InVivoMAb anti-mouse/rat/rabbit TNFα
Product Description
Specifications
| Isotype | Armenian Hamster IgG, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
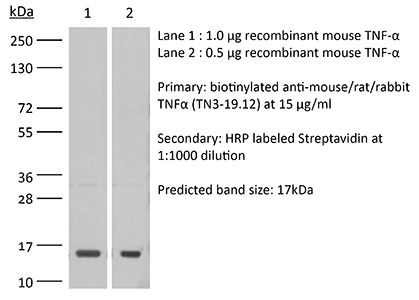
| Immunogen | Recombinant mouse TNFα |
| Reported Applications |
in vivo TNFα neutralization Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687725 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo TNFα neutralization
Xiong, H., et al (2016). "Innate Lymphocyte/Ly6C Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance" Cell. doi : 10.1016/j.cell.2016.03.017.
PubMed
Increasing antibiotic resistance among bacterial pathogens has rendered some infections untreatable with available antibiotics. Klebsiella pneumoniae, a bacterial pathogen that has acquired high-level antibiotic resistance, is a common cause of pulmonary infections. Optimal clearance of K. pneumoniae from the host lung requires TNF and IL-17A. Herein, we demonstrate that inflammatory monocytes are rapidly recruited to the lungs of K. pneumoniae-infected mice and produce TNF, which markedly increases the frequency of IL-17-producing innate lymphoid cells. While pulmonary clearance of K. pneumoniae is preserved in neutrophil-depleted mice, monocyte depletion or TNF deficiency impairs IL-17A-dependent resolution of pneumonia. Monocyte-mediated bacterial uptake and killing is enhanced by ILC production of IL-17A, indicating that innate lymphocytes engage in a positive-feedback loop with monocytes that promotes clearance of pneumonia. Innate immune defense against a highly antibiotic-resistant bacterial pathogen depends on crosstalk between inflammatory monocytes and innate lymphocytes that is mediated by TNF and IL-17A.
in vivo TNFα neutralization
Brasseit, J., et al (2015). "CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis" Mucosal Immunol. doi : 10.1038/mi.2015.93.
PubMed
Current therapies to treat inflammatory bowel diseases have limited efficacy, significant side effects, and often wane over time. Little is known about the cellular and molecular mechanisms operative in the process of mucosal healing from colitis. To study such events, we developed a new model of reversible colitis in which adoptive transfer of CD4+CD45RBhi T cells into Helicobacter typhlonius-colonized lymphopenic mice resulted in a rapid onset of colonic inflammation that was reversible through depletion of colitogenic T cells. Remission was associated with an improved clinical and histopathological score, reduced immune cell infiltration to the intestinal mucosa, altered intestinal gene expression profiles, regeneration of the colonic mucus layer, and the restoration of epithelial barrier integrity. Notably, colitogenic T cells were not only critical for induction of colitis but also for maintenance of disease. Depletion of colitogenic T cells resulted in a rapid drop in tumor necrosis factor alpha (TNFalpha) levels associated with reduced infiltration of inflammatory immune cells to sites of inflammation. Although neutralization of TNFalpha prevented the onset of colitis, anti-TNFalpha treatment of mice with established disease failed to resolve colonic inflammation. Collectively, this new model of reversible colitis provides an important research tool to study the dynamics of mucosal healing in chronic intestinal remitting-relapsing disorders.
in vivo TNFα neutralization
Flow Cytometry
Khmaladze, I., et al (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo TNFα neutralization
Gopinath, S., et al (2014). "Role of disease-associated tolerance in infectious superspreaders" Proc Natl Acad Sci U S A 111(44): 15780-15785.
PubMed
Natural populations show striking heterogeneity in their ability to transmit disease. For example, a minority of infected individuals known as superspreaders carries out the majority of pathogen transmission events. In a mouse model of Salmonella infection, a subset of infected hosts becomes superspreaders, shedding high levels of bacteria (>10(8) cfu per g of feces) but remain asymptomatic with a dampened systemic immune state. Here we show that superspreader hosts remain asymptomatic when they are treated with oral antibiotics. In contrast, nonsuperspreader Salmonella-infected hosts that are treated with oral antibiotics rapidly shed superspreader levels of the pathogen but display signs of morbidity. This morbidity is linked to an increase in inflammatory myeloid cells in the spleen followed by increased production of acute-phase proteins and proinflammatory cytokines. The degree of colonic inflammation is similar in antibiotic-treated superspreader and nonsuperspreader hosts, indicating that the superspreader hosts are tolerant of antibiotic-mediated perturbations in the intestinal tract. Importantly, neutralization of acute-phase proinflammatory cytokines in antibiotic-induced superspreaders suppresses the expansion of inflammatory myeloid cells and reduces morbidity. We describe a unique disease-associated tolerance to oral antibiotics in superspreaders that facilitates continued transmission of the pathogen.
Product Citations
-
-
Genetics
-
Biochemistry and Molecular biology
ACSS2-Mediated Histone H4 Lysine 12 Crotonylation (H4K12cr) Alleviates Colitis via Enhancing Transcription of CLDN7.
In Adv Sci (Weinh) on 1 August 2025 by Yuan, M., Chen, S., et al.
PubMed
Histone lysine crotonylation (Kcr), a highly conserved posttranslational modification, plays critical roles in various biological processes. Nevertheless, the dynamic alterations and functions of histone Kcr in inflammatory bowel disease (IBD) remain poorly explored. Herein, a notable decrease of both Pan-Kcr and ACSS2 (acyl-CoA synthetase short-chain family member 2), the key enzyme for crotonyl-CoA generation, is revealed in inflamed intestinal epithelial cells. Genetic or pharmacological inhibition of ACSS2 dramatically impairs mouse intestinal barrier integrity and exacerbates colitis. Mechanistically, ACSS2-mediated histone H4 lysine 12 crotonylation (H4K12cr) upregulates CLDN7 expression to fortify intestinal epithelial barrier, which can be augmented by crotonate supplementation. Furthermore, tumor necrosis factor-α (TNF-α) is revealed to enhance the m6A modification of ACSS2 mRNA, consequently destabilizing and downregulating ACSS2. Combinational therapy involving anti-TNF-α and crotonate can significantly ameliorate colitis. Overall, ACSS2-mediated H4K12cr emerges as a pivotal modulator governing intestinal barrier function during IBD progression.
-
-
-
Immunology and Microbiology
Engineered Escherichia coli for the in situ secretion of therapeutic nanobodies in the gut.
In Cell Host Microbe on 12 April 2023 by Lynch, J. P., González-Prieto, C., et al.
PubMed
Drug platforms that enable the directed delivery of therapeutics to sites of diseases to maximize efficacy and limit off-target effects are needed. Here, we report the development of PROT3EcT, a suite of commensal Escherichia coli engineered to secrete proteins directly into their surroundings. These bacteria consist of three modular components: a modified bacterial protein secretion system, the associated regulatable transcriptional activator, and a secreted therapeutic payload. PROT3EcT secrete functional single-domain antibodies, nanobodies (Nbs), and stably colonize and maintain an active secretion system within the intestines of mice. Furthermore, a single prophylactic dose of a variant of PROT3EcT that secretes a tumor necrosis factor-alpha (TNF-α)-neutralizing Nb is sufficient to ablate pro-inflammatory TNF levels and prevent the development of injury and inflammation in a chemically induced model of colitis. This work lays the foundation for developing PROT3EcT as a platform for the treatment of gastrointestinal-based diseases.
-
-
-
Immunology and Microbiology
GATA4 controls regionalization of tissue immunity and commensal-driven immunopathology.
In Immunity on 10 January 2023 by Earley, Z. M., Lisicka, W., et al.
PubMed
There is growing recognition that regionalization of bacterial colonization and immunity along the intestinal tract has an important role in health and disease. Yet, the mechanisms underlying intestinal regionalization and its dysregulation in disease are not well understood. This study found that regional epithelial expression of the transcription factor GATA4 controls bacterial colonization and inflammatory tissue immunity in the proximal small intestine by regulating retinol metabolism and luminal IgA. Furthermore, in mice without jejunal GATA4 expression, the commensal segmented filamentous bacteria promoted pathogenic inflammatory immune responses that disrupted barrier function and increased mortality upon Citrobacter rodentium infection. In celiac disease patients, low GATA4 expression was associated with metabolic alterations, mucosal Actinobacillus, and increased IL-17 immunity. Taken together, these results reveal broad impacts of GATA4-regulated intestinal regionalization on bacterial colonization and tissue immunity, highlighting an elaborate interdependence of intestinal metabolism, immunity, and microbiota in homeostasis and disease.
-
-
-
Rattus norvegicus (Rat)
-
Immunology and Microbiology
-
Neuroscience
Lidocaine Ameliorates Psoriasis by Obstructing Pathogenic CGRP Signaling‒Mediated Sensory Neuron‒Dendritic Cell Communication.
In J Invest Dermatol on 1 August 2022 by Yin, Q., Sun, L., et al.
PubMed
Psoriasis is a chronic immune-mediated skin disorder with the nervous system contributing to its pathology. The neurogenic mediators of psoriasis are elusive, and whether the intervention of the cutaneous nervous system can treat psoriasis remains to be determined. In this study, we conducted a pilot study using an epidural injection of lidocaine to treat patients with psoriasis. Lidocaine treatment markedly reduced patients' clinical scores and improved an imiquimod-induced rat model of psoriasis as competent as systemic delivery of a TNF-α antibody. Imiquimod application elicited aberrant cutaneous nerve outgrowth and excessive generation of neuropeptide calcitonin gene-related peptide from dorsal root ganglion neurons, both of which were inhibited by epidural lidocaine treatment. Single-cell RNA sequencing unveiled the overrepresentation of calcitonin gene-related peptide receptors in dermal dendritic cell populations of patients with psoriasis. Through disturbing calcitonin gene-related peptide signaling, lidocaine inhibited IL-23 production by dendritic cells cocultured with dorsal root ganglion neurons. Thus, epidural nerve block with lidocaine demonstrates an effective therapy for psoriasis, which suppresses both inordinate sensory nerve growth in the inflamed skin and calcitonin gene-related peptide-mediated IL-23 production from psoriatic dendritic cells.
-
-
-
Immunology and Microbiology
Ifnar gene variants influence gut microbial production of palmitoleic acid and host immune responses to tuberculosis.
In Nat Metab on 1 March 2022 by Chen, L., Zhang, G., et al.
PubMed
Both host genetics and the gut microbiome have important effects on human health, yet how host genetics regulates gut bacteria and further determines disease susceptibility remains unclear. Here, we find that the gut microbiome pattern of participants with active tuberculosis is characterized by a reduction of core species found across healthy individuals, particularly Akkermansia muciniphila. Oral treatment of A. muciniphila or A. muciniphila-mediated palmitoleic acid strongly inhibits tuberculosis infection through epigenetic inhibition of tumour necrosis factor in mice infected with Mycobacterium tuberculosis. We use three independent cohorts comprising 6,512 individuals and identify that the single-nucleotide polymorphism rs2257167 'G' allele of type I interferon receptor 1 (encoded by IFNAR1 in humans) contributes to stronger type I interferon signalling, impaired colonization and abundance of A. muciniphila, reduced palmitoleic acid production, higher levels of tumour necrosis factor, and more severe tuberculosis disease in humans and transgenic mice. Thus, host genetics are critical in modulating the structure and functions of gut microbiome and gut microbial metabolites, which further determine disease susceptibility.
-
-
-
Immunology and Microbiology
Engineered E. coli for the targeted deposition of therapeutic payloads to sites of disease
In Research Square on 21 January 2022 by Lynch, J., González-Prieto, C. C., et al.
-
-
An Ifnar1 allele impairs the colonization of gut bacteria and promotes tuberculosis
In Research Square on 2 September 2021 by Chen, L., Zhang, G., et al.
-
-
Biochemistry and Molecular biology
Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23.
In Cell Metab on 6 July 2021 by He, Z., Chen, L., et al.
PubMed
Both genetic predisposition and environmental factors appear to play a role in inflammatory bowel disease (IBD) development. Genetic studies in humans have linked the interleukin (IL)-23 signaling pathway with IBD, but the environmental factors contributing to disease have remained elusive. Here, we show that the azo dyes Red 40 and Yellow 6, the most abundant food colorants in the world, can trigger an IBD-like colitis in mice conditionally expressing IL-23, or in two additional animal models in which IL-23 expression was augmented. Increased IL-23 expression led to generation of activated CD4+ T cells that expressed interferon-γ and transferred disease to mice exposed to Red 40. Colitis induction was dependent on the commensal microbiota promoting the azo reduction of Red 40 and generation of a metabolite, 1-amino-2-naphthol-6-sulfonate sodium salt. Together these findings suggest that specific food colorants represent novel risk factors for development of colitis in mice with increased IL-23 signaling.
-
-
-
Immunology and Microbiology
Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor.
In Gastroenterology on 1 October 2017 by Holvoet, T., Devriese, S., et al.
PubMed
Intestinal fibrosis resulting in (sub)obstruction is a common complication of Crohn's disease (CD). Rho kinases (ROCKs) play multiple roles in TGFβ-induced myofibroblast activation that could be therapeutic targets. Because systemic ROCK inhibition causes cardiovascular side effects, we evaluated the effects of a locally acting ROCK inhibitor (AMA0825) on intestinal fibrosis.
-
-
-
Immunology and Microbiology
CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis.
In Mucosal Immunol on 1 May 2016 by Brasseit, J., Althaus-Steiner, E., et al.
PubMed
Current therapies to treat inflammatory bowel diseases have limited efficacy, significant side effects, and often wane over time. Little is known about the cellular and molecular mechanisms operative in the process of mucosal healing from colitis. To study such events, we developed a new model of reversible colitis in which adoptive transfer of CD4(+)CD45RB(hi) T cells into Helicobacter typhlonius-colonized lymphopenic mice resulted in a rapid onset of colonic inflammation that was reversible through depletion of colitogenic T cells. Remission was associated with an improved clinical and histopathological score, reduced immune cell infiltration to the intestinal mucosa, altered intestinal gene expression profiles, regeneration of the colonic mucus layer, and the restoration of epithelial barrier integrity. Notably, colitogenic T cells were not only critical for induction of colitis but also for maintenance of disease. Depletion of colitogenic T cells resulted in a rapid drop in tumor necrosis factor α (TNFα) levels associated with reduced infiltration of inflammatory immune cells to sites of inflammation. Although neutralization of TNFα prevented the onset of colitis, anti-TNFα treatment of mice with established disease failed to resolve colonic inflammation. Collectively, this new model of reversible colitis provides an important research tool to study the dynamics of mucosal healing in chronic intestinal remitting-relapsing disorders.
-
-
-
Flow cytometry/Cell sorting
-
Pathology
-
Mus musculus (Mouse)
Role of disease-associated tolerance in infectious superspreaders.
In Proc Natl Acad Sci U S A on 4 November 2014 by Gopinath, S., Lichtman, J. S., et al.
PubMed
Natural populations show striking heterogeneity in their ability to transmit disease. For example, a minority of infected individuals known as superspreaders carries out the majority of pathogen transmission events. In a mouse model of Salmonella infection, a subset of infected hosts becomes superspreaders, shedding high levels of bacteria (>10(8) cfu per g of feces) but remain asymptomatic with a dampened systemic immune state. Here we show that superspreader hosts remain asymptomatic when they are treated with oral antibiotics. In contrast, nonsuperspreader Salmonella-infected hosts that are treated with oral antibiotics rapidly shed superspreader levels of the pathogen but display signs of morbidity. This morbidity is linked to an increase in inflammatory myeloid cells in the spleen followed by increased production of acute-phase proteins and proinflammatory cytokines. The degree of colonic inflammation is similar in antibiotic-treated superspreader and nonsuperspreader hosts, indicating that the superspreader hosts are tolerant of antibiotic-mediated perturbations in the intestinal tract. Importantly, neutralization of acute-phase proinflammatory cytokines in antibiotic-induced superspreaders suppresses the expansion of inflammatory myeloid cells and reduces morbidity. We describe a unique disease-associated tolerance to oral antibiotics in superspreaders that facilitates continued transmission of the pathogen.
-