation strongly stimulated inflammatory cytokine production in the vaccine and tumor draining lymph nodes and in the tumor itself. The addition of CTLA-4 blockade further increased IFN-gamma production from CD4+ effector T-cells in the vaccine draining node and the tumor. Anti 4-1BB treatment, with or without CTLA-4 blockade, induced approximately 75% of CD8+ and 45% of CD4+ effector T-cells in the tumor to express the killer cell lectin-like receptor G1 (KLRG1). Tumors treated with combination antibody therapy showed 1.7-fold greater infiltration by these KLRG1+CD4+ effector T-cells than did those treated with alpha4-1BB alone. CONCLUSIONS/SIGNIFICANCE: This study shows that combining T-cell co-inhibitory blockade with alphaCTLA-4 and active co-stimulation with alpha4-1BB promotes rejection of B16 melanoma in the context of a suitable vaccine. In addition, we identify KLRG1 as a useful marker for monitoring the anti-tumor immune response elicited by this therapy. These findings should aid in the design of future trials for the immunotherapy of melanoma.
in vivo CTLA-4 neutralization
Balachandran, V. P., et al (2011). "Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido" Nat Med 17(9): 1094-1100.
PubMed
Imatinib mesylate targets mutated KIT oncoproteins in gastrointestinal stromal tumor (GIST) and produces a clinical response in 80% of patients. The mechanism is believed to depend predominantly on the inhibition of KIT-driven signals for tumor-cell survival and proliferation. Using a mouse model of spontaneous GIST, we found that the immune system contributes substantially to the antitumor effects of imatinib. Imatinib therapy activated CD8(+) T cells and induced regulatory T cell (T(reg) cell) apoptosis within the tumor by reducing tumor-cell expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (Ido). Concurrent immunotherapy augmented the efficacy of imatinib in mouse GIST. In freshly obtained human GIST specimens, the T cell profile correlated with imatinib sensitivity and IDO expression. Thus, T cells are crucial to the antitumor effects of imatinib in GIST, and concomitant immunotherapy may further improve outcomes in human cancers treated with targeted agents.
Product Citations
-
-
Cancer Research
Nodal Expansion, Tumor Infiltration and Exhaustion of Neoepitope-Specific Th Cells After Prophylactic Peptide Vaccination and Anti-CTLA4 Therapy in Mouse Melanoma B16.
In Int J Mol Sci on 4 July 2025 by Shabalkina, A. V., Izosimova, A. V., et al.
PubMed
Peptide vaccines possess several advantages over mRNA vaccines but are generally less effective at inducing antitumor immunity. The bottlenecks limiting peptide vaccine efficacy could be elucidated by tracking and comparing vaccine-induced T-lymphocytes in successful and unsuccessful cases. Here we have applied our recent database of neoantigen-specific T cell receptors (TCRs) to profile tumor-specific T cells following vaccination with a neoantigen peptide vaccine and to correlate this with the response. Mice were vaccinated prophylactically with p30 peptide encoding B16 melanoma neoantigen (K739N mutation in Kif18b gene). The B16F0 melanoma in the vaccinated mice was additionally treated by a CTLA-4 checkpoint blockade. T cells from the tumors, tumor-draining lymph nodes (tdLNs) and vaccine depots were isolated, phenotyped, sorted by subsets and sequenced for TCR repertoires. The vaccine induced the accumulation of tumor-specific CD4+ Th cells in the tdLNs, while in the tumors these cells were present and their frequencies were not changed by the vaccine. These cells also accumulated at the vaccine depots, where they were phenotypically skewed by the vaccine components; however, these effects were minor due to approximately 50-fold lower cell quantities compared to the tdLNs. Only some of the p30-specific Th cells showed tumoricidal activity, as revealed by the reverse correlation of their frequencies in the tdLNs with the tumor size. The CTLA-4 blockade did not affect the tumor growth or the frequencies of tumor-specific cells but did stimulate Th cell motility. Thus, we have shown that tumor-specific Th clones accumulate and/or expand in the tdLNs, which correlates with tumor suppression but only for some of these clones. Tumor infiltration by these clones is not correlated with the growth rate.
-
-
-
Immunology and Microbiology
Mitoxantrone-Encapsulated ZIF-8 Enhances Chemo-Immunotherapy via Amplified Immunogenic Cell Death.
In Adv Sci (Weinh) on 1 April 2025 by Li, J., Lv, W., et al.
PubMed
Chemo-immunotherapy, combining systemic chemotherapeutic drugs and immune checkpoint blockers, is a promising paradigm in cancer treatment. However, challenges such as limited induction of immune responses and systemic immune toxicity have hindered its clinical applications. Here, a zeolite imidazolate framework-8 (ZIF-8) that encapsulates mitoxantrone (MIT), an immune cell death (ICD)-inducing chemotherapeutic agent (MIT@ZIF-8), is synthesized using a one-pot aqueous-phase process. ZIF-8 serves as a dual-functional nanomaterial for chemo-immunotherapy: a carrier to enhance tumor uptake of MIT for improved chemotherapy efficacy, and a pyroptosis inducer to amplify MIT-induced ICD for augmented anti-tumor immune responses. As a result, in vivo administration of MIT@ZIF-8 markedly inhibits tumor growth in both immunologically "hot" colon cancer and immunologically "cold" prostate cancer. Moreover, MIT@ZIF-8 treatment increases the abundance of cytotoxic CD8+ T cells and reduces the amount of immunosuppressive regulatory T cells in tumors, thereby enhancing anti-tumor immunity and sensitizing prostate cancer to anti-CTLA-4 immunotherapy. In summary, MIT@ZIF-8 offers a highly translational approach for chemo-immunotherapy.
-
-
-
Cancer Research
An activin receptor-like kinase 1-governed monocytic lineage shapes an immunosuppressive landscape in breast cancer metastases.
In J Clin Invest on 14 January 2025 by Safaee Talkhoncheh, M., Sjölund, J., et al.
PubMed
The biology centered around the TGF-β type I receptor activin receptor-like kinase (ALK) 1 (encoded by ACVRL1) has been almost exclusively based on its reported endothelial expression pattern since its first functional characterization more than 2 decades ago. Here, in efforts to better define the therapeutic context in which to use ALK1 inhibitors, we uncover a population of tumor-associated macrophages (TAMs) that, by virtue of their unanticipated Acvrl1 expression, are effector targets for adjuvant antiangiogenic immunotherapy in mouse models of metastatic breast cancer. The combinatorial benefit depended on ALK1-mediated modulation of the differentiation potential of bone marrow-derived granulocyte-macrophage progenitors, the release of CD14+ monocytes into circulation, and their eventual extravasation. Notably, ACVRL1+ TAMs coincided with an immunosuppressive phenotype and were overrepresented in human cancers progressing on therapy. Accordingly, breast cancer patients with a prominent ACVRL1hi TAM signature exhibited a significantly shorter survival. In conclusion, we shed light on an unexpected multimodal regulation of tumorigenic phenotypes by ALK1 and demonstrate its utility as a target for antiangiogenic immunotherapy.
-
-
CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors.
In Nature on 1 November 2024 by Skoulidis, F., Araújo, H. A., et al.
PubMed
For patients with advanced non-small-cell lung cancer (NSCLC), dual immune checkpoint blockade (ICB) with CTLA4 inhibitors and PD-1 or PD-L1 inhibitors (hereafter, PD-(L)1 inhibitors) is associated with higher rates of anti-tumour activity and immune-related toxicities, when compared with treatment with PD-(L)1 inhibitors alone. However, there are currently no validated biomarkers to identify which patients will benefit from dual ICB1,2. Here we show that patients with NSCLC who have mutations in the STK11 and/or KEAP1 tumour suppressor genes derived clinical benefit from dual ICB with the PD-L1 inhibitor durvalumab and the CTLA4 inhibitor tremelimumab, but not from durvalumab alone, when added to chemotherapy in the randomized phase III POSEIDON trial3. Unbiased genetic screens identified loss of both of these tumour suppressor genes as independent drivers of resistance to PD-(L)1 inhibition, and showed that loss of Keap1 was the strongest genomic predictor of dual ICB efficacy-a finding that was confirmed in several mouse models of Kras-driven NSCLC. In both mouse models and patients, KEAP1 and STK11 alterations were associated with an adverse tumour microenvironment, which was characterized by a preponderance of suppressive myeloid cells and the depletion of CD8+ cytotoxic T cells, but relative sparing of CD4+ effector subsets. Dual ICB potently engaged CD4+ effector cells and reprogrammed the tumour myeloid cell compartment towards inducible nitric oxide synthase (iNOS)-expressing tumoricidal phenotypes that-together with CD4+ and CD8+ T cells-contributed to anti-tumour efficacy. These data support the use of chemo-immunotherapy with dual ICB to mitigate resistance to PD-(L)1 inhibition in patients with NSCLC who have STK11 and/or KEAP1 alterations.
-
-
Cancer Research
Toward a CRISPR-based mouse model of Vhl-deficient clear cell kidney cancer: Initial experience and lessons learned.
In Proc Natl Acad Sci U S A on 8 October 2024 by Stransky, L. A., Gao, W., et al.
PubMed
CRISPR is revolutionizing the ability to do somatic gene editing in mice for the purpose of creating new cancer models. Inactivation of the VHL tumor suppressor gene is the signature initiating event in the most common form of kidney cancer, clear cell renal cell carcinoma (ccRCC). Such tumors are usually driven by the excessive HIF2 activity that arises when the VHL gene product, pVHL, is defective. Given the pressing need for a robust immunocompetent mouse model of human ccRCC, we directly injected adenovirus-associated viruses (AAVs) encoding sgRNAs against VHL and other known/suspected ccRCC tumor suppressor genes into the kidneys of C57BL/6 mice under conditions where Cas9 was under the control of one of two different kidney-specific promoters (Cdh16 or Pax8) to induce kidney tumors. An AAV targeting Vhl, Pbrm1, Keap1, and Tsc1 reproducibly caused macroscopic ccRCCs that partially resembled human ccRCC tumors with respect to transcriptome and cell of origin and responded to a ccRCC standard-of-care agent, axitinib. Unfortunately, these tumors, like those produced by earlier genetically engineered mouse ccRCCs, are HIF2 independent.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
In vivo experiments
NAT10/ac4C/JunB facilitates TNBC malignant progression and immunosuppression by driving glycolysis addiction.
In J Exp Clin Cancer Res on 4 October 2024 by Li, G., Ma, X., et al.
PubMed
N4-Acetylcytidine (ac4C), a highly conserved post-transcriptional mechanism, plays a pivotal role in RNA modification and tumor progression. However, the molecular mechanism by which ac4C modification mediates tumor immunosuppression remains elusive in triple-negative breast cancer (TNBC).
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Aberrant cytoplasmic expression of UHRF1 restrains the MHC-I-mediated anti-tumor immune response.
In Nat Commun on 3 October 2024 by Tan, L. M., Yin, T., et al.
PubMed
Immunotherapy successfully complements traditional cancer treatment. However, primary and acquired resistance might limit efficacy. Reduced antigen presentation by MHC-I has been identified as potential resistance factor. Here we show that the epigenetic regulator ubiquitin-like with PHD and ring finger domains 1 (UHRF1), exhibits altered expression and aberrant cytosolic localization in cancerous tissues, where it promotes MHC-I ubiquitination and degradation. Cytoplasmic translocation of UHRF1 is induced by its phosphorylation on a specific serine in response to signals provided by factors present in the tumor microenvironment (TME), such as TGF-β, enabling UHRF1 to bind MHC-I. Downregulation of MHC-I results in suppression of the antigen presentation pathway to establish an immune hostile TME. UHRF1 inactivation by genetic deletion synergizes with immune checkpoint blockade (ICB) treatment and induces an anti-tumour memory response by evoking low-affinity T cells. Our study adds to the understanding of UHRF1 in cancer immune evasion and provides a potential target to synergize with immunotherapy and overcome immunotherapeutic resistance.
-
-
-
Immunology and Microbiology
Validation of the C-X-C chemokine receptor 3 (CXCR3) as a target for PET imaging of T cell activation.
In EJNMMI Res on 28 August 2024 by Martin, S., Wendlinger, L., et al.
PubMed
CXCR3 is expressed on activated T cells and plays a crucial role in T-cell recruitment to the tumor microenvironment (TME) during cell-based and immune checkpoint inhibitor (ICI) immunotherapy. This study utilized a 64Cu-labeled NOTA-α-CXCR3 antibody to assess CXCR3 expression in the TME and validate it as a potential T cell activation biomarker in vivo.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Inhibition of MER proto-oncogene tyrosine kinase by an antisense oligonucleotide enhances treatment efficacy of immunoradiotherapy.
In J Exp Clin Cancer Res on 6 March 2024 by Hu, Y., Revenko, A., et al.
PubMed
The combination of radiotherapy and immunotherapy (immunoradiotherapy) has been increasingly used for treating a wide range of cancers. However, some tumors are resistant to immunoradiotherapy. We have previously shown that MER proto-oncogene tyrosine kinase (MerTK) expressed on macrophages mediates resistance to immunoradiotherapy. We therefore sought to develop therapeutics that can mitigate the negative impact of MerTK. We designed and developed a MerTK specific antisense oligonucleotide (ASO) and characterized its effects on eliciting an anti-tumor immune response in mice.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Aldehyde dehydrogenase 2-mediated aldehyde metabolism promotes tumor immune evasion by regulating the NOD/VISTA axis.
In J Immunother Cancer on 7 December 2023 by Chen, Y., Sun, J., et al.
PubMed
Aldehyde dehydrogenase 2 (ALDH2) is a crucial enzyme involved in endogenous aldehyde detoxification and has been implicated in tumor progression. However, its role in tumor immune evasion remains unclear.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Loss of p53 and mutational heterogeneity drives immune resistance in an autochthonous mouse lung cancer model with high tumor mutational burden.
In Cancer Cell on 9 October 2023 by Zhu, M., Kim, J., et al.
PubMed
The role of tumor mutational burden (TMB) in shaping tumor immunity is a key question that has not been addressable using genetically engineered mouse models (GEMMs) of lung cancer. To induce TMB in lung GEMMs, we expressed an ultra-mutator variant of DNA polymerase-E (POLE)P286R in lung epithelial cells. Introduction of PoleP286R allele into KrasG12D and KrasG12D; p53L/L (KP) models significantly increase their TMB. Immunogenicity and sensitivity to immune checkpoint blockade (ICB) induced by Pole is partially dependent on p53. Corroborating these observations, survival of NSCLC patients whose tumors have TP53truncating mutations is shorter than those with TP53WT with immunotherapy. Immune resistance is in part through reduced antigen presentation and in part due to mutational heterogeneity. Total STING protein levels are elevated in Pole mutated KP tumors creating a vulnerability. A stable polyvalent STING agonist or p53 induction increases sensitivity to immunotherapy offering therapeutic options in these polyclonal tumors.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Immune checkpoint activity regulates polycystic kidney disease progression.
In JCI Insight on 22 June 2023 by Kleczko, E. K., Nguyen, D. T., et al.
PubMed
Innate and adaptive immune cells modulate the severity of autosomal dominant polycystic kidney disease (ADPKD), a common kidney disease with inadequate treatment options. ADPKD has parallels with cancer, in which immune checkpoint inhibitors have been shown to reactivate CD8+ T cells and slow tumor growth. We have previously shown that in PKD, CD8+ T cell loss worsens disease. This study used orthologous early-onset and adult-onset ADPKD models (Pkd1 p.R3277C) to evaluate the role of immune checkpoints in PKD. Flow cytometry of kidney cells showed increased levels of programmed cell death protein 1 (PD-1)/cytotoxic T lymphocyte associated protein 4 (CTLA-4) on T cells and programmed cell death ligand 1 (PD-L1)/CD80 on macrophages and epithelial cells in Pkd1RC/RC mice versus WT, paralleling disease severity. PD-L1/CD80 was also upregulated in ADPKD human cells and patient kidney tissue versus controls. Genetic PD-L1 loss or treatment with an anti-PD-1 antibody did not impact PKD severity in early-onset or adult-onset ADPKD models. However, treatment with anti-PD-1 plus anti-CTLA-4, blocking 2 immune checkpoints, improved PKD outcomes in adult-onset ADPKD mice; neither monotherapy altered PKD severity. Combination therapy resulted in increased kidney CD8+ T cell numbers/activation and decreased kidney regulatory T cell numbers correlative with PKD severity. Together, our data suggest that immune checkpoint activation is an important feature of and potential novel therapeutic target in ADPKD.
-
-
-
Cancer Research
-
Immunology and Microbiology
Melanoma clonal subline analysis uncovers heterogeneity-driven immunotherapy resistance mechanisms
In bioRxiv on 5 April 2023 by Gruen, C., Yang, H. H., et al.
-
-
-
Cancer Research
-
Mus musculus (Mouse)
Hyaluronan driven by epithelial aPKC deficiency remodels the microenvironment and creates a vulnerability in mesenchymal colorectal cancer.
In Cancer Cell on 13 February 2023 by Martinez-Ordoñez, A., Duran, A., et al.
PubMed
Mesenchymal colorectal cancer (mCRC) is microsatellite stable (MSS), highly desmoplastic, with CD8+ T cells excluded to the stromal periphery, resistant to immunotherapy, and driven by low levels of the atypical protein kinase Cs (aPKCs) in the intestinal epithelium. We show here that a salient feature of these tumors is the accumulation of hyaluronan (HA) which, along with reduced aPKC levels, predicts poor survival. HA promotes epithelial heterogeneity and the emergence of a tumor fetal metaplastic cell (TFMC) population endowed with invasive cancer features through a network of interactions with activated fibroblasts. TFMCs are sensitive to HA deposition, and their metaplastic markers have prognostic value. We demonstrate that in vivo HA degradation with a clinical dose of hyaluronidase impairs mCRC tumorigenesis and liver metastasis and enables immune checkpoint blockade therapy by promoting the recruitment of B and CD8+ T cells, including a proportion with resident memory features, and by blocking immunosuppression.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Pharmaceutical targeting Th2-mediated immunity enhances immunotherapy response in breast cancer.
In J Transl Med on 23 December 2022 by Chen, Y., Sun, J., et al.
PubMed
Breast cancer is a complex disease with a highly immunosuppressive tumor microenvironment, and has limited clinical response to immune checkpoint blockade (ICB) therapy. T-helper 2 (Th2) cells, an important component of the tumor microenvironment (TME), play an essential role in regulation of tumor immunity. However, the deep relationship between Th2-mediated immunity and immune evasion in breast cancer remains enigmatic.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Immune cells and their inflammatory mediators modify β cells and cause checkpoint inhibitor-induced diabetes.
In JCI Insight on 8 September 2022 by Perdigoto, A. L., Deng, S., et al.
PubMed
Checkpoint inhibitors (CPIs) targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) have revolutionized cancer treatment but can trigger autoimmune complications, including CPI-induced diabetes mellitus (CPI-DM), which occurs preferentially with PD-1 blockade. We found evidence of pancreatic inflammation in patients with CPI-DM with shrinkage of pancreases, increased pancreatic enzymes, and in a case from a patient who died with CPI-DM, peri-islet lymphocytic infiltration. In the NOD mouse model, anti-PD-L1 but not anti-CTLA-4 induced diabetes rapidly. RNA sequencing revealed that cytolytic IFN-γ+CD8+ T cells infiltrated islets with anti-PD-L1. Changes in β cells were predominantly driven by IFN-γ and TNF-α and included induction of a potentially novel β cell population with transcriptional changes suggesting dedifferentiation. IFN-γ increased checkpoint ligand expression and activated apoptosis pathways in human β cells in vitro. Treatment with anti-IFN-γ and anti-TNF-α prevented CPI-DM in anti-PD-L1-treated NOD mice. CPIs targeting the PD-1/PD-L1 pathway resulted in transcriptional changes in β cells and immune infiltrates that may lead to the development of diabetes. Inhibition of inflammatory cytokines can prevent CPI-DM, suggesting a strategy for clinical application to prevent this complication.
-
-
-
Cancer Research
-
Immunology and Microbiology
Antitumor efficacy of 90Y-NM600 targeted radionuclide therapy and PD-1 blockade is limited by regulatory T cells in murine prostate tumors.
In J Immunother Cancer on 1 August 2022 by Potluri, H. K., Ferreira, C. A., et al.
PubMed
Systemic radiation treatments that preferentially irradiate cancer cells over normal tissue, known as targeted radionuclide therapy (TRT), have shown significant potential for treating metastatic prostate cancer. Preclinical studies have demonstrated the ability of external beam radiation therapy (EBRT) to sensitize tumors to T cell checkpoint blockade. Combining TRT approaches with immunotherapy may be more feasible than combining with EBRT to treat widely metastatic disease, however the effects of TRT on the prostate tumor microenvironment alone and in combinfation with checkpoint blockade have not yet been studied.
-
-
-
Cancer Research
-
Immunology and Microbiology
Engineering immunoproteasome-expressing mesenchymal stromal cells: A potent cellular vaccine for lymphoma and melanoma in mice.
In Cell Rep Med on 21 December 2021 by Abusarah, J., Khodayarian, F., et al.
PubMed
Dendritic cells (DCs) excel at cross-presenting antigens, but their effectiveness as cancer vaccine is limited. Here, we describe a vaccination approach using mesenchymal stromal cells (MSCs) engineered to express the immunoproteasome complex (MSC-IPr). Such modification instills efficient antigen cross-presentation abilities associated with enhanced major histocompatibility complex class I and CD80 expression, de novo production of interleukin-12, and higher chemokine secretion. This cross-presentation capacity of MSC-IPr is highly dependent on their metabolic activity. Compared with DCs, MSC-IPr hold the ability to cross-present a vastly different epitope repertoire, which translates into potent re-activation of T cell immunity against EL4 and A20 lymphomas and B16 melanoma tumors. Moreover, therapeutic vaccination of mice with pre-established tumors efficiently controls cancer growth, an effect further enhanced when combined with antibodies targeting PD-1, CTLA4, LAG3, or 4-1BB under both autologous and allogeneic settings. Therefore, MSC-IPr constitute a promising subset of non-hematopoietic antigen-presenting cells suitable for designing universal cell-based cancer vaccines.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
Neuroscience
A radioenhancing nanoparticle mediated immunoradiation improves survival and generates long-term antitumor immune memory in an anti-PD1-resistant murine lung cancer model.
In J Nanobiotechnology on 11 December 2021 by Hu, Y., Paris, S., et al.
PubMed
Combining radiotherapy with PD1 blockade has had impressive antitumor effects in preclinical models of metastatic lung cancer, although anti-PD1 resistance remains problematic. Here, we report results from a triple-combination therapy in which NBTXR3, a clinically approved nanoparticle radioenhancer, is combined with high-dose radiation (HDXRT) to a primary tumor plus low-dose radiation (LDXRT) to a secondary tumor along with checkpoint blockade in a mouse model of anti-PD1-resistant metastatic lung cancer.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Conditional PD-1/PD-L1 Probody Therapeutics Induce Comparable Antitumor Immunity but Reduced Systemic Toxicity Compared with Traditional Anti-PD-1/PD-L1 Agents.
In Cancer Immunol Res on 1 December 2021 by Assi, H. H., Wong, C., et al.
PubMed
Immune-checkpoint blockade has revolutionized cancer treatment. However, most patients do not respond to single-agent therapy. Combining checkpoint inhibitors with other immune-stimulating agents increases both efficacy and toxicity due to systemic T-cell activation. Protease-activatable antibody prodrugs, known as Probody therapeutics (Pb-Tx), localize antibody activity by attenuating capacity to bind antigen until protease activation in the tumor microenvironment. Herein, we show that systemic administration of anti-programmed cell death ligand 1 (anti-PD-L1) and anti-programmed cell death protein 1 (anti-PD-1) Pb-Tx to tumor-bearing mice elicited antitumor activity similar to that of traditional PD-1/PD-L1-targeted antibodies. Pb-Tx exhibited reduced systemic activity and an improved nonclinical safety profile, with markedly reduced target occupancy on peripheral T cells and reduced incidence of early-onset autoimmune diabetes in nonobese diabetic mice. Our results confirm that localized PD-1/PD-L1 inhibition by Pb-Tx can elicit robust antitumor immunity and minimize systemic immune-mediated toxicity. These data provide further preclinical rationale to support the ongoing development of the anti-PD-L1 Pb-Tx CX-072, which is currently in clinical trials.
-