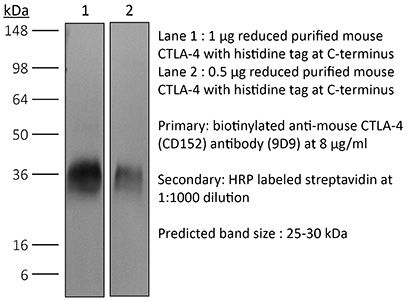
InVivoMAb anti-mouse CTLA-4 (CD152)
Product Description
Specifications
| Isotype | Mouse IgG2b |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2b isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo CTLA-4 neutralization Western blot in vivo intra-tumoral regulatory T cell depletion in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_10949609 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro Organoids/Organ-on-Chip
Sivakumar R, Chan M, Shin JS, Nishida-Aoki N, Kenerson HL, Elemento O, Beltran H, Yeung R, Gujral TS (2019). "Organotypic tumor slice cultures provide a versatile platform for immuno-oncology and drug discovery" Oncoimmunology 8(12):e1670019.
PubMed
Organotypic tumor slices represent a physiologically-relevant culture system for studying the tumor microenvironment. Systematic characterization of the tumor slice culture system will enable its effective application for translational research. Here, using flow cytometry-based immunophenotyping, we performed a comprehensive characterization of the immune cell composition in organotypic tumor slices prepared from four syngeneic mouse tumor models and a human liver tumor. We found that the immune cell compositions of organotypic tumor slices prepared on the same day as the tumor cores were harvested are similar. Differences were primarily observed in the lymphocyte population of a clinical hepatocellular carcinoma case. Viable populations of immune cells persisted in the tumor slices for 7 days. Despite some changes in the immune cell populations, we showed the utility of mouse tumor slices for assessing responses to immune-modulatory agents. Further, we demonstrated the ability to use patient-derived xenograft tumor slices for assessing responses to targeted and cytotoxic drugs. Overall, tumor slices provide a broadly useful platform for studying the tumor microenvironment and evaluating the preclinical efficacy of cancer therapeutics.
in vivo CTLA-4 neutralization
Dai, M., et al (2015). "Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies" Clin Cancer Res 21(5): 1127-1138.
PubMed
PURPOSE: Immunomodulatory mAbs can treat cancer, but cures are rare except for small tumors. Our objective was to explore whether the therapeutic window increases by combining mAbs with different modes of action and injecting them into tumors. EXPERIMENTAL DESIGN: Combinations of mAbs to CD137/PD-1/CTLA-4 or CD137/PD-1/CTLA-4/CD19 were administrated intratumorally to mice with syngeneic tumors (B16 and SW1 melanoma, TC1 lung carcinoma), including tumors with a mean surface of approximately 80 mm(2). Survival and tumor growth were assessed. Immunologic responses were evaluated using flow cytometry and qRT-PCR. RESULTS: More than 50% of tumor-bearing mice had complete regression and long-term survival after tumor injection with mAbs recognizing CD137/PD-1/CTLA-4/CD19 with similar responses in three models. Intratumoral injection was more efficacious than intraperitoneal injection in causing rejection also of untreated tumors in the same mice. The three-mAb combination could also induce regression, but was less efficacious. There were few side effects, and therapy-resistant tumors were not observed. Transplanted tumor cells rapidly caused a Th2 response with increased CD19 cells. Successful therapy shifted this response to the Th1 phenotype with decreased CD19 cells and increased numbers of long-term memory CD8 effector cells and T cells making IFNgamma and TNFalpha. CONCLUSIONS: Intratumoral injection of mAbs recognizing CD137/PD-1/CTLA-4/CD19 can eradicate established tumors and reverse a Th2 response with tumor-associated CD19 cells to Th1 immunity, whereas a combination lacking anti-CD19 is less effective. There are several human cancers for which a similar approach may provide clinical benefit.
in vivo CTLA-4 neutralization
Zippelius, A., et al (2015). "Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment" Cancer Immunol Res 3(3): 236-244.
PubMed
CD40 stimulation on antigen-presenting cells (APC) allows direct activation of CD8(+) cytotoxic T cells, independent of CD4(+) T-cell help. Agonistic anti-CD40 antibodies have been demonstrated to induce beneficial antitumor T-cell responses in mouse models of cancer and early clinical trials. We report here that anti-CD40 treatment induces programmed death ligand-1 (PD-L1) upregulation on tumor-infiltrating monocytes and macrophages, which was strictly dependent on T cells and IFNgamma. PD-L1 expression could be counteracted by coadministration of antibodies blocking the PD-1 (programmed death-1)/PD-L1 axis as shown for T cells from tumor models and human donors. The combined treatment was highly synergistic and induced complete tumor rejection in about 50% of mice bearing MC-38 colon and EMT-6 breast tumors. Mechanistically, this was reflected by a strong increase of IFNgamma and granzyme-B production in intratumoral CD8(+) T cells. Concomitant CTLA-4 blockade further improved rejection of established tumors in mice. This study uncovers a novel mechanism of acquired resistance upon agonistic CD40 stimulation and proposes that the concomitant blockade of the PD-1/PD-L1 axis is a viable therapeutic strategy to optimize clinical outcomes.
in vivo CTLA-4 neutralization
Redmond, W. L., et al (2014). "Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity" Cancer Immunol Res 2(2): 142-153.
PubMed
Ligation of the TNF receptor family costimulatory molecule OX40 (CD134) with an agonist anti-OX40 monoclonal antibody (mAb) enhances antitumor immunity by augmenting T-cell differentiation as well as turning off the suppressive activity of the FoxP3(+)CD4(+) regulatory T cells (Treg). In addition, antibody-mediated blockade of the checkpoint inhibitor CTLA-4 releases the “brakes” on T cells to augment tumor immunotherapy. However, monotherapy with these agents has limited therapeutic benefit particularly against poorly immunogenic murine tumors. Therefore, we examined whether the administration of agonist anti-OX40 therapy in the presence of CTLA-4 blockade would enhance tumor immunotherapy. Combined anti-OX40/anti-CTLA-4 immunotherapy significantly enhanced tumor regression and the survival of tumor-bearing hosts in a CD4 and CD8 T cell-dependent manner. Mechanistic studies revealed that the combination immunotherapy directed the expansion of effector T-bet(high)/Eomes(high) granzyme B(+) CD8 T cells. Dual immunotherapy also induced distinct populations of Th1 [interleukin (IL)-2, IFN-gamma], and, surprisingly, Th2 (IL-4, IL-5, and IL-13) CD4 T cells exhibiting increased T-bet and Gata-3 expression. Furthermore, IL-4 blockade inhibited the Th2 response, while maintaining the Th1 CD4 and effector CD8 T cells that enhanced tumor-free survival. These data demonstrate that refining the global T-cell response during combination immunotherapy can further enhance the therapeutic efficacy of these agents.
in vivo CTLA-4 neutralization
Condamine, T., et al (2014). "ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis" J Clin Invest 124(6): 2626-2639.
PubMed
Myeloid-derived suppressor cells (MDSCs) dampen the immune response thorough inhibition of T cell activation and proliferation and often are expanded in pathological conditions. Here, we studied the fate of MDSCs in cancer. Unexpectedly, MDSCs had lower viability and a shorter half-life in tumor-bearing mice compared with neutrophils and monocytes. The reduction of MDSC viability was due to increased apoptosis, which was mediated by increased expression of TNF-related apoptosis-induced ligand receptors (TRAIL-Rs) in these cells. Targeting TRAIL-Rs in naive mice did not affect myeloid cell populations, but it dramatically reduced the presence of MDSCs and improved immune responses in tumor-bearing mice. Treatment of myeloid cells with proinflammatory cytokines did not affect TRAIL-R expression; however, induction of ER stress in myeloid cells recapitulated changes in TRAIL-R expression observed in tumor-bearing hosts. The ER stress response was detected in MDSCs isolated from cancer patients and tumor-bearing mice, but not in control neutrophils or monocytes, and blockade of ER stress abrogated tumor-associated changes in TRAIL-Rs. Together, these data indicate that MDSC pathophysiology is linked to ER stress, which shortens the lifespan of these cells in the periphery and promotes expansion in BM. Furthermore, TRAIL-Rs can be considered as potential targets for selectively inhibiting MDSCs.
in vivo CTLA-4 neutralization
Muller, P., et al (2014). "Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells" Cancer Immunol Res 2(8): 741-755.
PubMed
Antibody-drug conjugates (ADC) are emerging as powerful treatment strategies with outstanding target-specificity and high therapeutic activity in patients with cancer. Brentuximab vedotin represents a first-in-class ADC directed against CD30(+) malignancies. We hypothesized that its sustained clinical responses could be related to the stimulation of an anticancer immune response. In this study, we demonstrate that the dolastatin family of microtubule inhibitors, from which the cytotoxic component of brentuximab vedotin is derived, comprises potent inducers of phenotypic and functional dendritic cell (DC) maturation. In addition to the direct cytotoxic effect on tumor cells, dolastatins efficiently promoted antigen uptake and migration of tumor-resident DCs to the tumor-draining lymph nodes. Exposure of murine and human DCs to dolastatins significantly increased their capacity to prime T cells. Underlining the requirement of an intact host immune system for the full therapeutic benefit of dolastatins, the antitumor effect was far less pronounced in immunocompromised mice. We observed substantial therapeutic synergies when combining dolastatins with tumor antigen-specific vaccination or blockade of the PD-1-PD-L1 and CTLA-4 coinhibitory pathways. Ultimately, treatment with ADCs using dolastatins induces DC homing and activates cellular antitumor immune responses in patients. Our data reveal a novel mechanism of action for dolastatins and provide a strong rationale for clinical treatment regimens combining dolastatin-based therapies, such as brentuximab vedotin, with immune-based therapies.
in vivo CTLA-4 neutralization
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo CTLA-4 neutralization
Wei, H., et al (2013). "Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin" PLoS One 8(12): e84927.
PubMed
90 days (and was probably curative) by a mechanism which included a systemic CD8(+) T cell response with tumor specificity and immunological memory. Strikingly, combined treatment of cisplatin and CD137/PD-1 mAb also gave rise to the long-term survival of mice with established TC1 lung tumors. A similar combination of the 2 mAbs and cisplatin should be considered for clinical ‘translation’.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>There is an urgent need for improved therapy for advanced ovarian carcinoma, which may be met by administering immune-modulatory monoclonal antibodies (mAbs) to generate a tumor-destructive immune response. Using the ID8 mouse ovarian cancer model, we investigated the therapeutic efficacy of various mAb combinations in mice with intraperitoneal (i.p.) tumor established by transplanting 3 x 10(6) ID8 cells 10 days previously. While most of the tested mAbs were ineffective when given individually or together, the data confirm our previous finding that 2 i.p. injections of a combination of anti-CD137 with anti-PD-1 mAbs doubles overall survival. Mice treated with this mAb combination have a significantly increased frequency and total number of CD8(+) T cells both in the peritoneal lavage and spleens, and these cells are functional as demonstrated by antigen-specific cytolytic activity and IFN-gamma production. While administration of anti-CD137 mAb as a single agent similarly increases CD8(+) T cells, these have no functional activity, which may be attributed to up-regulation of co-inhibitory PD-1 and TIM-3 molecules induced by CD137. Addition of the anti-cancer drug cisplatin to the 2 mAb combination increased overall survival >90 days (and was probably curative) by a mechanism which included a systemic CD8(+) T cell response with tumor specificity and immunological memory. Strikingly, combined treatment of cisplatin and CD137/PD-1 mAb also gave rise to the long-term survival of mice with established TC1 lung tumors. A similar combination of the 2 mAbs and cisplatin should be considered for clinical ‘translation’.
in vivo CTLA-4 neutralization
Bulliard, Y., et al (2013). "Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies" J Exp Med 210(9): 1685-1693.
PubMed
Fc gamma receptor (FcgammaR) coengagement can facilitate antibody-mediated receptor activation in target cells. In particular, agonistic antibodies that target tumor necrosis factor receptor (TNFR) family members have shown dependence on expression of the inhibitory FcgammaR, FcgammaRIIB. It remains unclear if engagement of FcgammaRIIB also extends to the activities of antibodies targeting immunoregulatory TNFRs expressed by T cells. We have explored the requirement for activating and inhibitory FcgammaRs for the antitumor effects of antibodies targeting the TNFR glucocorticoid-induced TNFR-related protein (GITR; TNFRSF18; CD357) expressed on activated and regulatory T cells (T reg cells). We found that although FcgammaRIIB was dispensable for the in vivo efficacy of anti-GITR antibodies, in contrast, activating FcgammaRs were essential. Surprisingly, the dependence on activating FcgammaRs extended to an antibody targeting the non-TNFR receptor CTLA-4 (CD152) that acts as a negative regulator of T cell immunity. We define a common mechanism that correlated with tumor efficacy, whereby antibodies that coengaged activating FcgammaRs expressed by tumor-associated leukocytes facilitated the selective elimination of intratumoral T cell populations, particularly T reg cells. These findings may have broad implications for antibody engineering efforts aimed at enhancing the therapeutic activity of immunomodulatory antibodies.
in vivo CTLA-4 neutralization
Hooijkaas, A., et al (2012). "Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma" Oncoimmunology 1(5): 609-617.
PubMed
The development of targeted therapies and immunotherapies has markedly advanced the treatment of metastasized melanoma. While treatment with selective BRAF(V600E) inhibitors (like vemurafenib or dabrafenib) leads to high response rates but short response duration, CTLA-4 blocking therapies induce sustained responses, but only in a limited number of patients. The combination of these diametric treatment approaches may further improve survival, but pre-clinical data concerning this approach is limited. We investigated, using Tyr::CreER(T2)PTEN(F-/-)BRAF(F-V600E/+) inducible melanoma mice, whether BRAF(V600E) inhibition can synergize with anti-CTLA-4 mAb treatment, focusing on the interaction between the BRAF(V600E) inhibitor PLX4720 and the immune system. While PLX4720 treatment strongly decreased tumor growth, it did not induce cell death in BRAF(V600E)/PTEN(-/-) melanomas. More strikingly, PLX4720 treatment led to a decreased frequency of tumor-resident T cells, NK-cells, MDSCs and macrophages, which could not be restored by the addition of anti-CTLA-4 mAb. As this effect was not observed upon treatment of BRAF wild-type B16F10 tumors, we conclude that the decreased frequency of immune cells correlates to BRAF(V600E) inhibition in tumor cells and is not due to an off-target effect of PLX4720 on immune cells. Furthermore, anti-CTLA-4 mAb treatment of inducible melanoma mice treated with PLX4720 did not result in enhanced tumor control, while anti-CTLA-4 mAb treatment did improve the effect of tumor-vaccination in B16F10-inoculated mice. Our data suggest that vemurafenib may negatively affect the immune activity within the tumor. Therefore, the potential effect of targeted therapy on the tumor-microenvironment should be taken into consideration in the design of clinical trials combining targeted and immunotherapy.
in vivo CTLA-4 neutralization
Curran, M. A., et al (2011). "Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production" PLoS One 6(4): e19499.
PubMed
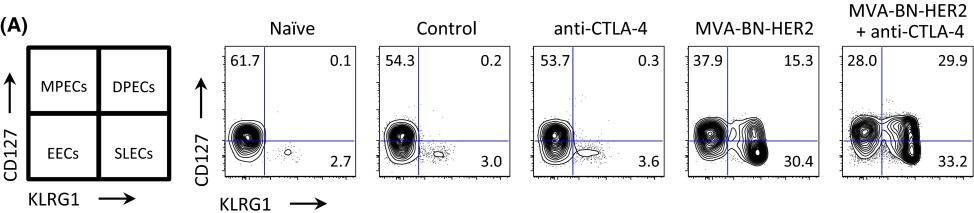
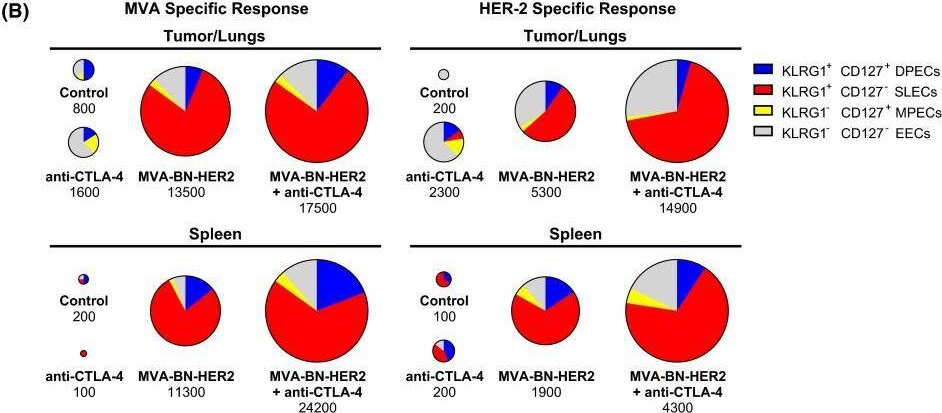
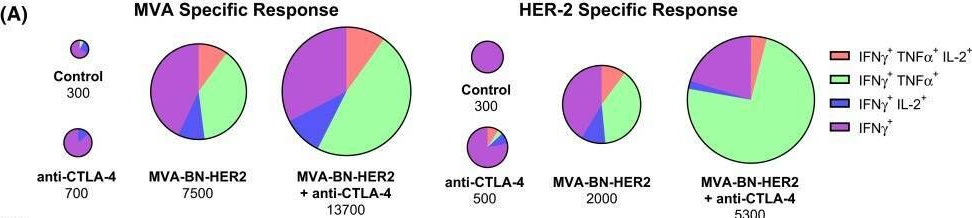
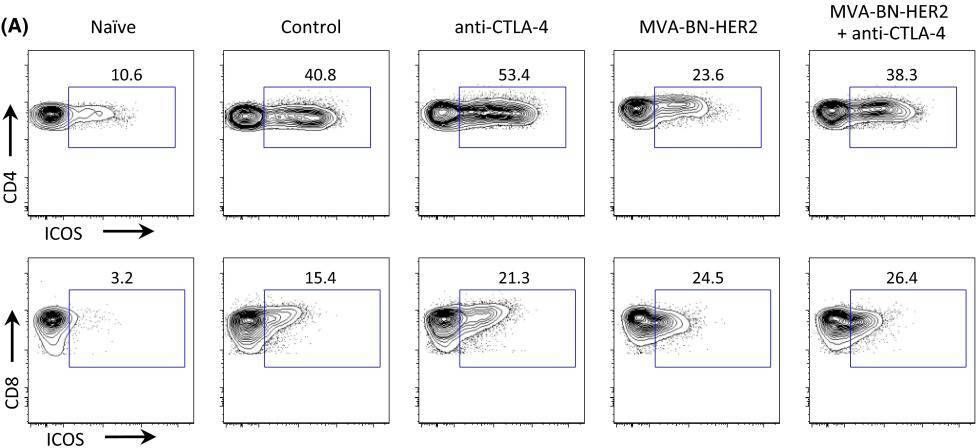
BACKGROUND: The co-inhibitory receptor Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) attenuates immune responses and prevent autoimmunity, however, tumors exploit this pathway to evade the host T-cell response. The T-cell co-stimulatory receptor 4-1BB is transiently upregulated on T-cells following activation and increases their proliferation and inflammatory cytokine production when engaged. Antibodies which block CTLA-4 or which activate 4-1BB can promote the rejection of some murine tumors, but fail to cure poorly immunogenic tumors like B16 melanoma as single agents. METHODOLOGY/PRINCIPAL FINDINGS: We find that combining alphaCTLA-4 and alpha4-1BB antibodies in the context of a Flt3-ligand, but not a GM-CSF, based B16 melanoma vaccine promoted synergistic levels of tumor rejection. 4-1BB activation elicited strong infiltration of CD8+ T-cells into the tumor and drove the proliferation of these cells, while CTLA-4 blockade did the same for CD4+ effector T-cells. Anti-4-1BB also depressed regulatory T-cell infiltration of tumors. 4-1BB activation strongly stimulated inflammatory cytokine production in the vaccine and tumor draining lymph nodes and in the tumor itself. The addition of CTLA-4 blockade further increased IFN-gamma production from CD4+ effector T-cells in the vaccine draining node and the tumor. Anti 4-1BB treatment, with or without CTLA-4 blockade, induced approximately 75% of CD8+ and 45% of CD4+ effector T-cells in the tumor to express the killer cell lectin-like receptor G1 (KLRG1). Tumors treated with combination antibody therapy showed 1.7-fold greater infiltration by these KLRG1+CD4+ effector T-cells than did those treated with alpha4-1BB alone. CONCLUSIONS/SIGNIFICANCE: This study shows that combining T-cell co-inhibitory blockade with alphaCTLA-4 and active co-stimulation with alpha4-1BB promotes rejection of B16 melanoma in the context of a suitable vaccine. In addition, we identify KLRG1 as a useful marker for monitoring the anti-tumor immune response elicited by this therapy. These findings should aid in the design of future trials for the immunotherapy of melanoma.
in vivo CTLA-4 neutralization
Balachandran, V. P., et al (2011). "Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido" Nat Med 17(9): 1094-1100.
PubMed
Imatinib mesylate targets mutated KIT oncoproteins in gastrointestinal stromal tumor (GIST) and produces a clinical response in 80% of patients. The mechanism is believed to depend predominantly on the inhibition of KIT-driven signals for tumor-cell survival and proliferation. Using a mouse model of spontaneous GIST, we found that the immune system contributes substantially to the antitumor effects of imatinib. Imatinib therapy activated CD8(+) T cells and induced regulatory T cell (T(reg) cell) apoptosis within the tumor by reducing tumor-cell expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (Ido). Concurrent immunotherapy augmented the efficacy of imatinib in mouse GIST. In freshly obtained human GIST specimens, the T cell profile correlated with imatinib sensitivity and IDO expression. Thus, T cells are crucial to the antitumor effects of imatinib in GIST, and concomitant immunotherapy may further improve outcomes in human cancers treated with targeted agents.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
Rescue of Tolerant CD8+ T Cells during Cancer Immunotherapy with IL2:Antibody Complexes.
In Cancer Immunology Research on 1 December 2016 by Klevorn, L. E., Berrien-Elliott, M. M., et al.
PubMed
Interleukin-2 (IL2) was among the earliest reagents used for cancer immunotherapy due to its ability to support the survival and function of tumor-reactive T cells. However, treatment with IL2 is accompanied by off-target toxicity and low response rates in patients. In mouse models, these issues are largely overcome when IL2 is administered as a cytokine/antibody complex (IL2c). The complex has a longer serum half-life and can be designed for preferential cytokine delivery to specific cells of interest. Early studies showed IL2c could boost antitumor immunity in mice by activating tumor-reactive CD8+ T cells. But such functional T cells are often limited in the tumor microenvironment, where instead unresponsive tolerant T cells are eventually eliminated by apoptosis, representing a major obstacle to the success of cancer immunotherapy. We found that IL2c treatment rescued tumor-specific CD8+ T cells from a state of established tolerance, providing effective immunotherapy in tumor-bearing mice. Expression of the transcription factor T-bet was necessary to drive intratumoral IFNγ production and effector activity by T cells rescued with IL2c. Furthermore, IL2c promoted T-bet expression in human CD4+ and CD8+ T cells in humanized tumor-bearing mice, but also increased the frequency of Foxp3+ regulatory T cells. Our study reveals a novel role for IL2c as a powerful immunotherapeutic reagent capable of reversing tolerance in tumor-reactive T cells, and provides the first evidence that IL2c influences human T cells in vivo, highlighting the translational potential to modulate human antitumor immune responses. Cancer Immunol Res; 4(12); 1016-26. ©2016 AACR. ©2016 American Association for Cancer Research.
-
-
-
Immunology and Microbiology
-
Cancer Research
β-adrenergic signaling blockade attenuates metastasis through activation of cytotoxic CD4 T cells.
In Nat Commun on 17 November 2025 by Fjæstad, K. Y., Johansen, A. Z., et al.
PubMed
β-adrenergic signaling has been suggested to promote tumor growth, and β-blockers are being evaluated for repurposing for cancer treatment. Here, we identify a β-adrenergic signaling axis involved in metastasis formation. We show that the β-blocker propranolol has strong anti-metastatic activity in multiple murine models, with this effect being completely dependent on CD4 + T cells and independent of NK or CD8 + T cells. We also observe that CD4 + T cells are required for the anti-tumor effect of propranolol in a syngeneic subcutaneous model of colon cancer. Mechanistically, propranolol induces a Th1-polarized and cytotoxic CD4 + T cell response, which requires MHC class II expression by cancer cells for full efficacy. We also report propanolol-driven systemic changes in the monocyte compartment, and upon depletion of monocytes, propranolol loses its anti-tumor effects. Finally, we show that propranolol treatment synergizes with anti-CTLA-4 therapy to further enhance CD4 + T cell infiltration and control metastasis. Thus, we show that β-adrenergic signaling limits CD4 T cell-mediated anti-tumor immunity, highlighting the potential of repurposing β-blockers for cancer treatment.
-
-
-
Immunology and Microbiology
-
Cancer Research
225Actinium-armed antibody targeting CCR8+ regulatory T cells synergizes with immunotherapy to promote tumor rejection in syngeneic colorectal cancer models.
In Front Immunol on 2 October 2025 by Frank, C., Xiao, Z., et al.
PubMed
Colorectal cancer (CRC) remains a formidable threat to health worldwide. Immunotherapy with immune checkpoint inhibitors results in only a minority of CRC patients experiencing long-term progression-free survival, at the expense of significant autoimmune toxicity. Development of new therapeutics to "wake up" the immune system to fight CRC is necessary. Here we investigated for the first time radioimmunotherapy (RIT) directed towards CCR8, a marker of tumor-infiltrating immunosuppressive T-regulatory cells (ti-Tregs) as a method to recover anti-tumor immunity followed by immunotherapy in CRC models.
-
-
STAT3 sustains tumorigenicity following mutant KRAS ablation.
In EMBO Rep on 1 October 2025 by D'Amico, S., Kirillov, V., et al.
PubMed
Oncogenic KRAS mutations underlie some of the deadliest human cancers. Genetic or pharmacological KRAS inactivation produces mixed outcomes and frequent relapse. Mechanisms of tumor resistance to KRAS inhibition remain poorly understood. We present evidence that STAT3 supports tumor growth following KRAS depletion. Using a conceptual framework of pancreatic ductal adenocarcinoma, we show that cancer cells that survive CRISPR-mediated ablation of mutant KRAS are dependent on STAT3 function to maintain tumorigenicity. Mechanistically, the combined loss of mutant KRAS and STAT3 disrupts a core transcriptional program of cancer cells critical to oncogenic competence. This in turn impairs tumor growth in mice and enhances immune rejection, leading to tumor clearance. We propose that the STAT3 transcriptional program operating in cancer cells enforces their malignant identity, rather than providing classical features of transformation, and shapes cancer persistence following KRAS inactivation. Our findings establish STAT3 as a critical enforcer of oncogenic identity in KRAS-ablated tumors, revealing a key vulnerability.
-
-
Immunology and Microbiology
-
Cancer Research
FSTL3 is a biomarker of poor prognosis and associated with immunotherapy resistance in ovarian cancer.
In J Exp Clin Cancer Res on 30 September 2025 by Chauvin, M., Tromelin, E., et al.
PubMed
High-grade serous ovarian carcinoma (HGSOC) is associated with high mortality rates due to late-stage diagnosis and limited treatment options. We investigated the role of FSTL3 in ovarian cancer progression both as a prognostic biomarker and as a potential therapeutic target.We measured levels of follistatin (FST) and follistatin-like 3 (FSTL3) in 96 ovarian cancer patient ascites samples and found that FSTL3 overexpression was more predominant than FST and associated with poorer survival outcomes. Mice implanted with an HGSOC syngeneic cell line bearing common alterations in ovarian cancer (KRASG12 V, P53R172H, CCNE1oe, AKT2oe) had increasing levels of FST and FSTL3 in serum during tumor growth. Further alteration of this model to generate a knockout of FST (KPCA.FSTKO) and an overexpression of human FSTL3 (KPCA.FSTKO_hFSTL3) revealed that FSTL3 expression was associated with a more fibrotic tumor microenvironment, correlating with an increased abundance of cancer-associated myofibroblasts (myCAFs), and cancer cells with a more mesenchymal phenotype. Tumors overexpressing FSTL3 also had significantly less immunocyte infiltration, reduced intratumoral T-cell abundance, and increased CD8+ T cell exhaustion. FSTL3 overexpression completely abrogated tumor response to PPC treatment (Prexasertib combined with PD-1 and CTLA-4 blockade) compared to controls, suggesting that FSTL3 may be involved in immunotherapy resistance. In conclusion, this study suggests a role for FSTL3 as a prognostic marker and as therapeutic target in HGSOC, where it may play a role in promoting a mesenchymal tumor phenotype, maintaining an immunosuppressive tumor microenvironment, and driving immunotherapy resistance.
-
-
-
Immunology and Microbiology
-
Cancer Research
CD137L promotes immune surveillance in melanoma via HLTF regulation.
In Nat Commun on 26 September 2025 by Liang, L., Zhu, L., et al.
PubMed
Immune checkpoint blockers (ICBs) have demonstrated substantial efficacy across various malignancies, yet the benefits of ICBs are limited to a subset of patients. Therefore, it is essential to identify novel therapeutic targets. By integrating multi-omics data from cohorts of patients with melanoma treated with ICBs, a positive correlation is observed between tumor CD137L expression and the efficacy of PD-1 blockade. Functionally, CD137L induction in cancer cells significantly enhances anti-tumor immunity by promoting CD8+ T cell survival, both in vivo and in vitro. Mechanistically, helicase-like transcription factor (HLTF) is identified as a pivotal transcriptional regulator of CD137L, controlling its expression through phosphorylation of serine at position 398. Therapeutically, the AMPK agonist AICAR (acadesine) as an inducer of CD137L, exhibiting synergistic effects with PD-1 or CTLA-4 blockade. In summary, our findings elucidate a mechanism controlling CD137L expression and highlight a promising combination therapy to enhance the efficacy of ICBs in melanoma. One Sentence Summary: Inducing co-stimulatory immune checkpoint CD137L expression in melanoma cells enhances T cell-mediated anti-tumor immunity.
-
-
Distinct TIME Modulation by Anti-PD-1/PD-L1, VEGF, and CTLA-4 Blockade Provides a Rationale for Triplet Therapy in HCC.
In Clin Mol Hepatol on 25 September 2025 by Iwamoto, H., Koga, H., et al.
PubMed
-
-
Immunology and Microbiology
-
Cancer Research
Identification of anti-TIM-3 based checkpoint inhibitor combinations with activity in immunotherapy refractory melanoma models.
In J Immunother Cancer on 18 August 2025 by Phadke, M. S., Li, J., et al.
PubMed
A significant percentage of melanomas are refractory to immune checkpoint inhibitor (ICI) monotherapies and combinations. As there are currently no effective second-line therapies available for ICI-resistant patients, we sought to identify novel checkpoint inhibitor combinations for future clinical evaluation.
-
-
-
Cancer Research
-
Immunology and Microbiology
BRAF/MEK inhibition induces cell state transitions boosting immune checkpoint sensitivity in BRAFV600E-mutant glioma.
In Cell Rep Med on 17 June 2025 by Xing, Y. L., Panovska, D., et al.
PubMed
Resistance to v-raf murine sarcoma viral oncogene homolog B1 (BRAF) plus mitogen-activated protein kinase kinase (MEK) inhibition (BRAFi+MEKi) in BRAFV600E-mutant gliomas drives rebound, progression, and high mortality, yet it remains poorly understood. This study addresses the urgent need to develop treatments for BRAFi+MEKi-resistant glioma using preclinical mouse models and patient-derived materials. BRAFi+MEKi reveals glioma plasticity by heightening cell state transitions along glial differentiation trajectories, giving rise to astrocyte- and immunomodulatory oligodendrocyte (OL)-like states. PD-L1 upregulation in OL-like cells links cell state transitions to immune evasion, possibly orchestrated by Galectin-3. BRAFi+MEKi induces interferon response signatures, tumor infiltration, and suppression of T cells. Combining BRAFi+MEKi with immune checkpoint inhibition enhances survival in a T cell-dependent manner, reinvigorates T cells, and outperforms individual or sequential therapies in mice. Elevated PD-L1 expression in BRAF-mutant versus BRAF-wild-type glioblastoma supports the rationale for PD-1 inhibition in patients. These findings underscore the potential of targeting glioma plasticity and highlight combination strategies to overcome therapy resistance in BRAFV600E-mutant high-grade glioma.
-
-
-
Cancer Research
Fc-optimized anti-CTLA-4 antibodies increase tumor-associated high endothelial venules and sensitize refractory tumors to PD-1 blockade.
In Cell Rep Med on 17 June 2025 by Blanchard, L., Vina, E., et al.
PubMed
The lack of T cells in tumors is a major hurdle to successful immune checkpoint therapy (ICT). Therefore, therapeutic strategies promoting T cell recruitment into tumors are warranted to improve the treatment efficacy. Here, we report that Fc-optimized anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies are potent remodelers of tumor vasculature that increase tumor-associated high endothelial venules (TA-HEVs), specialized blood vessels supporting lymphocyte entry into tumors. Mechanistically, this effect is dependent on the Fc domain of anti-CTLA-4 antibodies and CD4+ T cells and involves interferon gamma (IFNγ). Unexpectedly, we find that the human anti-CTLA-4 antibody ipilimumab fails to increase TA-HEVs in a humanized mouse model. However, increasing its Fc effector function rescues the modulation of TA-HEVs, promotes CD4+ and CD8+ T cell infiltration into tumors, and sensitizes recalcitrant tumors to programmed cell death protein 1 (PD-1) blockade. Our findings suggest that Fc-optimized anti-CTLA-4 antibodies could be used to reprogram tumor vasculature in poorly immunogenic cold tumors and improve the efficacy of ICT.
-
-
-
Cancer Research
Cancer therapy via neoepitope-specific monoclonal antibody cocktails.
In Cancer Immunol Immunother on 31 May 2025 by Hartman, C. J., Mohamed, A. O., et al.
PubMed
Cellular heterogeneity presents a significant challenge to cancer treatment. Antibody therapies targeting individual tumor-associated antigens can be extremely effective but are not suited for all patients and often fail against tumors with heterogeneous expression as tumor cells with low or no antigen expression escape targeting and develop resistance. Simultaneously targeting multiple tumor-specific proteins with multiple antibodies has the potential to overcome this barrier and improve efficacy, but relatively few widely expressed cancer-specific antigens are known. In contrast, neoepitopes, which arise from mutations unique to tumor cells, are considerably more abundant. However, since neoepitopes are not commonly shared between individuals, a patient-customized approach is necessary and motivates efforts to develop an efficient means to identify suitable target mutations and isolate neoepitope-specific monoclonal antibodies. Here, focusing on the latter goal, we use directed evolution in yeast and phage display systems to engineer antibodies from nonimmune, human antibody fragment libraries that are specific for neoepitopes previously reported in the B16F10 melanoma model. We demonstrate proof-of-concept for a pipeline that supports rapid isolation and functional enhancement of multiple neoepitope peptide-targeted monoclonal antibodies and demonstrate their robust binding to B16F10 cells and potent effector functions in vitro. These antibodies were combined and evaluated in vivo for anticancer activity in tumor-bearing mice, where they suppressed B16F10 tumor growth and prolonged survival. These findings emphasize the potential for clinical application of patient-customized antibody cocktails in the treatment of the many cancers poorly addressed by current therapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
Simultaneous co-delivery of Ginsenoside Rg3 and imiquimod from PLGA nanoparticles for effective breast cancer immunotherapy.
In iScience on 16 May 2025 by Hu, C., Nong, S., et al.
PubMed
Breast cancer is a fatal malignancy facing human health, with most patients experiencing recurrence and resistance to chemotherapy. The immunosuppressive tumor microenvironment (TME) greatly limits the actual outcome of immunotherapy. This study aimed to develop a modality of theranostics nanoparticles for breast cancer based on a near-infrared light-triggered nanoparticle for the targeted delivery of ginsenoside Rg3 and immune adjuvants imiquimod (R837) for effective breast cancer immunotherapy. Folate-receptor (FA) targeting IR780-R837/ginsenoside Rg3-perfluorohexane (PFH) @ polyethylene glycol (PEG)-poly (lactide-co-glycolic acid) (PLGA) nanoparticles (FA-NPs) can be activated by near-infrared laser irradiation in tumors, which leads to rapid release of ginsenoside Rg3 and R837 in the regions with high expression of folate receptors and glucose transporter 1 (GLUT1). Meanwhile, the nanoparticles can be used as dual-mode contrast agents for photoacoustic and ultrasound imaging. This strategy provides a strong immune memory effect, which can prevent tumor recurrence after eliminating the initial tumor.
-
-
-
Flow cytometry/Cell sorting
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Loss of MNX1 Sensitizes Tumors to Cytotoxic T Cells by Degradation of PD-L1 mRNA.
In Adv Sci (Weinh) on 1 March 2025 by Li, Z., Chen, L., et al.
PubMed
Immune checkpoint blockade (ICB) therapy, targeting programmed cell death ligand-1 (PD-L1)/programmed cell death protein 1 (PD-1) axis and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), has exhibited amazing clinical outcomes in various types of cancers. However, only a small portion of patients benefit from ICB therapy, indicating that the mechanism underlying immune checkpoint is still unclear. Here, it is reported that motor neuron and pancreas homeobox 1 (MNX1), a homeobox domain-containing transcription factor, contributes to the tumor immune escape. MNX1 increases PD-L1 expression in cancer cells by stabilizing PD-L1 mRNA rather than activating transcription. Mechanistically, MNX1 exists in the cytoplasm of cancer cells and interacts with Y-box binding protein 1 (YBX1), a multifunctional DNA/RNA-binding protein, to enhance the binding of YBX1 to PD-L1 mRNA. MNX1 ablation activates cytotoxic T cell-mediated anti-tumor immunity and sensitizes CTLA-4 blockade therapy. Moreover, MNX1 also facilitates tumor progression in an immune-independent manner in cancer cells. In addition, MNX1 is upregulated by its adjacent long non-coding RNA MNX1-AS1 via HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2). Together, these results reveal MNX1 as a novel immune checkpoint regulator with promising therapeutic potential.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Alterations in PD-L1 succinylation shape anti-tumor immune responses in melanoma.
In Nat Genet on 1 March 2025 by Liang, L., Kuang, X., et al.
PubMed
Tumors undergo metabolic reprogramming to meet the energetic, synthetic and redox demands essential for malignancy, often characterized by increased glycolysis and lactate production. However, the role of mitochondrial metabolism in tumor immunity remains unclear. The present study integrates spatial transcriptomics, bulk transcriptomics and proteomics, revealing a strong link between the metabolite succinyl-CoA and tumor immunity as well as the efficacy of anti-programmed cell death protein-1 (PD-1) therapy in patients with melanoma. Elevated succinyl-CoA levels, through α-ketoglutarate or succinate supplementation, enhanced T cell-mediated tumor elimination, both in vitro and in vivo. Mechanistically, succinylation of the ligand of PD-1 (PD-L1) at lysine 129 led to its degradation. Increased carnitine palmitoyltransferase 1A (CPT1A), identified as a succinyltransferase for PD-L1, boosted anti-tumor activity. Preclinically, bezafibrate, a hyperlipidemia drug, upregulated CPT1A and synergized with CTLA-4 monoclonal antibody to inhibit tumor growth. Clinically, higher PD-L1 and lower CPT1A levels in tumors correlated with better anti-PD-1 therapy responses, suggesting potential biomarkers for prediction of treatment efficacy.
-
-
Intratumor heterogeneity in KRAS signaling shapes treatment resistance.
In iScience on 21 February 2025 by Petrenko, O., Kirillov, V., et al.
PubMed
KRAS mutations are linked to some of the deadliest forms of cancer. Pharmacological studies suggest that co-targeting KRAS with feedback/bypass pathways could lead to enhanced anti-tumor activity. The underlying premise is that cancers display a deep-rooted hypersensitivity to KRAS inactivation. Here, we investigate the role of intratumor heterogeneity in pancreatic ductal adenocarcinoma, focusing on oncogenic KRAS addiction and treatment resistance. Integrated analysis of single-cell and bulk RNA sequencing data reveals that most tumors display a mixture of cells with vastly different degrees of KRAS dependency. We identify distinct cell populations that vary in their gene expression patterns pertaining to the predicted level of KRAS signaling activity, cell growth, and differentiation commitment within each tumor. Selective targeting of mutant KRAS suppresses the growth of tumor cells with high RAS/mitogen-activated protein kinase (MAPK) activity while sparing pre-existing subsets with low RAS signaling activity, necessitating alternative treatments. Combination immunotherapy leads to durable tumor regression in preclinical models.
-
-
Cancer Research
-
Immunology and Microbiology
Development of Syngeneic Murine Glioma Models with Somatic Mismatch Repair Deficiency to Study Therapeutic Responses to Alkylating Agents and Immunotherapy.
In Curr Protoc on 1 February 2025 by Bhatt, D., Sundaram, R. K., et al.
PubMed
Glioblastoma (GBM) carries a dismal prognosis, with a median survival of less than 15 months. Temozolomide (TMZ), the standard frontline chemotherapeutic for GBM, is an alkylating agent that generates DNA O6-methylguanine (O6MeG) lesions. Without O6MeG-methyltransferase (MGMT), this lesion triggers the mismatch repair (MMR) pathway and leads to cytotoxicity via futile cycling. TMZ resistance frequently arises via the somatic acquisition of MMR deficiency (MMRd). Moreover, DNA-damaging agents have been shown capable of increasing tumor immunogenicity and improving response to immune checkpoint blockade (ICB), which has had limited success in glioma. The study of how alkylating chemotherapy such as TMZ impacts antitumor immunity in glioma has been hindered by a lack of immunocompetent models that incorporate relevant DNA repair genotypes. Here, we used CRISPR/Cas9 to generate models isogenic for knockout (KO) of Mlh1 in the syngeneic SB28 murine glioma cell line. MMR KO models readily formed intracranial tumors and exhibited in vitro and in vivo resistance to TMZ. In contrast, MMR KO cells maintained sensitivity to KL-50, a newly developed alkylating compound that exerts MGMT-dependent, MMR-independent cytotoxicity. Lastly, MMR KO tumors remained resistant to ICB, mirroring the lack of response seen in patients with somatic MMRd GBM. The development of syngeneic, immunologically cold glioma models with somatic loss of MMR will facilitate future studies on the immunomodulatory effects of alkylating agents in relevant DNA repair contexts, which will be vital for optimizing combinations with ICB. © 2025 Wiley Periodicals LLC. Basic Protocol 1: Validation of mismatch repair knockouts and in vitro sensitivity to alkylating agents Basic Protocol 2: Stereotaxic injection of isogenic SB28 cells in female C57BL/6J mice and in vivo treatment.
-
-
-
Immunology and Microbiology
Polymeric Multivalent Fc Binding Peptides-Fabricated Clinical Compounding Bispecific Antibody Potentiates Dual Immunotherapy Targeting PD1 and CTLA-4.
In Adv Sci (Weinh) on 1 January 2025 by Liu, Z., Chu, H., et al.
PubMed
Dual Opdivo plus Yervoy immunotherapy, targeting the immune checkpoints PD1 and CTLA-4, is successful in clinical use. However, it is associated with a high incidence of adverse events, and its therapeutic efficacy needs improving. In this study, polymeric multivalent Fc-binding peptides (PLG-Fc-III-4C) are employed to fabricate a bispecific antibody (PD1/CTLA-4 BsAb) to potentiate dual immunotherapy targeting PD1 and CTLA-4. The PD1/CTLA-4 BsAb is prepared by mixing PLG-Fc-III-4C with aPD1 and aCTLA-4 in an aqueous solution for 3 h using the clinically optimal 3:1 proportion of aPD1 to aCTLA-4. PD1/CTLA-4 BsAb significantly inhibits tumors in MC38 colon cancer-bearing mice more effectively than the combination of aPD1 and aCTLA-4, with tumor suppression rates of 96.8% and 77.3%, respectively. It also induces a higher percentage of CD8+ T cells and increases the secretion of effector cytokines while reducing Treg levels in tumors compared to phosphate-buffered saline, indicating significant tumor immunity regulation. Mechanistically, a 6.3-fold increase in PD1/CTLA-4 BsAb accumulation in tumors due to the tumor targeting ability of aPD1, and the PD1/CTLA-4 BsAb significantly reduces the adverse colitis event in healthy mice, compared to aPD1 and aCTLA-4. Thus, these findings provide a novel approach to enhance antitumor therapy using aPD1 and aCTLA-4.
-
-
-
Cancer Research
-
Immunology and Microbiology
FSTL3 is a biomarker of poor prognosis and is associated with immunotherapy resistance in ovarian cancer
In bioRxiv on 12 December 2024 by Chauvin, M., Tromelin, E., et al.
-
-
-
Cancer Research
Identification of VISTA regulators in macrophages mediating cancer cell survival.
In Sci Adv on 29 November 2024 by Abdrabou, A. M., Ahmed, S., et al.
PubMed
Numerous human cancers have exhibited the ability to elude immune checkpoint blockade (ICB) therapies. This type of resistance can be mediated by immune-suppressive macrophages that limit antitumor immunity in the tumor microenvironment (TME). Here, we elucidate a strategy to shift macrophages into a proinflammatory state that down-regulates V domain immunoglobulin suppressor of T cell activation (VISTA) via inhibiting AhR and IRAK1. We used a high-throughput microfluidic platform combined with a genome-wide CRISPR knockout screen to identify regulators of VISTA levels. Functional characterization showed that the knockdown of these hits diminished VISTA surface levels on macrophages and sustained an antitumor phenotype. Furthermore, targeting of both AhR and IRAK1 in mouse models overcame resistance to ICB treatment. Tumor immunophenotyping indicated that infiltration of cytotoxic CD8+ cells, natural killer cells, and antitumor macrophages was substantially increased in treated mice. Collectively, AhR and IRAK1 are implicated as regulators of VISTA that coordinate a multifaceted barrier to antitumor immune responses.
-
-
-
Cancer Research
-
Immunology and Microbiology
Calcium nanoparticles target and activate T cells to enhance anti-tumor function.
In Nat Commun on 21 November 2024 by Yang, W., Feng, Z., et al.
PubMed
Calcium signaling plays a crucial role in the activation of T lymphocytes. However, modulating calcium levels to control T cell activation in vivo remains a challenge. In this study, we investigate T cell activation using 12-myristate 13-acetate (PMA)-encapsulated CaCO3 nanoparticles. We find that anti-PD-1 antibody-conjugated CaCO3 nanoparticles can be internalized by T cells via receptor-mediated endocytosis and then gradually release calcium. This results in an increase in cytosolic calcium, which triggers the activation of NFAT and NF-κB pathways, especially when the surface of the CaCO3 nanoparticles is loaded with PMA. Animal studies demonstrate that the PMA-loaded calcium nanoparticles enhance the activation and proliferation of cytotoxic T cells, leading to improved tumor suppression without additional toxicity. When tested in metastatic tumor models, T cells loaded with the calcium nanoparticles prior to adoptive cell transfer control tumor growth better, resulting in prolonged animal survival. Our approach offers an alternative T cell activation strategy to potentiate immunotherapy by targeting a fundamental signaling pathway.
-