InVivoMAb anti-mouse TNFα
Product Description
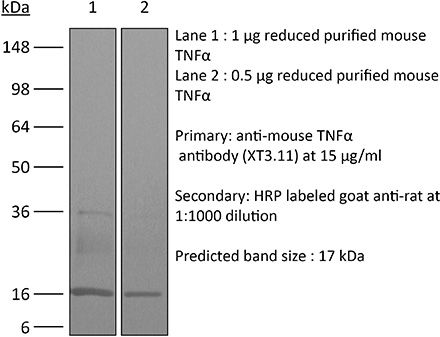
Specifications
| Isotype | Rat IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse TNFα |
| Reported Applications |
in vivo TNFα neutralization in vitro TNFα neutralization Western blot |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107764 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo TNFα neutralization
Shaabani, N., et al (2018). "The probacterial effect of type I interferon signaling requires its own negative regulator USP18" Sci Immunol 3(27).
PubMed
Type I interferon (IFN-I) signaling paradoxically impairs host immune responses during many primary and secondary bacterial infections. Lack of IFN-I receptor reduces bacterial replication and/or bacterial persistence during infection with several bacteria. However, the mechanisms that mediate the adverse IFN-I effect are incompletely understood. Here, we show that Usp18, an interferon-stimulated gene that negatively regulates IFN-I signaling, is primarily responsible for the deleterious effect of IFN-I signaling during infection of mice with Listeria monocytogenes or Staphylococcus aureus Mechanistically, USP18 promoted bacterial replication by inhibiting antibacterial tumor necrosis factor-alpha (TNF-alpha) signaling. Deleting IFNAR1 or USP18 in CD11c-Cre(+) cells similarly reduced bacterial titers in multiple organs and enhanced survival. Our results demonstrate that inhibiting USP18 function can promote control of primary and secondary bacterial infection by enhancing the antibacterial effect of TNF-alpha, which correlates with induction of reactive oxygen species (ROS). These findings suggest that USP18 could be targeted therapeutically in patients to ameliorate disease caused by serious bacterial infections.
in vivo TNFα neutralization
Baeyens, A., et al (2015). "Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context" J Immunol 194(3): 999-1010.
PubMed
CD4(+)CD25(+)Foxp3(+) regulatory T (Treg) cells play a major role in peripheral tolerance. Multiple environmental factors and cell types affect their biology. Among them, activated effector CD4(+) T cells can boost Treg cell expansion through TNF or IL-2. In this study, we further characterized this effector T (Teff) cell-dependent Treg cell boost in vivo in mice. This phenomenon was observed when both Treg and Teff cells were activated by their cognate Ag, with the latter being the same or different. Also, when Treg cells highly proliferated on their own, there was no additional Treg cell boost by Teff cells. In a condition of low inflammation, the Teff cell-mediated Treg cell boost involved TNF, OX40L, and plasmacytoid dendritic cells, whereas in a condition of high inflammation, it involved TNF and IL-2. Thus, this feedback mechanism in which Treg cells are highly activated by their Teff cell counterparts depends on the immune context for its effectiveness and mechanism. This Teff cell-dependent Treg cell boost may be crucial to limit inflammatory and autoimmune responses.
in vivo TNFα neutralization
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo TNFα neutralization
Grinberg-Bleyer, Y., et al (2015). "Cutting edge: NF-kappaB p65 and c-Rel control epidermal development and immune homeostasis in the skin" J Immunol 194(6): 2472-2476.
PubMed
Psoriasis is an inflammatory skin disease in which activated immune cells and the proinflammatory cytokine TNF are well-known mediators of pathogenesis. The transcription factor NF-kappaB is a key regulator of TNF production and TNF-induced proinflammatory gene expression, and both the psoriatic transcriptome and genetic susceptibility further implicate NF-kappaB in psoriasis etiopathology. However, the role of NF-kappaB in psoriasis remains controversial. We analyzed the function of canonical NF-kappaB in the epidermis using CRE-mediated deletion of p65 and c-Rel in keratinocytes. In contrast to animals lacking p65 or c-Rel alone, mice lacking both subunits developed severe dermatitis after birth. Consistent with its partial histological similarity to human psoriasis, this condition could be prevented by anti-TNF treatment. Moreover, regulatory T cells in lesional skin played an important role in disease remission. Our results demonstrate that canonical NF-kappaB in keratinocytes is essential for the maintenance of skin immune homeostasis and is protective against spontaneous dermatitis.
in vivo TNFα neutralization
Deng, L., et al (2014). "Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice" J Clin Invest 124(2): 687-695.
PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
in vitro TNFα neutralization
Beug, S. T., et al (2014). "Smac mimetics and innate immune stimuli synergize to promote tumor death" Nat Biotechnol 32(2): 182-190.
PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
in vivo TNFα neutralization
DeBerge, M. P., et al (2014). "Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology" J Immunol 192(12): 5839-5851.
PubMed
TNF-alpha is a pleotropic cytokine that has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-alpha is first expressed as a transmembrane protein that is proteolytically processed to release a soluble form. Transmembrane TNF-alpha (memTNF-alpha) and soluble TNF-alpha (solTNF-alpha) have been shown to exert distinct tissue-protective or tissue-pathologic effects in several disease models. However, the relative contributions of memTNF-alpha or solTNF-alpha in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing noncleavable memTNF-alpha or lacking TNF-alpha entirely and examined the outcomes. We found that solTNF-alpha, but not memTNF-alpha, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-alpha, there was a significant increase in the CD8(+) T cell response, including virus-specific CD8(+) T cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-alpha mediates these immunoregulatory effects primarily through TNFR1, because mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-alpha. We also found that solTNF-alpha expression was required early during infection to regulate the magnitude of the CD8(+) T cell response, indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-alpha to release solTNF-alpha is a critical event regulating the immune response during influenza infection.
in vivo TNFα neutralization
Maltby, S., et al (2014). "Production and differentiation of myeloid cells driven by proinflammatory cytokines in response to acute pneumovirus infection in mice" J Immunol 193(8): 4072-4082.
PubMed
Respiratory virus infections are often pathogenic, driving severe inflammatory responses. Most research has focused on localized effects of virus infection and inflammation. However, infection can induce broad-reaching, systemic changes that are only beginning to be characterized. In this study, we assessed the impact of acute pneumovirus infection in C57BL/6 mice on bone marrow hematopoiesis. We hypothesized that inflammatory cytokine production in the lung upregulates myeloid cell production in response to infection. We demonstrate a dramatic increase in the percentages of circulating myeloid cells, which is associated with pronounced elevations in inflammatory cytokines in serum (IFN-gamma, IL-6, CCL2), bone (TNF-alpha), and lung tissue (TNF-alpha, IFN-gamma, IL-6, CCL2, CCL3, G-CSF, osteopontin). Increased hematopoietic stem/progenitor cell percentages (Lineage(-)Sca-I(+)c-kit(+)) were also detected in the bone marrow. This increase was accompanied by an increase in the proportions of committed myeloid progenitors, as determined by colony-forming unit assays. However, no functional changes in hematopoietic stem cells occurred, as assessed by competitive bone marrow reconstitution. Systemic administration of neutralizing Abs to either TNF-alpha or IFN-gamma blocked expansion of myeloid progenitors in the bone marrow and also limited virus clearance from the lung. These findings suggest that acute inflammatory cytokines drive production and differentiation of myeloid cells in the bone marrow by inducing differentiation of committed myeloid progenitors. Our findings provide insight into the mechanisms via which innate immune responses regulate myeloid cell progenitor numbers in response to acute respiratory virus infection.
in vivo TNFα neutralization
Yoo, J. K. and T. J. Braciale (2014). "IL-21 promotes late activator APC-mediated T follicular helper cell differentiation in experimental pulmonary virus infection" PLoS One 9(9): e105872.
PubMed
IL-21 is a type-I cytokine that has pleiotropic immuno-modulatory effects. Primarily produced by activated T cells including NKT and TFH cells, IL-21 plays a pivotal role in promoting TFH differentiation through poorly understood cellular and molecular mechanisms. Here, employing a mouse model of influenza A virus (IAV) infection, we demonstrate that IL-21, initially produced by NKT cells, promotes TFH differentiation by promoting the migration of late activator antigen presenting cell (LAPC), a recently identified TFH inducer, from the infected lungs into the draining lymph nodes (dLN). LAPC migration from IAV-infected lung into the dLN is CXCR3-CXCL9 dependent. IL-21-induced TNF-alpha production by conventional T cells is critical to stimulate CXCL9 expression by DCs in the dLN, which supports LAPC migration into the dLN and ultimately facilitates TFH differentiation. Our results reveal a previously unappreciated mechanism for IL-21 modulation of TFH responses during respiratory virus infection.
in vivo TNFα neutralization
Dietze, K. K., et al (2013). "Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads" PLoS Pathog 9(12): e1003798.
PubMed
Chronic infections with human viruses, such as HIV and HCV, or mouse viruses, such as LCMV or Friend Virus (FV), result in functional exhaustion of CD8(+) T cells. Two main mechanisms have been described that mediate this exhaustion: expression of inhibitory receptors on CD8(+) T cells and expansion of regulatory T cells (Tregs) that suppress CD8(+) T cell activity. Several studies show that blockage of one of these pathways results in reactivation of CD8(+) T cells and partial reduction in chronic viral loads. Using blocking antibodies against PD-1 ligand and Tim-3 and transgenic mice in which Tregs can be selectively ablated, we compared these two treatment strategies and combined them for the first time in a model of chronic retrovirus infection. Blocking inhibitory receptors was more efficient than transient depletion of Tregs in reactivating exhausted CD8(+) T cells and reducing viral set points. However, a combination therapy was superior to any single treatment and further augmented CD8(+) T cell responses and resulted in a sustained reduction in chronic viral loads. These results demonstrate that Tregs and inhibitory receptors are non-overlapping factors in the maintenance of chronic viral infections and that immunotherapies targeting both pathways may be a promising strategy to treat chronic infectious diseases.
in vivo TNFα neutralization
Kugler, D. G., et al (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927.
PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo TNFα neutralization
Weinlich, R., et al (2013). "Protective roles for caspase-8 and cFLIP in adult homeostasis" Cell Rep 5(2): 340-348.
PubMed
Caspase-8 or cellular FLICE-like inhibitor protein (cFLIP) deficiency leads to embryonic lethality in mice due to defects in endothelial tissues. Caspase-8(-/-) and receptor-interacting protein kinase-3 (RIPK3)(-/-), but not cFLIP(-/-) and RIPK3(-/-), double-knockout animals develop normally, indicating that caspase-8 antagonizes the lethal effects of RIPK3 during development. Here, we show that the acute deletion of caspase-8 in the gut of adult mice induces enterocyte death, disruption of tissue homeostasis, and inflammation, resulting in sepsis and mortality. Likewise, acute deletion of caspase-8 in a focal region of the skin induces local keratinocyte death, tissue disruption, and inflammation. Strikingly, RIPK3 ablation rescues both phenotypes. However, acute loss of cFLIP in the skin produces a similar phenotype that is not rescued by RIPK3 ablation. TNF neutralization protects from either acute loss of caspase-8 or cFLIP. These results demonstrate that caspase-8-mediated suppression of RIPK3-induced death is required not only during development but also for adult homeostasis. Furthermore, RIPK3-dependent inflammation is dispensable for the skin phenotype.
in vivo TNFα neutralization
Bradley, L. M., et al (2012). "Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling" PLoS Pathog 8(4): e1002641.
PubMed
The early inflammatory response to influenza virus infection contributes to severe lung disease and continues to pose a serious threat to human health. The mechanisms by which neutrophils gain entry to the respiratory tract and their role during pathogenesis remain unclear. Here, we report that neutrophils significantly contributed to morbidity in a pathological mouse model of influenza virus infection. Using extensive immunohistochemistry, bone marrow transfers, and depletion studies, we identified neutrophils as the predominant pulmonary cellular source of the gelatinase matrix metalloprotease (MMP) 9, which is capable of digesting the extracellular matrix. Furthermore, infection of MMP9-deficient mice showed that MMP9 was functionally required for neutrophil migration and control of viral replication in the respiratory tract. Although MMP9 release was toll-like receptor (TLR) signaling-dependent, MyD88-mediated signals in non-hematopoietic cells, rather than neutrophil TLRs themselves, were important for neutrophil migration. These results were extended using multiplex analyses of inflammatory mediators to show that neutrophil chemotactic factor, CCL3, and TNFalpha were reduced in the Myd88(-)/(-) airways. Furthermore, TNFalpha induced MMP9 secretion by neutrophils and blocking TNFalpha in vivo reduced neutrophil recruitment after infection. Innate recognition of influenza virus therefore provides the mechanisms to induce recruitment of neutrophils through chemokines and to enable their motility within the tissue via MMP9-mediated cleavage of the basement membrane. Our results demonstrate a previously unknown contribution of MMP9 to influenza virus pathogenesis by mediating excessive neutrophil migration into the respiratory tract in response to viral replication that could be exploited for therapeutic purposes.
in vivo TNFα neutralization
Quezada, S. A., et al (2010). "Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts" J Exp Med 207(3): 637-650.
PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
Product Citations
-
Unexpected Role of TNFα Signaling in the Resolution of Postoperative Pain in Mice.
In J Pain Res on 19 November 2025 by Laumet, S., Edwards, A. M., et al.
PubMed
The mechanisms that govern the transition from acute to chronic pain remain poorly defined. Emerging evidence suggests that immune cells and acute inflammatory responses are not merely pathological but actively contribute to pain resolution and the prevention of chronic pain. Using a mouse model of postoperative pain induced by plantar incision, we demonstrate that inhibition of tumor necrosis factor (TNFα) signaling prolongs pain hypersensitivity. Intraplantar administration of either monoclonal or polyclonal neutralizing anti-TNFα antibodies or Etanercept, a TNF receptor decoy, significantly delayed the resolution of pain in both female and male mice. Unexpectedly, early blockade of TNFα signaling did not reduce pain hypersensitivity but instead extended its duration. These findings underscore a paradoxical yet critical role for TNFα and immune signaling in promoting the resolution of acute pain and preventing its persistence. Together it supports the concept that acute inflammation and immune cells are essential for initiating the resolution of pain.
-
-
Immunology and Microbiology
Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity.
In Cell Rep Med on 19 August 2025 by Ahn, J. J., Dudics, S., et al.
PubMed
Wild-type interleukin (IL)-2 induces anti-tumor immunity and toxicity, predominated by vascular leak syndrome (VLS) leading to edema, hypotension, organ toxicity, and regulatory T cell (Treg) expansion. Efforts to uncouple IL-2 toxicity from its potency have failed in the clinic. We hypothesize that IL-2 toxicity is driven by cytokine release syndrome (CRS) followed by VLS and that coupling IL-2 with IL-10 will ameliorate toxicity. Our data, generated using human primary cells, mouse models, and non-human primates, suggest that coupling of these cytokines prevents toxicity while retaining cytotoxic T cell activation and limiting Treg expansion. In syngeneic murine tumor models, DK210 epidermal growth factor receptor (EGFR), an IL-2/IL-10 fusion molecule targeted to EGFR via an anti-EGFR single-chain variable fragment (scFV), potently activates T cells and natural killer (NK) cells and elicits interferon (IFN)γ-dependent anti-tumor function without peripheral inflammatory toxicity or Treg accumulation. Therefore, combining IL-2 with IL-10 uncouples toxicity from immune activation, leading to a balanced and pleiotropic anti-tumor immune response.
-
-
-
Immunology and Microbiology
Engineered bacteria launch and control an oncolytic virus.
In Nat Biomed Eng on 15 August 2025 by Singer, Z. S., Pabon, J., et al.
PubMed
The ability of bacteria and viruses to selectively replicate in tumours has led to synthetic engineering of new microbial therapies. Here we design a cooperative strategy whereby Salmonella typhimurium bacteria transcribe and deliver the Senecavirus A RNA genome inside host cells, launching a potent oncolytic viral infection. 'Encapsidated' by bacteria, the viral genome can further bypass circulating antiviral antibodies to reach the tumour and initiate replication and spread within immune mice. Finally, we engineer the virus to require a bacterially delivered protease to achieve virion maturation, demonstrating bacterial control over the virus. Together, we refer to this platform as 'CAPPSID' for Coordinated Activity of Prokaryote and Picornavirus for Safe Intracellular Delivery. This work extends bacterially delivered therapeutics to viral genomes, and shows how a consortium of microbes can achieve a cooperative aim.
-
-
-
Immunology and Microbiology
A bacterial network of T3SS effectors counteracts host pro-inflammatory responses and cell death to promote infection.
In EMBO J on 1 May 2025 by Yeap, H. W., Goh, G. R., et al.
PubMed
Innate immune signalling and cell death pathways are highly interconnected processes involving receptor-interacting protein kinases (RIPKs) as mediators of potent anti-microbial responses. However, these processes are often antagonised by bacterial type III secretion system (T3SS) effectors, and the cellular mechanisms by which the host retaliates are not completely understood. Here, we demonstrate that during Citrobacter rodentium infection, murine macrophages and colonic epithelial cells exhibit RIPK1 kinase-dependent caspase-8 activation to counteract NleE effector-mediated suppression of pro-inflammatory signalling. While C. rodentium injects into the host cells a second effector, NleB, to block caspase-8 signalling, macrophages respond by triggering RIPK3-mediated necroptosis, whereupon a third T3SS effector, EspL, acts to inactivate necroptosis. We further show that NleB and EspL collaborate to suppress caspase-8 and NLRP3 inflammasome activation in macrophages. Our findings suggest that C. rodentium has evolved to express a complex network of effectors as an adaptation to the importance of cell death for anti-bacterial defence in the host-pathogen arms race.
-
-
-
Immunology and Microbiology
B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine.
In Nat Immunol on 1 May 2025 by Cembellin-Prieto, A., Luo, Z., et al.
PubMed
The rapid onset of innate immune defenses is critical for early control of viral replication in an infected host and yet it can also lead to irreversible tissue damage, especially in the respiratory tract. Sensitive regulators must exist that modulate inflammation, while controlling the infection. In the present study, we identified acetylcholine (ACh)-producing B cells as such early regulators. B cells are the most prevalent ACh-producing leukocyte population in the respiratory tract demonstrated with choline acetyltransferase (ChAT)-green fluorescent protein (GFP) reporter mice, both before and after infection with influenza A virus. Mice lacking ChAT in B cells, disabling their ability to generate ACh (ChatBKO), but not those lacking ChAT in T cells, significantly, selectively and directly suppressed α7-nicotinic-ACh receptor-expressing interstitial, but not alveolar, macrophage activation and their ability to secrete tumor necrosis factor (TNF), while better controlling virus replication at 1 d postinfection. Conversely, TNF blockade via monoclonal antibody treatment increased viral loads at that time. By day 10 of infection, ChatBKO mice showed increased local and systemic inflammation and reduced signs of lung epithelial repair despite similar viral loads and viral clearance. Thus, B cells are key participants of an immediate early regulatory cascade that controls lung tissue damage after viral infection, shifting the balance toward reduced inflammation at the cost of enhanced early viral replication.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Discrete and conserved inflammatory signatures drive thrombosis in different organs after Salmonella infection.
In Nat Commun on 10 March 2025 by Perez-Toledo, M., Beristain-Covarrubias, N., et al.
PubMed
Inflammation-induced thrombosis is a common consequence of bacterial infections, such as those caused by Salmonella Typhimurium (STm). The presentation of multi-organ thrombosis post-infection that develops and resolves with organ-specific kinetics raises significant challenges for its therapeutic control. Here, we identify specific inflammatory events driving thrombosis in the spleens and livers of STm-infected mice. IFN-γ or platelet expression of C-type lectin-like receptor CLEC-2, key drivers of thrombosis in liver, are dispensable for thrombosis in the spleen. Platelets, monocytes, and neutrophils are identified as core constituents of thrombi in both organs. Depleting either neutrophils or monocytic cells abrogates thrombus formation. Neutrophils and monocytes secrete TNF and blocking TNF diminishes both thrombosis and inflammation, which correlates with reduced endothelial expression of E-selectin and leukocyte infiltration. Moreover, inhibiting tissue factor and P-selectin glycoprotein ligand-1 pathways impairs thrombosis in both spleen and liver. Therefore, we identify organ-specific, and shared mechanisms driving thrombosis within a single infection. This may inform on tailoring treatments towards infection-induced inflammation, and single- or multi-organ thrombosis, based on the clinical need.
-
-
Cooperation of TRADD- and RIPK1-dependent cell death pathways in maintaining intestinal homeostasis.
In Nat Commun on 22 February 2025 by Sun, Z., Ye, J., et al.
PubMed
Dysfunctional NF-κB signaling is critically involved in inflammatory bowel disease (IBD). We investigated the mechanism by which RIPK1 and TRADD, two key mediators of NF-κB signaling, in mediating intestinal pathology using TAK1 IEC deficient model. We show that phosphorylation of TRADD by TAK1 modulates RIPK1-dependent apoptosis. TRADD and RIPK1 act cooperatively to mediate cell death regulated by TNF and TLR signaling. We demonstrate the pathological evolution from RIPK1-dependent ileitis to RIPK1- and TRADD-co-dependent colitis in TAK1 IEC deficient condition. Combined RIPK1 inhibition and TRADD knockout completely protect against intestinal pathology and lethality in TAK1 IEC KO mice. Furthermore, we identify distinctive microbiota dysbiosis biomarkers for RIPK1-dependent ileitis and TRADD-dependent colitis. These findings reveal the cooperation between RIPK1 and TRADD in mediating cell death and inflammation in IBD with NF-κB deficiency and suggest the possibility of combined inhibition of RIPK1 kinase and TRADD as a new therapeutic strategy for IBD.
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Dynamics of tissue repair regulatory T cells and damage in acute Trypanosoma cruzi infection.
In PLoS Pathog on 1 January 2025 by Boccardo, S., Rodriguez, C., et al.
PubMed
Tissue-repair regulatory T cells (trTregs) comprise a specialized cell subset essential for tissue homeostasis and repair. While well-studied in sterile injury models, their role in infection-induced tissue damage and antimicrobial immunity is less understood. We investigated trTreg dynamics during acute Trypanosoma cruzi infection, marked by extensive tissue damage and strong CD8+ immunity. Unlike sterile injury models, trTregs significantly declined in secondary lymphoid organs and non-lymphoid target tissues during infection, correlating with systemic and local tissue damage, and downregulation of function-associated genes in skeletal muscle. This decline was linked to decreased systemic IL-33 levels, a key trTreg growth factor, and promoted by the Th1 cytokine IFN-γ. Early recombinant IL-33 treatment increased trTregs, type 2 innate lymphoid cells, and parasite-specific CD8+ cells at specific time points after infection, leading to reduced tissue damage, lower parasite burden, and improved disease outcome. Our findings not only provide novel insights into trTregs during infection but also highlight the potential of optimizing immune balance by modulating trTreg responses to promote tissue repair while maintaining effective pathogen control during infection-induced injury.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Neuroscience
Macrophages and nociceptor neurons form a sentinel unit around fenestrated capillaries to defend the synovium from circulating immune challenge.
In Nat Immunol on 1 December 2024 by Hasegawa, T., Lee, C. Y. C., et al.
PubMed
A wide variety of systemic pathologies, including infectious and autoimmune diseases, are accompanied by joint pain or inflammation, often mediated by circulating immune complexes (ICs). How such stimuli access joints and trigger inflammation is unclear. Whole-mount synovial imaging revealed PV1+ fenestrated capillaries at the periphery of the synovium in the lining-sublining interface. Circulating ICs extravasated from these PV1+ capillaries, and nociceptor neurons and three distinct macrophage subsets formed a sentinel unit around them. Macrophages showed subset-specific responses to systemic IC challenge; LYVE1+CX3CR1+ macrophages orchestrated neutrophil recruitment and activated calcitonin gene-related peptide+ (CGRP+) nociceptor neurons via interleukin-1β. In contrast, major histocompatibility complex class II+CD11c+ (MHCII+CD11c+) and MHCII+CD11c- interstitial macrophages formed tight clusters around PV1+ capillaries in response to systemic immune stimuli, a feature enhanced by nociceptor-derived CGRP. Altogether, we identify the anatomical location of synovial PV1+ capillaries and subset-specific macrophage-nociceptor cross-talk that forms a blood-joint barrier protecting the synovium from circulating immune challenges.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
VSV∆M51 drives CD8+ T cell-mediated tumour regression through infection of both cancer and non-cancer cells.
In Nat Commun on 15 November 2024 by Rajwani, J., Vishnevskiy, D. A., et al.
PubMed
Oncolytic viruses (OV) are designed to selectively infect and kill cancer cells, while simultaneously eliciting antitumour immunity. The mechanism is expected to originate from infected cancer cells. However, recent reports of tumour regression unaccompanied by cancer cell infection suggest a more complex mechanism of action. Here, we engineered vesicular stomatitis virus (VSV)ΔM51-sensitive and VSVΔM51-resistant tumour lines to elucidate the role of OV-infected cancer and non-cancer cells. We found that, while cancer cell infections elicit oncolysis and antitumour immunity as expected, infection of non-cancer cells alone can also contribute to tumour regression. This effect is partly attributed to the systemic production of cytokines that promote dendritic cell (DC) activation, migration and antigen cross-presentation, leading to magnified antitumour CD8+ T cell activation and tumour regression. Such OV-induced antitumour immunity is complementary to PD-1 blockade. Overall, our results reveal mechanistic insights into OV-induced antitumour immunity that can be leveraged to improve OV-based therapeutics.
-
-
-
Immunology and Microbiology
-
Neuroscience
MrgprA3 neurons drive cutaneous immunity against helminths through selective control of myeloid-derived IL-33.
In Nat Immunol on 1 November 2024 by Inclan-Rico, J. M., Napuri, C. M., et al.
PubMed
Skin uses interdependent cellular networks for barrier integrity and host immunity, but most underlying mechanisms remain obscure. Herein, we demonstrate that the human parasitic helminth Schistosoma mansoni inhibited pruritus evoked by itch-sensing afferents bearing the Mas-related G-protein-coupled receptor A3 (MrgprA3) in mice. MrgprA3 neurons controlled interleukin (IL)-17+ γδ T cell expansion, epidermal hyperplasia and host resistance against S. mansoni through shaping cytokine expression in cutaneous antigen-presenting cells. MrgprA3 neuron activation downregulated IL-33 but induced IL-1β and tumor necrosis factor in macrophages and type 2 conventional dendritic cells partially through the neuropeptide calcitonin gene-related peptide. Macrophages exposed to MrgprA3-derived secretions or bearing cell-intrinsic IL-33 deletion showed increased chromatin accessibility at multiple inflammatory cytokine loci, promoting IL-17/IL-23-dependent changes to the epidermis and anti-helminth resistance. This study reveals a previously unrecognized intercellular communication mechanism wherein itch-inducing MrgprA3 neurons initiate host immunity against skin-invasive parasites by directing cytokine expression patterns in myeloid antigen-presenting cell subsets.
-
-
-
Immunology and Microbiology
TBK1 and IKKε protect target cells from IFNγ-mediated T cell killing via an inflammatory apoptotic mechanism
In bioRxiv on 8 August 2024 by Sun, N. D., Carr, A. R., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Chimeric antigen receptor dendritic cells targeted delivery of a single tumoricidal factor for cancer immunotherapy.
In Cancer Immunol Immunother on 6 August 2024 by Duan, R., Milton, P., et al.
PubMed
Chimeric antigen receptor (CAR)-T cells have been used to treat blood cancers by producing a wide variety of cytokines. However, they are not effective in treating solid cancers and can cause severe side-effects, including cytokine release syndrome. TNFα is a tumoricidal cytokine, but it markedly increases the protein levels of cIAP1 and cIAP2, the members of inhibitor of apoptosis protein (IAP) family of E3 ubiquitin ligase that limits caspase-induced apoptosis. Degradation of IAP proteins by an IAP antagonist does not effectively kill cancer cells but enables TNFα to strongly induce cancer cell apoptosis. It would be a promising approach to treat cancers by targeted delivery of TNFα through an inactive adoptive cell in combination with an IAP antagonist.
-
-
-
COVID-19
-
Immunology and Microbiology
Differential action modes of Neutrophil Extracellular Trap-targeted drugs define T cell responses in SARS-CoV-2 infection
In bioRxiv on 1 July 2024 by Bonilha, C. S., Veras, F. P., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Granzyme B PET Imaging for Assessment of Disease Activity in Inflammatory Bowel Disease.
In J Nucl Med on 1 July 2024 by Heidari, P., Haj-Mirzaian, A., et al.
PubMed
Developing a noninvasive imaging method to detect immune system activation with a high temporal resolution is key to improving inflammatory bowel disease (IBD) management. In this study, granzyme B (GZMB), typically released from cytotoxic T and natural killer cells, was targeted using PET with 68Ga-NOTA-GZP (where GZP is β-Ala-Gly-Gly-Ile-Glu-Phe-Asp-CHO) to detect early intestinal inflammation in murine models of colitis. Methods: Bioinformatic analysis was used to assess the potential of GZMB as a biomarker for detecting IBD and predicting response to treatment. Human active and quiescent Crohn disease and ulcerative colitis tissues were stained for GZMB. We used IL-10-/- mice treated with dextran sulfate sodium (DSS) as an IBD model, wild-type C57BL/6J mice as a control, and anti-tumor necrosis factor as therapy. We used a murine GZMB-binding peptide conjugated to a NOTA chelator (NOTA-GZP) labeled with 68Ga as the PET tracer. PET imaging was conducted at 1, 3, and 4 wk after colitis induction to evaluate temporal changes. Results: Bioinformatic analysis showed that GZMB gene expression is significantly upregulated in human ulcerative colitis and Crohn disease compared with the noninflamed bowel by 2.98-fold and 1.92-fold, respectively; its expression is lower by 2.16-fold in treatment responders than in nonresponders. Immunofluorescence staining of human tissues demonstrated a significantly higher GZMB in patients with active than with quiescent IBD (P = 0.032).68Ga-NOTA-GZP PET imaging showed significantly increased bowel uptake in IL-10-/- mice with DSS-induced colitis compared with vehicle-treated IL-10-/- mice (SUVmean, 0.75 vs. 0.24; P < 0.001) and both vehicle- and DSS-treated wild-type mice (SUVmean, 0.26 and 0.37; P < 0.001). In the IL-10-/- DSS-induced colitis model, the bowel PET probe uptake decreased in response to treatment with tumor necrosis factor-α (SUVmean, 0.32; P < 0.001). There was a 4-fold increase in colonic uptake of 68Ga-NOTA-GZP in the colitis model compared with the control 1 wk after colitis induction. The uptake gradually decreased to approximately 2-fold by 4 wk after IBD induction; however, the inflamed bowel uptake remained significantly higher than control at all time points (week 4 SUVmean, 0.23 vs. 0.08; P = 0.001). Conclusion: GZMB is a promising biomarker to detect active IBD and predict response to treatment. This study provides compelling evidence to translate GZMB PET for imaging IBD activity in clinical settings.
-
-
-
Immunology and Microbiology
B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine
In Research Square on 25 June 2024 by Baumgarth, N., Prieto, A. C., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Inhibition of IL-25/IL-17RA improves immune-related adverse events of checkpoint inhibitors and reveals antitumor activity.
In J Immunother Cancer on 21 March 2024 by Hu, X., Bukhari, S. M., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have improved outcomes and extended patient survival in several tumor types. However, ICIs often induce immune-related adverse events (irAEs) that warrant therapy cessation, thereby limiting the overall effectiveness of this class of therapeutic agents. Currently, available therapies used to treat irAEs might also blunt the antitumor activity of the ICI themselves. Therefore, there is an urgent need to identify treatments that have the potential to be administered alongside ICI to optimize their use.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
A pairwise cytokine code explains the organism-wide response to sepsis.
In Nat Immunol on 1 February 2024 by Takahama, M., Patil, A., et al.
PubMed
Sepsis is a systemic response to infection with life-threatening consequences. Our understanding of the molecular and cellular impact of sepsis across organs remains rudimentary. Here, we characterize the pathogenesis of sepsis by measuring dynamic changes in gene expression across organs. To pinpoint molecules controlling organ states in sepsis, we compare the effects of sepsis on organ gene expression to those of 6 singles and 15 pairs of recombinant cytokines. Strikingly, we find that the pairwise effects of tumor necrosis factor plus interleukin (IL)-18, interferon-gamma or IL-1β suffice to mirror the impact of sepsis across tissues. Mechanistically, we map the cellular effects of sepsis and cytokines by computing changes in the abundance of 195 cell types across 9 organs, which we validate by whole-mouse spatial profiling. Our work decodes the cytokine cacophony in sepsis into a pairwise cytokine message capturing the gene, cell and tissue responses of the host to the disease.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
TNFR1 signaling converging on FGF14 controls neuronal hyperactivity and sickness behavior in experimental cerebral malaria.
In J Neuroinflammation on 19 December 2023 by Dvorak, N. M., Domingo, N. D., et al.
PubMed
Excess tumor necrosis factor (TNF) is implicated in the pathogenesis of hyperinflammatory experimental cerebral malaria (eCM), including gliosis, increased levels of fibrin(ogen) in the brain, behavioral changes, and mortality. However, the role of TNF in eCM within the brain parenchyma, particularly directly on neurons, remains underdefined. Here, we investigate electrophysiological consequences of eCM on neuronal excitability and cell signaling mechanisms that contribute to observed phenotypes.
-
-
-
Immunology and Microbiology
A discrete 'early-responder' stromal-cell subtype orchestrates immunocyte recruitment to injured tissue.
In Nat Immunol on 1 December 2023 by Yaghi, O. K., Hanna, B. S., et al.
PubMed
Following acute injury, stromal cells promote tissue regeneration by a diversity of mechanisms. Time-resolved single-cell RNA sequencing of muscle mesenchymal stromal cells (MmSCs) responding to acute injury identified an 'early-responder' subtype that spiked on day 1 and expressed a notable array of transcripts encoding immunomodulators. IL-1β, TNF-α and oncostatin M each strongly and rapidly induced MmSCs transcribing this immunomodulatory program. Macrophages amplified the program but were not strictly required for its induction. Transfer of the inflammatory MmSC subtype, tagged with a unique surface marker, into healthy hindlimb muscle induced inflammation primarily driven by neutrophils and macrophages. Among the abundant inflammatory transcripts produced by this subtype, Cxcl5 was stroma-specific and highly upregulated with injury. Depletion of this chemokine early after injury revealed a substantial impact on recruitment of neutrophils, a prolongation of inflammation to later times and an effect on tissue regeneration. Mesenchymal stromal cell subtypes expressing a comparable inflammatory program were found in a mouse model of muscular dystrophy and in several other tissues and pathologies in both mice and humans. These 'early-responder' mesenchymal stromal cells, already in place, permit rapid and coordinated mobilization and amplification of critical cell collaborators in response to injury.
-