InVivoPlus anti-mouse TNFα
Product Description
Specifications
| Isotype | Rat IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse TNFα |
| Reported Applications |
in vivo TNFα neutralization in vitro TNFα neutralization Western blot |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
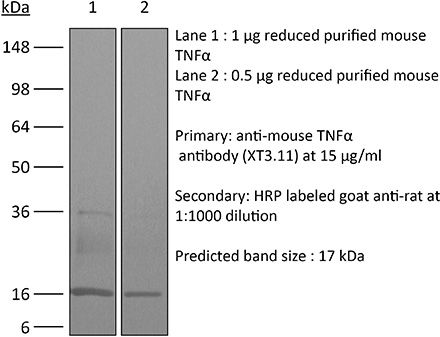
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107764 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo TNFα neutralization
Shaabani, N., et al (2018). "The probacterial effect of type I interferon signaling requires its own negative regulator USP18" Sci Immunol 3(27).
PubMed
Type I interferon (IFN-I) signaling paradoxically impairs host immune responses during many primary and secondary bacterial infections. Lack of IFN-I receptor reduces bacterial replication and/or bacterial persistence during infection with several bacteria. However, the mechanisms that mediate the adverse IFN-I effect are incompletely understood. Here, we show that Usp18, an interferon-stimulated gene that negatively regulates IFN-I signaling, is primarily responsible for the deleterious effect of IFN-I signaling during infection of mice with Listeria monocytogenes or Staphylococcus aureus Mechanistically, USP18 promoted bacterial replication by inhibiting antibacterial tumor necrosis factor-alpha (TNF-alpha) signaling. Deleting IFNAR1 or USP18 in CD11c-Cre(+) cells similarly reduced bacterial titers in multiple organs and enhanced survival. Our results demonstrate that inhibiting USP18 function can promote control of primary and secondary bacterial infection by enhancing the antibacterial effect of TNF-alpha, which correlates with induction of reactive oxygen species (ROS). These findings suggest that USP18 could be targeted therapeutically in patients to ameliorate disease caused by serious bacterial infections.
in vivo TNFα neutralization
Baeyens, A., et al (2015). "Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context" J Immunol 194(3): 999-1010.
PubMed
CD4(+)CD25(+)Foxp3(+) regulatory T (Treg) cells play a major role in peripheral tolerance. Multiple environmental factors and cell types affect their biology. Among them, activated effector CD4(+) T cells can boost Treg cell expansion through TNF or IL-2. In this study, we further characterized this effector T (Teff) cell-dependent Treg cell boost in vivo in mice. This phenomenon was observed when both Treg and Teff cells were activated by their cognate Ag, with the latter being the same or different. Also, when Treg cells highly proliferated on their own, there was no additional Treg cell boost by Teff cells. In a condition of low inflammation, the Teff cell-mediated Treg cell boost involved TNF, OX40L, and plasmacytoid dendritic cells, whereas in a condition of high inflammation, it involved TNF and IL-2. Thus, this feedback mechanism in which Treg cells are highly activated by their Teff cell counterparts depends on the immune context for its effectiveness and mechanism. This Teff cell-dependent Treg cell boost may be crucial to limit inflammatory and autoimmune responses.
in vivo TNFα neutralization
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo TNFα neutralization
Grinberg-Bleyer, Y., et al (2015). "Cutting edge: NF-kappaB p65 and c-Rel control epidermal development and immune homeostasis in the skin" J Immunol 194(6): 2472-2476.
PubMed
Psoriasis is an inflammatory skin disease in which activated immune cells and the proinflammatory cytokine TNF are well-known mediators of pathogenesis. The transcription factor NF-kappaB is a key regulator of TNF production and TNF-induced proinflammatory gene expression, and both the psoriatic transcriptome and genetic susceptibility further implicate NF-kappaB in psoriasis etiopathology. However, the role of NF-kappaB in psoriasis remains controversial. We analyzed the function of canonical NF-kappaB in the epidermis using CRE-mediated deletion of p65 and c-Rel in keratinocytes. In contrast to animals lacking p65 or c-Rel alone, mice lacking both subunits developed severe dermatitis after birth. Consistent with its partial histological similarity to human psoriasis, this condition could be prevented by anti-TNF treatment. Moreover, regulatory T cells in lesional skin played an important role in disease remission. Our results demonstrate that canonical NF-kappaB in keratinocytes is essential for the maintenance of skin immune homeostasis and is protective against spontaneous dermatitis.
in vivo TNFα neutralization
Deng, L., et al (2014). "Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice" J Clin Invest 124(2): 687-695.
PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
in vitro TNFα neutralization
Beug, S. T., et al (2014). "Smac mimetics and innate immune stimuli synergize to promote tumor death" Nat Biotechnol 32(2): 182-190.
PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
in vivo TNFα neutralization
DeBerge, M. P., et al (2014). "Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology" J Immunol 192(12): 5839-5851.
PubMed
TNF-alpha is a pleotropic cytokine that has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-alpha is first expressed as a transmembrane protein that is proteolytically processed to release a soluble form. Transmembrane TNF-alpha (memTNF-alpha) and soluble TNF-alpha (solTNF-alpha) have been shown to exert distinct tissue-protective or tissue-pathologic effects in several disease models. However, the relative contributions of memTNF-alpha or solTNF-alpha in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing noncleavable memTNF-alpha or lacking TNF-alpha entirely and examined the outcomes. We found that solTNF-alpha, but not memTNF-alpha, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-alpha, there was a significant increase in the CD8(+) T cell response, including virus-specific CD8(+) T cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-alpha mediates these immunoregulatory effects primarily through TNFR1, because mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-alpha. We also found that solTNF-alpha expression was required early during infection to regulate the magnitude of the CD8(+) T cell response, indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-alpha to release solTNF-alpha is a critical event regulating the immune response during influenza infection.
in vivo TNFα neutralization
Maltby, S., et al (2014). "Production and differentiation of myeloid cells driven by proinflammatory cytokines in response to acute pneumovirus infection in mice" J Immunol 193(8): 4072-4082.
PubMed
Respiratory virus infections are often pathogenic, driving severe inflammatory responses. Most research has focused on localized effects of virus infection and inflammation. However, infection can induce broad-reaching, systemic changes that are only beginning to be characterized. In this study, we assessed the impact of acute pneumovirus infection in C57BL/6 mice on bone marrow hematopoiesis. We hypothesized that inflammatory cytokine production in the lung upregulates myeloid cell production in response to infection. We demonstrate a dramatic increase in the percentages of circulating myeloid cells, which is associated with pronounced elevations in inflammatory cytokines in serum (IFN-gamma, IL-6, CCL2), bone (TNF-alpha), and lung tissue (TNF-alpha, IFN-gamma, IL-6, CCL2, CCL3, G-CSF, osteopontin). Increased hematopoietic stem/progenitor cell percentages (Lineage(-)Sca-I(+)c-kit(+)) were also detected in the bone marrow. This increase was accompanied by an increase in the proportions of committed myeloid progenitors, as determined by colony-forming unit assays. However, no functional changes in hematopoietic stem cells occurred, as assessed by competitive bone marrow reconstitution. Systemic administration of neutralizing Abs to either TNF-alpha or IFN-gamma blocked expansion of myeloid progenitors in the bone marrow and also limited virus clearance from the lung. These findings suggest that acute inflammatory cytokines drive production and differentiation of myeloid cells in the bone marrow by inducing differentiation of committed myeloid progenitors. Our findings provide insight into the mechanisms via which innate immune responses regulate myeloid cell progenitor numbers in response to acute respiratory virus infection.
in vivo TNFα neutralization
Yoo, J. K. and T. J. Braciale (2014). "IL-21 promotes late activator APC-mediated T follicular helper cell differentiation in experimental pulmonary virus infection" PLoS One 9(9): e105872.
PubMed
IL-21 is a type-I cytokine that has pleiotropic immuno-modulatory effects. Primarily produced by activated T cells including NKT and TFH cells, IL-21 plays a pivotal role in promoting TFH differentiation through poorly understood cellular and molecular mechanisms. Here, employing a mouse model of influenza A virus (IAV) infection, we demonstrate that IL-21, initially produced by NKT cells, promotes TFH differentiation by promoting the migration of late activator antigen presenting cell (LAPC), a recently identified TFH inducer, from the infected lungs into the draining lymph nodes (dLN). LAPC migration from IAV-infected lung into the dLN is CXCR3-CXCL9 dependent. IL-21-induced TNF-alpha production by conventional T cells is critical to stimulate CXCL9 expression by DCs in the dLN, which supports LAPC migration into the dLN and ultimately facilitates TFH differentiation. Our results reveal a previously unappreciated mechanism for IL-21 modulation of TFH responses during respiratory virus infection.
in vivo TNFα neutralization
Dietze, K. K., et al (2013). "Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads" PLoS Pathog 9(12): e1003798.
PubMed
Chronic infections with human viruses, such as HIV and HCV, or mouse viruses, such as LCMV or Friend Virus (FV), result in functional exhaustion of CD8(+) T cells. Two main mechanisms have been described that mediate this exhaustion: expression of inhibitory receptors on CD8(+) T cells and expansion of regulatory T cells (Tregs) that suppress CD8(+) T cell activity. Several studies show that blockage of one of these pathways results in reactivation of CD8(+) T cells and partial reduction in chronic viral loads. Using blocking antibodies against PD-1 ligand and Tim-3 and transgenic mice in which Tregs can be selectively ablated, we compared these two treatment strategies and combined them for the first time in a model of chronic retrovirus infection. Blocking inhibitory receptors was more efficient than transient depletion of Tregs in reactivating exhausted CD8(+) T cells and reducing viral set points. However, a combination therapy was superior to any single treatment and further augmented CD8(+) T cell responses and resulted in a sustained reduction in chronic viral loads. These results demonstrate that Tregs and inhibitory receptors are non-overlapping factors in the maintenance of chronic viral infections and that immunotherapies targeting both pathways may be a promising strategy to treat chronic infectious diseases.
in vivo TNFα neutralization
Kugler, D. G., et al (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927.
PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo TNFα neutralization
Weinlich, R., et al (2013). "Protective roles for caspase-8 and cFLIP in adult homeostasis" Cell Rep 5(2): 340-348.
PubMed
Caspase-8 or cellular FLICE-like inhibitor protein (cFLIP) deficiency leads to embryonic lethality in mice due to defects in endothelial tissues. Caspase-8(-/-) and receptor-interacting protein kinase-3 (RIPK3)(-/-), but not cFLIP(-/-) and RIPK3(-/-), double-knockout animals develop normally, indicating that caspase-8 antagonizes the lethal effects of RIPK3 during development. Here, we show that the acute deletion of caspase-8 in the gut of adult mice induces enterocyte death, disruption of tissue homeostasis, and inflammation, resulting in sepsis and mortality. Likewise, acute deletion of caspase-8 in a focal region of the skin induces local keratinocyte death, tissue disruption, and inflammation. Strikingly, RIPK3 ablation rescues both phenotypes. However, acute loss of cFLIP in the skin produces a similar phenotype that is not rescued by RIPK3 ablation. TNF neutralization protects from either acute loss of caspase-8 or cFLIP. These results demonstrate that caspase-8-mediated suppression of RIPK3-induced death is required not only during development but also for adult homeostasis. Furthermore, RIPK3-dependent inflammation is dispensable for the skin phenotype.
in vivo TNFα neutralization
Bradley, L. M., et al (2012). "Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling" PLoS Pathog 8(4): e1002641.
PubMed
The early inflammatory response to influenza virus infection contributes to severe lung disease and continues to pose a serious threat to human health. The mechanisms by which neutrophils gain entry to the respiratory tract and their role during pathogenesis remain unclear. Here, we report that neutrophils significantly contributed to morbidity in a pathological mouse model of influenza virus infection. Using extensive immunohistochemistry, bone marrow transfers, and depletion studies, we identified neutrophils as the predominant pulmonary cellular source of the gelatinase matrix metalloprotease (MMP) 9, which is capable of digesting the extracellular matrix. Furthermore, infection of MMP9-deficient mice showed that MMP9 was functionally required for neutrophil migration and control of viral replication in the respiratory tract. Although MMP9 release was toll-like receptor (TLR) signaling-dependent, MyD88-mediated signals in non-hematopoietic cells, rather than neutrophil TLRs themselves, were important for neutrophil migration. These results were extended using multiplex analyses of inflammatory mediators to show that neutrophil chemotactic factor, CCL3, and TNFalpha were reduced in the Myd88(-)/(-) airways. Furthermore, TNFalpha induced MMP9 secretion by neutrophils and blocking TNFalpha in vivo reduced neutrophil recruitment after infection. Innate recognition of influenza virus therefore provides the mechanisms to induce recruitment of neutrophils through chemokines and to enable their motility within the tissue via MMP9-mediated cleavage of the basement membrane. Our results demonstrate a previously unknown contribution of MMP9 to influenza virus pathogenesis by mediating excessive neutrophil migration into the respiratory tract in response to viral replication that could be exploited for therapeutic purposes.
in vivo TNFα neutralization
Quezada, S. A., et al (2010). "Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts" J Exp Med 207(3): 637-650.
PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
Product Citations
-
-
Immunology and Microbiology
Naturalized immune responses are stable over years in a colony of laboratory mice with wild-derived microbiota.
In Immunity on 9 September 2025 by Oh, J. H., Hild, B., et al.
PubMed
Free-living mammals carry complex microbiota that co-evolved with their hosts over eons of years. The transfer of such microbiota from wild mice to genetically tractable laboratory mice has been shown to enhance modeling of human immune responses in preclinical studies. Here, we assessed the long-term stability of microbiota and immune phenotype of the first C57BL/6 mouse colony with natural microbiota (wildling mice). The bacterial gut microbiota of wildling mice maintained its increased α-diversity and richness over 5 years, with significantly greater stability than the gut microbiota of laboratory mice. Wildling mice had increased myeloid cell numbers across organs and increased activation and function of natural killer, B, and T cells, which was transferable to laboratory mice via co-housing. Immunological readouts in two preclinical models remained stable throughout the follow-up. These results demonstrate the feasibility of maintaining mouse colonies with natural, wild-derived microbiota as a sharable resource for basic and preclinical research.
-
-
-
Immunology and Microbiology
MOSPD2 regulates the activation state of αLβ2 integrin to control monocyte migration: applicability for treatment of chronic inflammatory diseases.
In Immunol Res on 1 May 2025 by Salem, Y., Yacov, N., et al.
PubMed
Monocytes are innate immune cells that drive the chronicity of various inflammatory diseases. Monocyte migration to inflamed tissues involves multiple steps of interaction with the vascular endothelium and the extracellular matrix (ECM), a process mediated through conformational transitions in cell surface integrins. We previously described motile sperm domain-containing protein 2 (MOSPD2) as a surface protein expressed on myeloid cells that is essential for the migration of monocytes and a key regulator of inflammation. Investigating MOSPD2's mechanism of action, we assessed whether it plays a role in regulating integrin activation and monocyte adhesion. Data show that silencing of MOSPD2 expression in the THP-1 monocytic cell line significantly increased cell adhesion to various ECM molecules. Employing IW-601, a humanized anti-human MOSDP2 monoclonal antibody, on primary human monocytes increased adhesion to ECM molecules as well as to adhesion molecules. At the molecular level, silencing of MOSPD2 or blocking MOSPD2 using IW-601 led to a transition in integrin αLβ2 (CD11a/CD18, LFA-1) conformation into an active high-affinity binding form and to the induction of adhesion-associated signaling pathways. Co-immunoprecipitation experiments showed that MOSPD2 binds integrin-β2 (CD18), but not integrin-β1 (CD29). Our results reveal a novel mechanism controlling monocyte migration, in which MOSPD2 acts as an adhesion checkpoint that governs the balance between monocyte adhesion and release. By demonstrating the inhibitory effect of IW-601 on the migration of primary monocytes isolated from patients with chronic inflammatory diseases, we provide proof of concept for translating MOSPD2's mechanism into a potential treatment for inflammatory diseases, further supported by in vivo data in models of RA and IBD.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance.
In Nat Immunol on 1 January 2024 by Lee, A., Floyd, K., et al.
PubMed
Bacille Calmette-Guérin (BCG) vaccination can confer nonspecific protection against heterologous pathogens. However, the underlying mechanisms remain mysterious. We show that mice vaccinated intravenously with BCG exhibited reduced weight loss and/or improved viral clearance when challenged with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 B.1.351) or PR8 influenza. Protection was first evident between 14 and 21 d post-vaccination and lasted ∼3 months. Notably, BCG induced a biphasic innate response and robust antigen-specific type 1 helper T cell (TH1 cell) responses in the lungs. MyD88 signaling was essential for innate and TH1 cell responses, and protection against SARS-CoV-2. Depletion of CD4+ T cells or interferon (IFN)-γ activity before infection obliterated innate activation and protection. Single-cell and spatial transcriptomics revealed CD4-dependent expression of IFN-stimulated genes in lung myeloid and epithelial cells. Notably, BCG also induced protection against weight loss after mouse-adapted SARS-CoV-2 BA.5, SARS-CoV and SHC014 coronavirus infections. Thus, BCG elicits integrated organ immunity, where CD4+ T cells feed back on tissue myeloid and epithelial cells to imprint prolonged and broad innate antiviral resistance.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Integrated Organ Immunity: Antigen-specific CD4-T cell-derived IFN-γ induced by BCG imprints prolonged lung innate resistance against respiratory viruses
In bioRxiv on 2 August 2023 by Lee, A., Floyd, K., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
The enteric nervous system relays psychological stress to intestinal inflammation.
In Cell on 22 June 2023 by Schneider, K. M., Blank, N., et al.
PubMed
Mental health profoundly impacts inflammatory responses in the body. This is particularly apparent in inflammatory bowel disease (IBD), in which psychological stress is associated with exacerbated disease flares. Here, we discover a critical role for the enteric nervous system (ENS) in mediating the aggravating effect of chronic stress on intestinal inflammation. We find that chronically elevated levels of glucocorticoids drive the generation of an inflammatory subset of enteric glia that promotes monocyte- and TNF-mediated inflammation via CSF1. Additionally, glucocorticoids cause transcriptional immaturity in enteric neurons, acetylcholine deficiency, and dysmotility via TGF-β2. We verify the connection between the psychological state, intestinal inflammation, and dysmotility in three cohorts of IBD patients. Together, these findings offer a mechanistic explanation for the impact of the brain on peripheral inflammation, define the ENS as a relay between psychological stress and gut inflammation, and suggest that stress management could serve as a valuable component of IBD care.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Tumour extracellular vesicles and particles induce liver metabolic dysfunction.
In Nature on 1 June 2023 by Wang, G., Li, J., et al.
PubMed
Cancer alters the function of multiple organs beyond those targeted by metastasis1,2. Here we show that inflammation, fatty liver and dysregulated metabolism are hallmarks of systemically affected livers in mouse models and in patients with extrahepatic metastasis. We identified tumour-derived extracellular vesicles and particles (EVPs) as crucial mediators of cancer-induced hepatic reprogramming, which could be reversed by reducing tumour EVP secretion via depletion of Rab27a. All EVP subpopulations, exosomes and principally exomeres, could dysregulate hepatic function. The fatty acid cargo of tumour EVPs-particularly palmitic acid-induced secretion of tumour necrosis factor (TNF) by Kupffer cells, generating a pro-inflammatory microenvironment, suppressing fatty acid metabolism and oxidative phosphorylation, and promoting fatty liver formation. Notably, Kupffer cell ablation or TNF blockade markedly decreased tumour-induced fatty liver generation. Tumour implantation or pre-treatment with tumour EVPs diminished cytochrome P450 gene expression and attenuated drug metabolism in a TNF-dependent manner. We also observed fatty liver and decreased cytochrome P450 expression at diagnosis in tumour-free livers of patients with pancreatic cancer who later developed extrahepatic metastasis, highlighting the clinical relevance of our findings. Notably, tumour EVP education enhanced side effects of chemotherapy, including bone marrow suppression and cardiotoxicity, suggesting that metabolic reprogramming of the liver by tumour-derived EVPs may limit chemotherapy tolerance in patients with cancer. Our results reveal how tumour-derived EVPs dysregulate hepatic function and their targetable potential, alongside TNF inhibition, for preventing fatty liver formation and enhancing the efficacy of chemotherapy.
-
-
Phase I trial of the TNF-α inhibitor certolizumab plus chemotherapy in stage IV lung adenocarcinomas.
In Nat Commun on 15 October 2022 by Paik, P., Luo, J., et al.
PubMed
We previously identified a chemotherapy-induced paracrine inflammatory loop that paradoxically mitigates the anti-tumor effect of chemotherapy and triggers metastatic propagation in breast and lung cancer models. Therefore, we sought to further validate and translate these findings into patient care by coupling the anti-TNF-α drug certolizumab pegol with standard cisplatin doublet chemotherapy. Here we first validate the anti-metastatic effect of certolizumab in a liver-metastatic Lewis Lung Carcinoma model. We then evaluate the safety, efficacy, and pharmacodynamic effects of certolizumab with cisplatin and pemetrexed in an open label Phase 1 clinical trial (NCT02120807) of eighteen adult patients with stage IV lung adenocarcinomas. The primary outcome is maximum tolerated dose. Secondary outcomes are response rate and progression-free survival (PFS); pharmacodynamic changes in blood and tumor are evaluated as a correlative outcome. There were nine partial responses among 16 patients evaluable (56%, 95% CI 30 to 80%). The median duration of response was 9.0 months (range 5.9 to 42.6 months) and median PFS was 7.1 months (95% CI 6.3 to NR). The standard 400 mg dose of certolizumab, added to cisplatin and pemetrexed, is well-tolerated and, as a correlative endpoint, demonstrates potent pharmacodynamic inhibition of peripheral cytokines associated with the paracrine inflammatory loop.
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Immune cells and their inflammatory mediators modify β cells and cause checkpoint inhibitor-induced diabetes.
In JCI Insight on 8 September 2022 by Perdigoto, A. L., Deng, S., et al.
PubMed
Checkpoint inhibitors (CPIs) targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) have revolutionized cancer treatment but can trigger autoimmune complications, including CPI-induced diabetes mellitus (CPI-DM), which occurs preferentially with PD-1 blockade. We found evidence of pancreatic inflammation in patients with CPI-DM with shrinkage of pancreases, increased pancreatic enzymes, and in a case from a patient who died with CPI-DM, peri-islet lymphocytic infiltration. In the NOD mouse model, anti-PD-L1 but not anti-CTLA-4 induced diabetes rapidly. RNA sequencing revealed that cytolytic IFN-γ+CD8+ T cells infiltrated islets with anti-PD-L1. Changes in β cells were predominantly driven by IFN-γ and TNF-α and included induction of a potentially novel β cell population with transcriptional changes suggesting dedifferentiation. IFN-γ increased checkpoint ligand expression and activated apoptosis pathways in human β cells in vitro. Treatment with anti-IFN-γ and anti-TNF-α prevented CPI-DM in anti-PD-L1-treated NOD mice. CPIs targeting the PD-1/PD-L1 pathway resulted in transcriptional changes in β cells and immune infiltrates that may lead to the development of diabetes. Inhibition of inflammatory cytokines can prevent CPI-DM, suggesting a strategy for clinical application to prevent this complication.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages.
In J Exp Clin Cancer Res on 3 January 2022 by Chen, Y., Liu, Y., et al.
PubMed
Immune checkpoint inhibitor-related cardiotoxicity is one of the most lethal adverse effects, and thus, the identification of underlying mechanisms for developing strategies to overcome it has clinical importance. This study aimed to investigate whether microbiota-host interactions contribute to PD-1/PD-L1 inhibitor-related cardiotoxicity.
-
-
-
Immunology and Microbiology
Modular complement assemblies for mitigating inflammatory conditions.
In Proc Natl Acad Sci U S A on 13 April 2021 by Hainline, K. M., Shores, L. S., et al.
PubMed
Complement protein C3dg, a key linkage between innate and adaptive immunity, is capable of stimulating both humoral and cell-mediated immune responses, leading to considerable interest in its use as a molecular adjuvant. However, the potential of C3dg as an adjuvant is limited without ways of controllably assembling multiple copies of it into vaccine platforms. Here, we report a strategy to assemble C3dg into supramolecular nanofibers with excellent compositional control, using β-tail fusion tags. These assemblies were investigated as therapeutic active immunotherapies, which may offer advantages over existing biologics, particularly toward chronic inflammatory diseases. Supramolecular assemblies based on the Q11 peptide system containing β-tail-tagged C3dg, B cell epitopes from TNF, and the universal T cell epitope PADRE raised strong antibody responses against both TNF and C3dg, and prophylactic immunization with these materials significantly improved protection in a lethal TNF-mediated inflammation model. Additionally, in a murine model of psoriasis induced by imiquimod, the C3dg-adjuvanted nanofiber vaccine performed as well as anti-TNF monoclonal antibodies. Nanofibers containing only β-tail-C3dg and lacking the TNF B cell epitope also showed improvements in both models, suggesting that supramolecular C3dg, by itself, played an important therapeutic role. We observed that immunization with β-tail-C3dg caused the expansion of an autoreactive C3dg-specific T cell population, which may act to dampen the immune response, preventing excessive inflammation. These findings indicate that molecular assemblies displaying C3dg warrant further development as active immunotherapies.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
MicroRNAs of the miR-17~9 family maintain adipose tissue macrophage homeostasis by sustaining IL-10 expression.
In Elife on 5 November 2020 by Zhang, X., Liu, J., et al.
PubMed
Macrophages are critically involved in not only immune and inflammatory responses but also maintenance of metabolic fitness of organisms. Combined genetic deficiency of three clusters in the miR-17~92 family drastically shifted macrophage phenotypes toward the inflammatory spectrum characterized by heightened production of pro-inflammatory mediator TNF and diminished expression of anti-inflammatory cytokine IL-10. Consequently, macrophages residing in the adipose tissues from myeloid-specific miRNA triple knockout mice spontaneously developed inflammatory phenotypes and displayed alterations of overall physiological conditions as evidenced by obesity and compromised glucose tolerance. Mechanistically, miR-17~92 family miRNAs sustained IL-10 production by promoting transcription of the Fos gene, which is secondary to downregulation of Fos by transcription factor YY1, a direct target of miR-17~92 family miRNAs. Together, these results identified miR-17~92 family miRNAs as crucial regulators of the balance between pro- and anti-inflammatory cytokines and exemplified how macrophage-intrinsic regulatory circuit exerted impactful influence on general physiology.
-
-
-
Enzyme-linked immunosorbent assay
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
Metabolic profiling during malaria reveals the role of the aryl hydrocarbon receptor in regulating kidney injury.
In Elife on 6 October 2020 by Lissner, M. M., Cumnock, K., et al.
PubMed
Systemic metabolic reprogramming induced by infection exerts profound, pathogen-specific effects on infection outcome. Here, we detail the host immune and metabolic response during sickness and recovery in a mouse model of malaria. We describe extensive alterations in metabolism during acute infection, and identify increases in host-derived metabolites that signal through the aryl hydrocarbon receptor (AHR), a transcription factor with immunomodulatory functions. We find that Ahr-/- mice are more susceptible to malaria and develop high plasma heme and acute kidney injury. This phenotype is dependent on AHR in Tek-expressing radioresistant cells. Our findings identify a role for AHR in limiting tissue damage during malaria. Furthermore, this work demonstrates the critical role of host metabolism in surviving infection.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
CD4+ T Cells Cross-Reactive with Dengue and Zika Viruses Protect against Zika Virus Infection.
In Cell Rep on 28 April 2020 by Wen, J., Wang, Y. T., et al.
PubMed
The underlying mechanisms by which prior immunity to dengue virus (DENV) affords cross-protection against the related flavivirus Zika virus (ZIKV) are poorly understood. Here, we examine the ability of DENV/ZIKV-cross-reactive CD4+ T cells to protect against versus exacerbate ZIKV infection by using a histocompatibility leukocyte antigen (HLA)-DRB1∗0101 transgenic, interferon α/β receptor-deficient mouse model that supports robust DENV and ZIKV replication. By mapping the HLA-DRB1∗0101-restricted T cell response, we identify DENV/ZIKV-cross-reactive CD4+ T cell epitopes that stimulate interferon gamma (IFNγ) and/or tumor necrosis factor (TNF) production. Vaccination of naive HLA-DRB1∗0101 transgenic mice with these peptides induces a CD4+ T cell response sufficient to reduce tissue viral burden following ZIKV infection. Notably, this protective response requires IFNγ and/or TNF secretion but not anti-ZIKV immunoglobulin G (IgG) production. Thus, DENV/ZIKV-cross-reactive CD4+ T cells producing canonical Th1 cytokines can suppress ZIKV replication in an antibody-independent manner. These results may have important implications for increasing the efficacy and safety of DENV/ZIKV vaccines and for developing pan-flavivirus vaccines.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
TNFR2 Signaling Enhances ILC2 Survival, Function, and Induction of Airway Hyperreactivity.
In Cell Rep on 24 December 2019 by Hurrell, B. P., Galle-Treger, L., et al.
PubMed
Group 2 innate lymphoid cells (ILC2s) can initiate pathologic inflammation in allergic asthma by secreting copious amounts of type 2 cytokines, promoting lung eosinophilia and airway hyperreactivity (AHR), a cardinal feature of asthma. We discovered that the TNF/TNFR2 axis is a central immune checkpoint in murine and human ILC2s. ILC2s selectively express TNFR2, and blocking the TNF/TNFR2 axis inhibits survival and cytokine production and reduces ILC2-dependent AHR. The mechanism of action of TNFR2 in ILC2s is through the non-canonical NF-κB pathway as an NF-κB-inducing kinase (NIK) inhibitor blocks the costimulatory effect of TNF-α. Similarly, human ILC2s selectively express TNFR2, and using hILC2s, we show that TNFR2 engagement promotes AHR through a NIK-dependent pathway in alymphoid murine recipients. These findings highlight the role of the TNF/TNFR2 axis in pulmonary ILC2s, suggesting that targeting TNFR2 or relevant signaling is a different strategy for treating patients with ILC2-dependent asthma.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Mucosal infection rewires TNFɑ signaling dynamics to skew susceptibility to recurrence.
In Elife on 20 August 2019 by Yu, L., O'Brien, V. P., et al.
PubMed
A mucosal infectious disease episode can render the host either more or less susceptible to recurrent infection, but the specific mechanisms that tip the balance remain unclear. We investigated this question in a mouse model of recurrent urinary tract infection and found that a prior bladder infection resulted in an earlier onset of tumor necrosis factor-alpha (TNFɑ)-mediated bladder inflammation upon subsequent bacterial challenge, relative to age-matched naive mice. However, the duration of TNFɑ signaling activation differed according to whether the first infection was chronic (Sensitized) or self-limiting (Resolved). TNFɑ depletion studies revealed that transient early-phase TNFɑ signaling in Resolved mice promoted clearance of bladder-colonizing bacteria via rapid recruitment of neutrophils and subsequent exfoliation of infected bladder cells. In contrast, sustained TNFɑ signaling in Sensitized mice prolonged damaging inflammation, worsening infection. This work reveals how TNFɑ signaling dynamics can be rewired by a prior infection to shape diverse susceptibilities to future mucosal infections.
-
-
Specific sequences of infectious challenge lead to secondary hemophagocytic lymphohistiocytosis-like disease in mice.
In Proc Natl Acad Sci U S A on 5 February 2019 by Wang, A., Pope, S. D., et al.
PubMed
Secondary hemophagocytic lymphohistiocytosis (sHLH) is a highly mortal complication associated with sepsis. In adults, it is often seen in the setting of infections, especially viral infections, but the mechanisms that underlie pathogenesis are unknown. sHLH is characterized by a hyperinflammatory state and the presence hemophagocytosis. We found that sequential challenging of mice with a nonlethal dose of viral toll-like receptor (TLR) agonist followed by a nonlethal dose of TLR4 agonist, but not other permutations, produced a highly lethal state that recapitulates many aspects of human HLH. We found that this hyperinflammatory response could be recapitulated in vitro in bone marrow-derived macrophages. RNA sequencing analyses revealed dramatic up-regulation of the red-pulp macrophage lineage-defining transcription factor SpiC and its associated transcriptional program, which was also present in bone marrow macrophages sorted from patients with sHLH. Transcriptional profiling also revealed a unique metabolic transcriptional profile in these macrophages, and immunometabolic phenotyping revealed impaired mitochondrial function and oxidative metabolism and a reliance on glycolytic metabolism. Subsequently, we show that therapeutic administration of the glycolysis inhibitor 2-deoxyglucose was sufficient to rescue animals from HLH. Together, these data identify a potential mechanism for the pathogenesis of sHLH and a potentially useful therapeutic strategy for its treatment.
-
Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle.
In Nat Med on 1 June 2018 by Wang, G., Biswas, A. K., et al.
PubMed
Patients with metastatic cancer experience a severe loss of skeletal muscle mass and function known as cachexia. Cachexia is associated with poor prognosis and accelerated death in patients with cancer, yet its underlying mechanisms remain poorly understood. Here, we identify the metal-ion transporter ZRT- and IRT-like protein 14 (ZIP14) as a critical mediator of cancer-induced cachexia. ZIP14 is upregulated in cachectic muscles of mice and in patients with metastatic cancer and can be induced by TNF-α and TGF-β cytokines. Strikingly, germline ablation or muscle-specific depletion of Zip14 markedly reduces muscle atrophy in metastatic cancer models. We find that ZIP14-mediated zinc uptake in muscle progenitor cells represses the expression of MyoD and Mef2c and blocks muscle-cell differentiation. Importantly, ZIP14-mediated zinc accumulation in differentiated muscle cells induces myosin heavy chain loss. These results highlight a previously unrecognized role for altered zinc homeostasis in metastatic cancer-induced muscle wasting and implicate ZIP14 as a therapeutic target for its treatment.
-
TNF activation of NF-κB is essential for development of single-positive thymocytes.
In J Exp Med on 25 July 2016 by Webb, L. V., Ley, S. C., et al.
PubMed
NF-κB activation has been implicated at multiple stages of thymic development of T cells, during which it is thought to mediate developmental signals originating from the T cell receptor (TCR). However, the Card11-Bcl10-Malt1 (CBM) complex that is essential for TCR activation of NF-κB in peripheral T cells is not required for thymocyte development. It has remained unclear whether the TCR activates NF-κB independent of the CBM complex in thymocyte development or whether another NF-κB activating receptor is involved. In the present study, we generated mice in which T cells lacked expression of both catalytic subunits of the inhibitor of κB kinase (IKK) complex, IKK1 and IKK2, to investigate this question. Although early stages of T cell development were unperturbed, maturation of CD4 and CD8 single-positive (SP) thymocytes was blocked in mice lacking IKK1/2 in the T cell lineage. We found that IKK1/2-deficient thymocytes were specifically sensitized to TNF-induced cell death in vitro. Furthermore, the block in thymocyte development in IKK1/2-deficient mice could be rescued by blocking TNF with anti-TNF mAb or by ablation of TNFRI expression. These experiments reveal an essential role for TNF activation of NF-κB to promote the survival and development of single positive T cells in the thymus.
-
-
Immunology and Microbiology
Neutrophil and Alveolar Macrophage-Mediated Innate Immune Control of Legionella pneumophila Lung Infection via TNF and ROS.
In PLoS Pathog on 1 April 2016 by Ziltener, P., Reinheckel, T., et al.
PubMed
Legionella pneumophila is a facultative intracellular bacterium that lives in aquatic environments where it parasitizes amoeba. However, upon inhalation of contaminated aerosols it can infect and replicate in human alveolar macrophages, which can result in Legionnaires' disease, a severe form of pneumonia. Upon experimental airway infection of mice, L. pneumophila is rapidly controlled by innate immune mechanisms. Here we identified, on a cell-type specific level, the key innate effector functions responsible for rapid control of infection. In addition to the well-characterized NLRC4-NAIP5 flagellin recognition pathway, tumor necrosis factor (TNF) and reactive oxygen species (ROS) are also essential for effective innate immune control of L. pneumophila. While ROS are essential for the bactericidal activity of neutrophils, alveolar macrophages (AM) rely on neutrophil and monocyte-derived TNF signaling via TNFR1 to restrict bacterial replication. This TNF-mediated antibacterial mechanism depends on the acidification of lysosomes and their fusion with L. pneumophila containing vacuoles (LCVs), as well as caspases with a minor contribution from cysteine-type cathepsins or calpains, and is independent of NLRC4, caspase-1, caspase-11 and NOX2. This study highlights the differential utilization of innate effector pathways to curtail intracellular bacterial replication in specific host cells upon L. pneumophila airway infection.
-