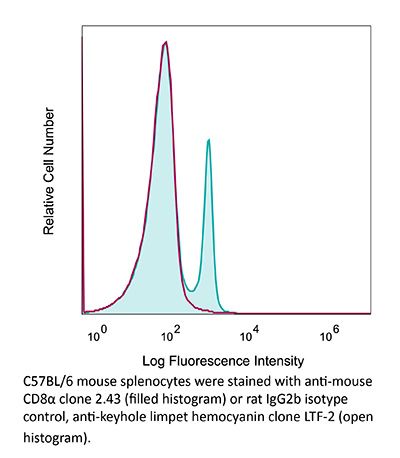
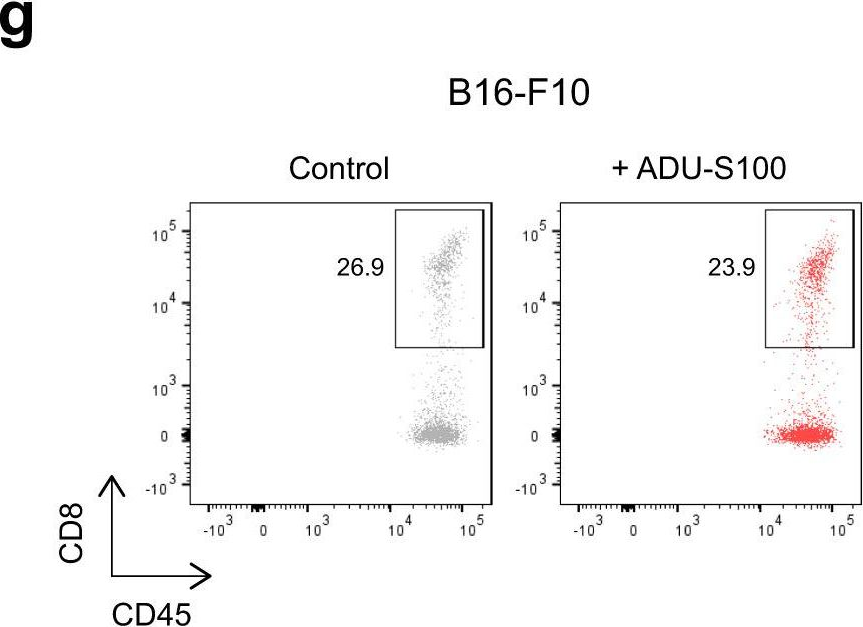
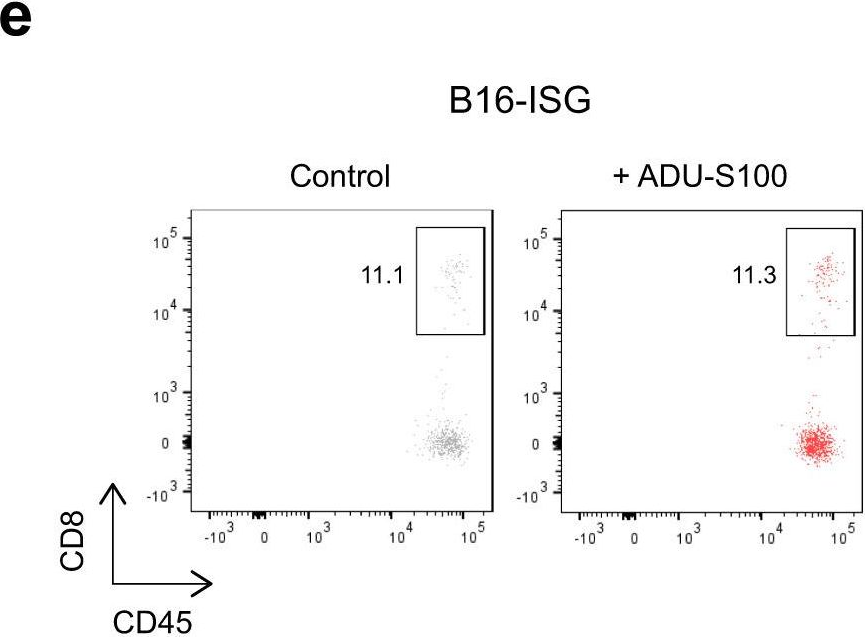
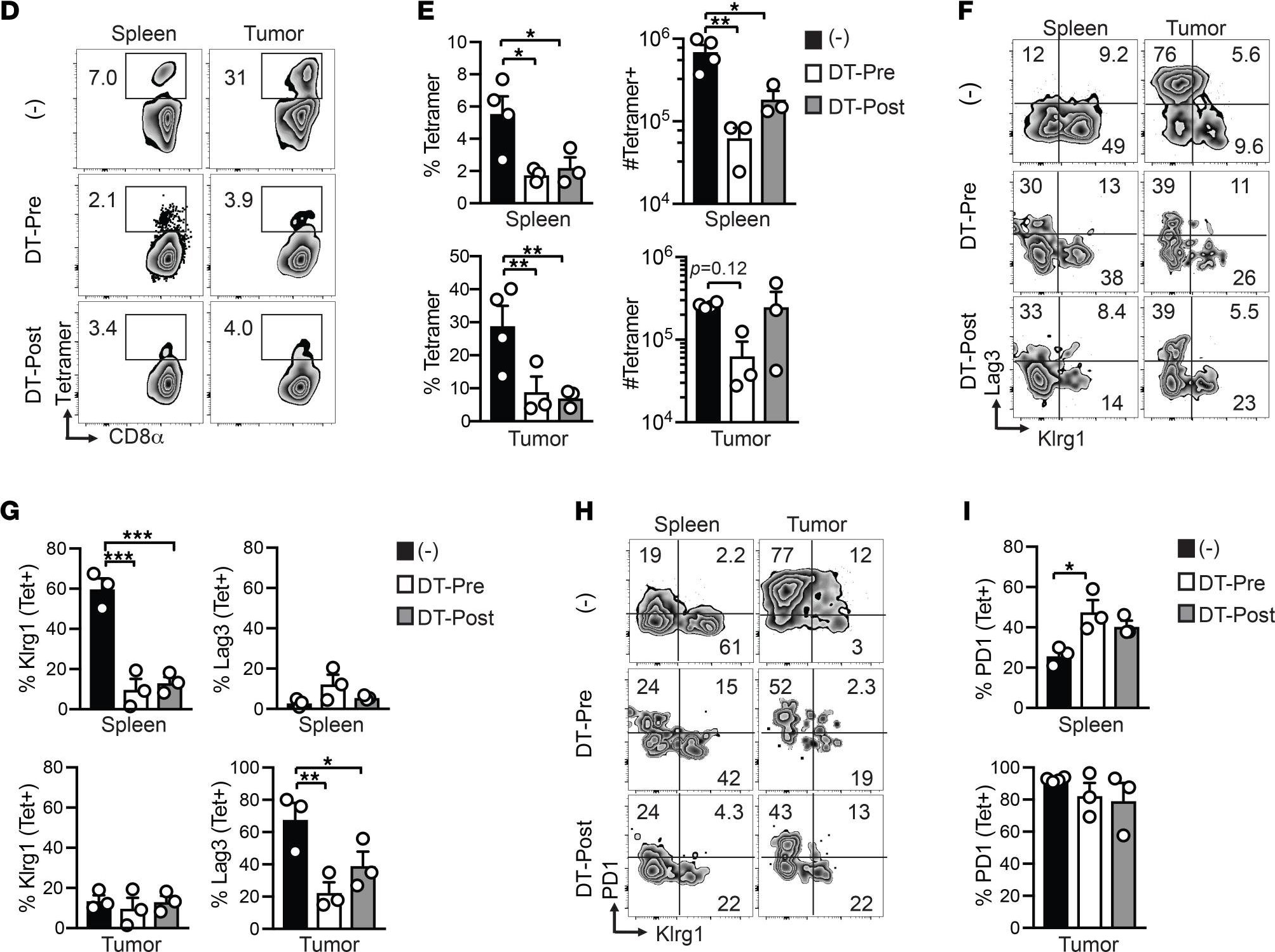
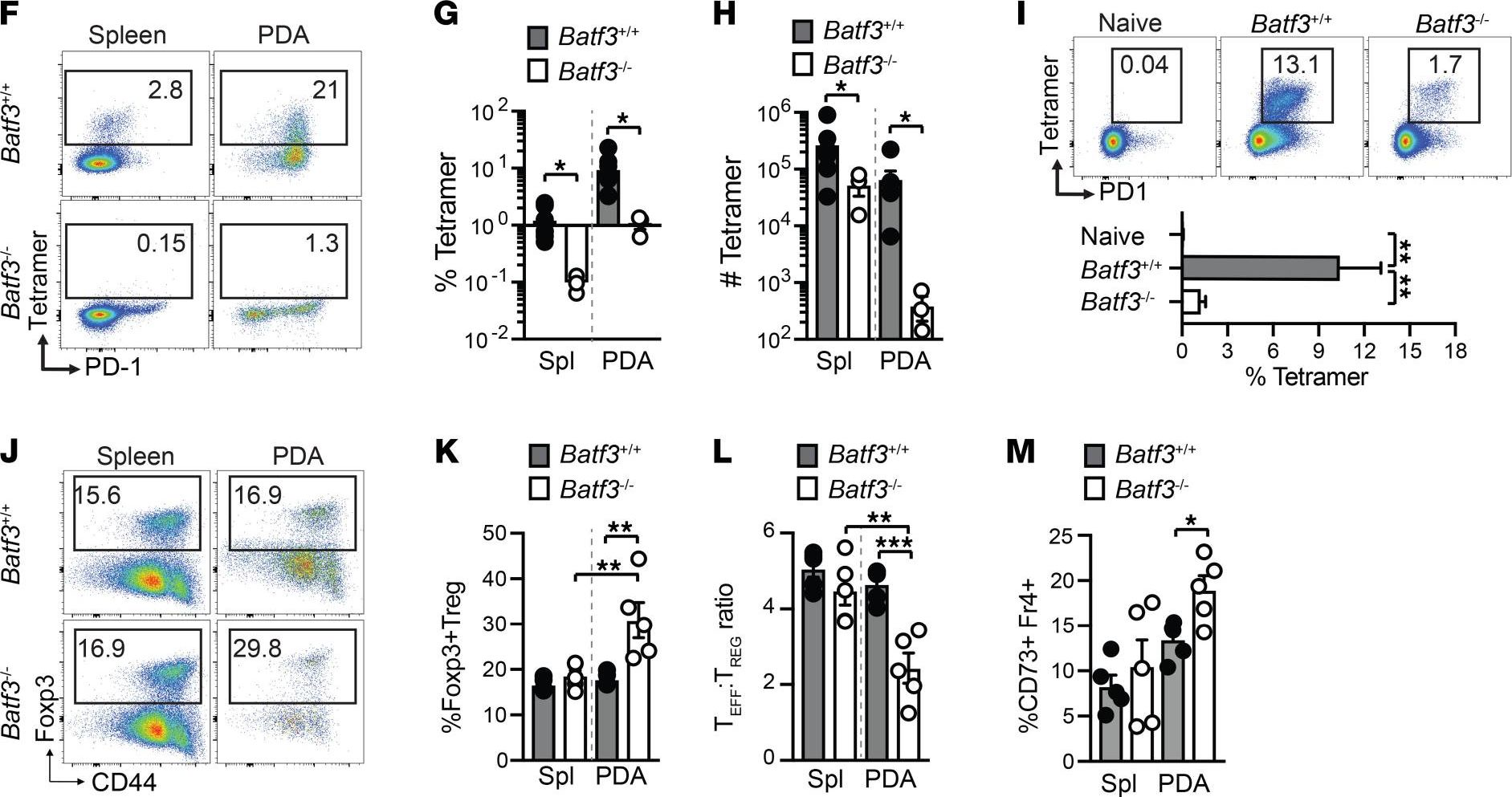
InVivoMAb anti-mouse CD8α
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CTL clone L3 |
| Reported Applications |
in vivo CD8+ T cell depletion Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1125541 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
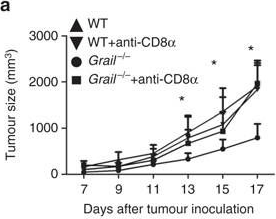
in vivo CD8+ T cell depletion
Balogh, K. N., et al. (2018). "Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses" PLoS One 13(6): e0197702.
PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo CD8+ T cell depletion
Li, J., et al. (2018). "Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8(+) T Cells" Immunity 48(4): 773-786 e775.
PubMed
The molecular mechanisms whereby CD8(+) T cells become “exhausted” in the tumor microenvironment remain unclear. Programmed death ligand-1 (PD-L1) is upregulated on tumor cells and PD-1-PD-L1 blockade has significant efficacy in human tumors; however, most patients do not respond, suggesting additional mechanisms underlying T cell exhaustion. B7 superfamily member 1 (B7S1), also called B7-H4, B7x, or VTCN1, negatively regulates T cell activation. Here we show increased B7S1 expression on myeloid cells from human hepatocellular carcinoma correlated with CD8(+) T cell dysfunction. B7S1 inhibition suppressed development of murine tumors. Putative B7S1 receptor was co-expressed with PD-1 but not T cell immunoglobulin and mucin-domain containing-3 (Tim-3) at an activated state of early tumor-infiltrating CD8(+) T cells, and B7S1 promoted T cell exhaustion, possibly through Eomes overexpression. Combinatorial blockade of B7S1 and PD-1 synergistically enhanced anti-tumor immune responses. Collectively, B7S1 initiates dysfunction of tumor-infiltrating CD8(+) T cells and may be targeted for cancer immunotherapy.
in vivo CD8+ T cell depletion
Moynihan, K. D., et al. (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo CD8+ T cell depletion
Voron, T., et al. (2015). "VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors" J Exp Med 212(2): 139-148.
PubMed
Immune escape is a prerequisite for tumor development. To avoid the immune system, tumors develop different mechanisms, including T cell exhaustion, which is characterized by expression of immune inhibitory receptors, such as PD-1, CTLA-4, Tim-3, and a progressive loss of function. The recent development of therapies targeting PD-1 and CTLA-4 have raised great interest since they induced long-lasting objective responses in patients suffering from advanced metastatic tumors. However, the regulation of PD-1 expression, and thereby of exhaustion, is unclear. VEGF-A, a proangiogenic molecule produced by the tumors, plays a key role in the development of an immunosuppressive microenvironment. We report in the present work that VEGF-A produced in the tumor microenvironment enhances expression of PD-1 and other inhibitory checkpoints involved in CD8(+) T cell exhaustion, which could be reverted by anti-angiogenic agents targeting VEGF-A-VEGFR. In view of these results, association of anti-angiogenic molecules with immunomodulators of inhibitory checkpoints may be of particular interest in VEGF-A-producing tumors.
in vivo CD8+ T cell depletion
Vanpouille-Box, C., et al. (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo CD8+ T cell depletion
Yamada, D. H., et al. (2015). "Suppression of Fcgamma-receptor-mediated antibody effector function during persistent viral infection" Immunity 42(2): 379-390.
PubMed
Understanding how viruses subvert host immunity and persist is essential for developing strategies to eliminate infection. T cell exhaustion during chronic viral infection is well described, but effects on antibody-mediated effector activity are unclear. Herein, we show that increased amounts of immune complexes generated in mice persistently infected with lymphocytic choriomeningitis virus (LCMV) suppressed multiple Fcgamma-receptor (FcgammaR) functions. The high amounts of immune complexes suppressed antibody-mediated cell depletion, therapeutic antibody-killing of LCMV infected cells and human CD20-expressing tumors, as well as reduced immune complex-mediated cross-presentation to T cells. Suppression of FcgammaR activity was not due to inhibitory FcgammaRs or high concentrations of free antibody, and proper FcgammaR functions were restored when persistently infected mice specifically lacked immune complexes. Thus, we identify a mechanism of immunosuppression during viral persistence with implications for understanding effective antibody activity aimed at pathogen control.
in vivo CD8+ T cell depletion
Twyman-Saint Victor, C., et al. (2015). "Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer" Nature 520(7547): 373-377.
PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
in vivo CD8+ T cell depletion
Coffelt, S. B., et al. (2015). "IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis" Nature 522(7556): 345-348.
PubMed
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1beta elicits IL-17 expression from gamma delta (gammadelta) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of gammadelta T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of gammadelta T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system–the gammadelta T cell/IL-17/neutrophil axis–represents a new strategy to inhibit metastatic disease.
in vivo CD8+ T cell depletion
Evans, E. E., et al. (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo CD8+ T cell depletion
Vegran, F., et al. (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vivo CD8+ T cell depletion
DeBerge, M. P., et al. (2014). "Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology" J Immunol 192(12): 5839-5851.
PubMed
TNF-alpha is a pleotropic cytokine that has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-alpha is first expressed as a transmembrane protein that is proteolytically processed to release a soluble form. Transmembrane TNF-alpha (memTNF-alpha) and soluble TNF-alpha (solTNF-alpha) have been shown to exert distinct tissue-protective or tissue-pathologic effects in several disease models. However, the relative contributions of memTNF-alpha or solTNF-alpha in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing noncleavable memTNF-alpha or lacking TNF-alpha entirely and examined the outcomes. We found that solTNF-alpha, but not memTNF-alpha, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-alpha, there was a significant increase in the CD8(+) T cell response, including virus-specific CD8(+) T cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-alpha mediates these immunoregulatory effects primarily through TNFR1, because mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-alpha. We also found that solTNF-alpha expression was required early during infection to regulate the magnitude of the CD8(+) T cell response, indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-alpha to release solTNF-alpha is a critical event regulating the immune response during influenza infection.
in vivo CD8+ T cell depletion
Deng, L., et al. (2014). "Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice" J Clin Invest 124(2): 687-695.
PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
in vivo CD8+ T cell depletion
Van der Jeught, K., et al. (2014). "Intratumoral administration of mRNA encoding a fusokine consisting of IFN-beta and the ectodomain of the TGF-beta receptor II potentiates antitumor immunity" Oncotarget 5(20): 10100-10113.
PubMed
It is generally accepted that the success of immunotherapy depends on the presence of tumor-specific CD8(+) cytotoxic T cells and the modulation of the tumor environment. In this study, we validated mRNA encoding soluble factors as a tool to modulate the tumor microenvironment to potentiate infiltration of tumor-specific T cells. Intratumoral delivery of mRNA encoding a fusion protein consisting of interferon-beta and the ectodomain of the transforming growth factor-beta receptor II, referred to as Fbeta(2), showed therapeutic potential. The treatment efficacy was dependent on CD8(+) T cells and could be improved through blockade of PD-1/PD-L1 interactions. In vitro studies revealed that administration of Fbeta(2) to tumor cells resulted in a reduced proliferation and increased expression of MHC I but also PD-L1. Importantly, Fbeta(2) enhanced the antigen presenting capacity of dendritic cells, whilst reducing the suppressive activity of myeloid-derived suppressor cells. In conclusion, these data suggest that intratumoral delivery of mRNA encoding soluble proteins, such as Fbeta(2), can modulate the tumor microenvironment, leading to effective antitumor T cell responses, which can be further potentiated through combination therapy.
in vivo CD8+ T cell depletion
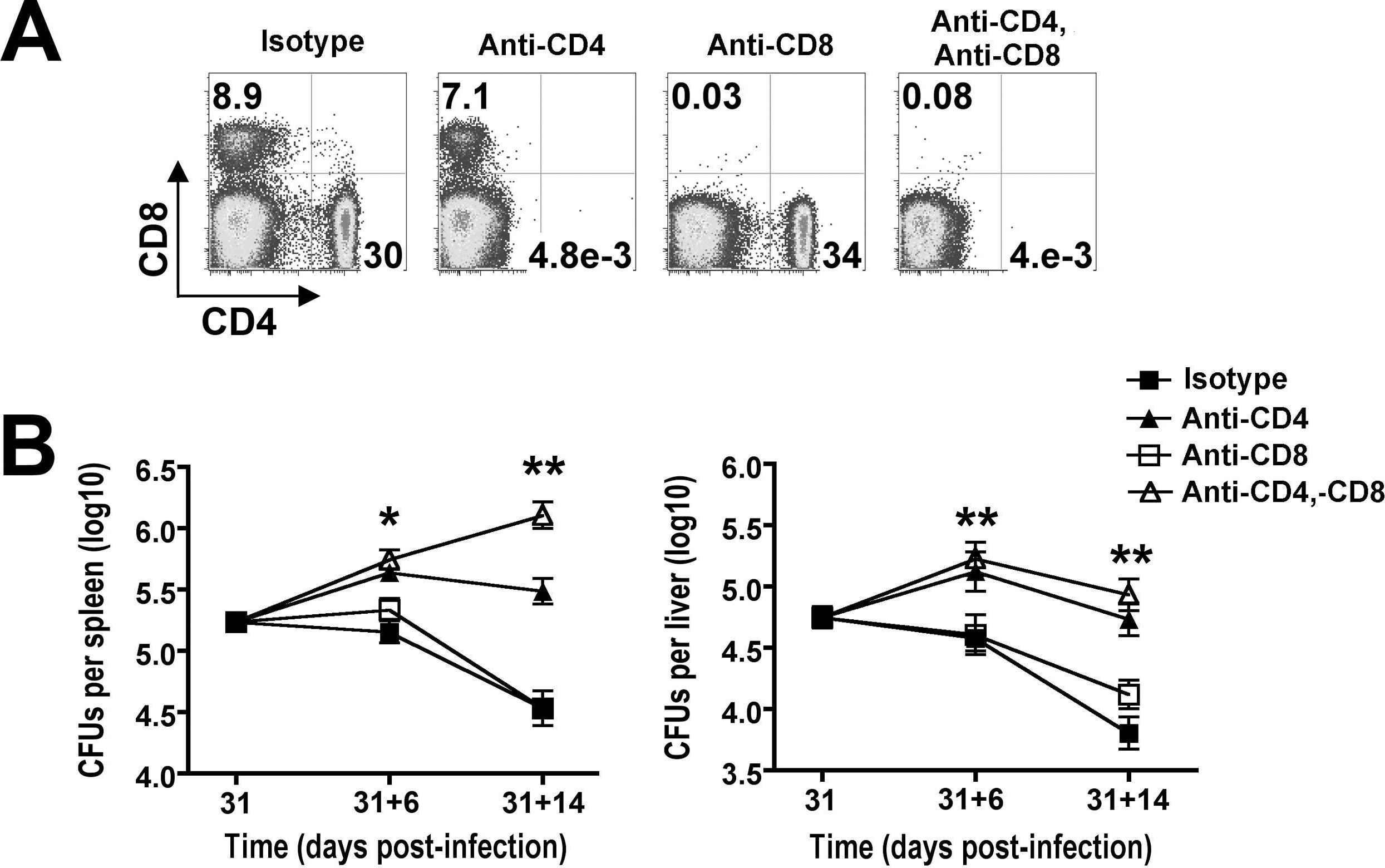
Kearl, T. J., et al. (2013). "Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma" J Immunol 190(11): 5620-5628.
PubMed
Early phase clinical trials targeting the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers. This pathway appears to play an important role in the failure of immune reactivity to malignant plasma cells in multiple myeloma patients, as the tumor cells express relatively high levels of PD-L1, and T cells show increased PD-1 expression. In the current study, we demonstrate that PD-1/PD-L1 blockade with a PD-L1-specific Ab elicits rejection of a murine myeloma when combined with lymphodepleting irradiation. This particular combined approach by itself has not previously been shown to be efficacious in other tumor models. The antitumor effect of lymphodepletion/anti-PD-L1 therapy was most robust when tumor Ag-experienced T cells were present either through cell transfer or survival after nonmyeloablative irradiation. In vivo depletion of CD4 or CD8 T cells completely eliminated antitumor efficacy of the lymphodepletion/anti-PD-L1 therapy, indicating that both T cell subsets are necessary for tumor rejection. Elimination of myeloma by T cells occurs relatively quickly as tumor cells in the bone marrow were nearly nondetectable by 5 d after the first anti-PD-L1 treatment, suggesting that antimyeloma reactivity is primarily mediated by preactivated T cells, rather than newly generated myeloma-reactive T cells. Anti-PD-L1 plus lymphodepletion failed to improve survival in two solid tumor models, but demonstrated significant efficacy in two hematologic malignancy models. In summary, our results support the clinical testing of lymphodepletion and PD-1/PD-L1 blockade as a novel approach for improving the survival of patients with multiple myeloma.
in vivo CD8+ T cell depletion
Sandoval, F., et al. (2013). "Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors" Sci Transl Med 5(172): 172ra120.
PubMed
Although many human cancers are located in mucosal sites, most cancer vaccines are tested against subcutaneous tumors in preclinical models. We therefore wondered whether mucosa-specific homing instructions to the immune system might influence mucosal tumor outgrowth. We showed that the growth of orthotopic head and neck or lung cancers was inhibited when a cancer vaccine was delivered by the intranasal mucosal route but not the intramuscular route. This antitumor effect was dependent on CD8(+) T cells. Indeed, only intranasal vaccination elicited mucosal-specific CD8(+) T cells expressing the mucosal integrin CD49a. Blockade of CD49a decreased intratumoral CD8(+) T cell infiltration and the efficacy of cancer vaccine on mucosal tumor. We then showed that after intranasal vaccination, dendritic cells from lung parenchyma, but not those from spleen, induced the expression of CD49a on cocultured specific CD8(+) T cells. Tumor-infiltrating lymphocytes from human mucosal lung cancer also expressed CD49a, which supports the relevance and possible extrapolation of these results in humans. We thus identified a link between the route of vaccination and the induction of a mucosal homing program on induced CD8(+) T cells that controlled their trafficking. Immunization route directly affected the efficacy of the cancer vaccine to control mucosal tumors.
in vivo CD8+ T cell depletion
Pasche, N., et al. (2012). "The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel" Clin Cancer Res 18(15): 4092-4103.
PubMed
PURPOSE: Interleukin-12 (IL12) is a potent proinflammatory cytokine with antitumor activity. Its heterodimeric nature makes it compatible with a large variety of different immunocytokine formats. Here we report the design, production, and characterization of a novel immunocytokine, based on the fusion of the F8 antibody (specific to the alternatively spliced EDA domain of fibronectin, a marker of tumor neovasculature) with IL12 (termed IL12-F8-F8). EXPERIMENTAL DESIGN: We developed a novel immunocytokine based on the sequential fusion of interleukin-12 as a single polypeptide with two F8 antibodies in single-chain Fv (scFv) format. The fusion protein was characterized in vitro, and its targeting performance was assessed in vivo. The immunocytokine antitumor activity was studied as monotherapy as well as in combination therapies in three different murine tumor models. Moreover, depletion experiments and tumor analysis revealed a dominant role of natural killer cells for the mechanism of action. RESULTS: IL12-F8-F8 can be produced in mammalian cells, yielding a product of good pharmaceutical quality, capable of selective localization on the tumor neovasculature in vivo, as judged by quantitative biodistribution analysis with radioiodinated protein preparations. The protein potently inhibited tumor growth in three different immunocompetent syngeneic models of cancer. The treatment was generally well tolerated. Moreover, the IL12-F8-F8 fusion protein could be produced both with murine IL12 (mIL12) and with human IL12 (hIL12). CONCLUSIONS: The potent antitumor activity of mIL12-F8-F8, studied alone or in combination with paclitaxel in different tumor models, paves the way to the clinical development of the fully human immunocytokine.
in vivo CD8+ T cell depletion
Quezada, S. A., et al. (2010). "Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts" J Exp Med 207(3): 637-650.
PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
Product Citations
-
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
FOXO1-driven metabolic reprogramming of hematomal CD8+ T cells drives the expansion of perihematomal edema following intracerebral hemorrhage.
In Cell Mol Immunol on 1 December 2025 by Lin, J., Ren, H., et al.
PubMed
Intracerebral hemorrhage (ICH) causes hematoma formation, leading to PHE, which is associated with leukocyte mobilization and increased inflammation at the site of brain injury. However, the fate of accumulated leukocytes within the hematoma and their impact on PHE expansion remain unknown. We performed single-cell immune profiling of hematoma cells from patients with acute ICH and reported a distinct phenotypic transformation of CD8+ T cells within the hematoma during the first 24 h after onset. In addition to enhanced IFN-γ production and migration capacity, these CD8+ T cells displayed remarkable glycolytic signatures. The metabolic fitness and functional reprogramming of hematomal CD8+ T cells are associated with the transcription factor FOXO1. Single-cell profiling of brain-infiltrating CD8+ T cells within the perihematomal tissues of ICH patients and cell culture assays revealed their capacity to activate microglia via the production of IFN-γ. Furthermore, the removal of hematomal CD8+ T cells reduced neuroinflammation, PHE expansion and neurological deficits in ICH mice. Thus, CD8+ T cells undergo metabolic and functional reprogramming within the hematoma during the acute phase of ICH, which contributes to PHE formation and neurological deterioration.
-
-
-
Immunology and Microbiology
Migration of CD8 + TSCM cells into intestine via PPBP-CXCR2 axis increases host stress susceptibility by inhibiting gut microbiome-derived homovanillic acid.
In Nat Commun on 19 November 2025 by Zhang, Y., Ju, M., et al.
PubMed
Psychosocial stress impacts immune system and brain function, yet mechanisms linking peripheral immune dysregulation to major depressive disorder remain unclear. Here, we demonstrate that a specific subset of T cells, the stem cell-like memory CD8+ T (TSCM) cells, is elevated in patients and stress-susceptible mice. CD8+ TSCM cells from patients display unique transcriptional programs and correlated with depression severity. Adoptive transfer of stress-derived CD8⁺ TSCM cells induced depressive-like behavior and neuroinflammation in recipients, without brain migration. Employing a whole-body immunolabeling technology, we discover CD8+ TSCM cells migrated to intestine via the interaction of pro-platelet basic protein and C-X-C motif chemokine receptor 2. CD8+ TSCM cells decrease the abundance of tyrosine-metabolizing bacteria to reducing homovanillic acid production, triggered neuroinflammation and depressive symptoms. Thus, our findings uncover a complex interplay between CD8+ TSCM cells and gut microbial metabolism, shedding light on potential mechanisms underlying depression and suggesting avenues for therapeutic intervention.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
USP22 drives tumor immune evasion and checkpoint blockade resistance through EZH2-mediated epigenetic silencing of MHC-I.
In J Clin Invest on 18 November 2025 by Liu, K., Iyer, R., et al.
PubMed
While immune checkpoint blockade (ICB) therapy has revolutionized the antitumor therapeutic landscape, it remains successful in only a small subset of cancer patients. Poor or loss of MHC-I expression has been implicated as a common mechanism of ICB resistance. Yet the molecular mechanisms underlying impaired MHC-I remain to be fully elucidated. Herein, we identified USP22 as a critical factor responsible for ICB resistance through suppressing MHC-I-mediated neoantigen presentation to CD8 T cells. Both genetic and pharmacologic USP22 inhibition increased immunogenicity and overcome anti-PD-1 immunotherapeutic resistance. At the molecular level, USP22 functions as a deubiquitinase for the methyltransferase EZH2, leading to transcriptional silencing of MHC-I gene expression. Targeted Usp22 inhibition resulted in increased tumoral MHC-I expression and consequently enhanced CD8 T cell killing, which was largely abrogated by Ezh2 reconstitution. Multiplexed immunofluorescence staining detected a strong reverse correlation between USP22 expression and both 2M expression and CD8+ T lymphocyte infiltration in solid tumors. Importantly, USP22 upregulation was associated with ICB immunotherapeutic resistance in patients with lung cancer. Collectively, this study highlights the role of USP22 as a diagnostic biomarker for ICB resistance and provides a potential therapeutic avenue to overcome the current ICB resistance through inhibition of USP22.
-
-
-
Immunology and Microbiology
Iron overload-induced ferroptosis in CD8+ T cells leads to functional abnormalities that promote endometriosis progression.
In BMC Med on 27 October 2025 by Wang, S., Xu, L., et al.
PubMed
Endometriosis (EM) exhibits localised iron overload. However, the contribution of ferroptosis to EM pathogenesis remains unclear. We investigated how iron overload affects CD8⁺ T cell immune function and the underlying ferroptotic mechanisms.
-
-
-
Immunology and Microbiology
-
Neuroscience
-
Pathology
Stage-specific roles of clonally expanded CD8+ T cells in regulating amyloid pathology in Alzheimer's disease models.
In Nat Commun on 27 October 2025 by Ohyagi, M., Ito, M., et al.
PubMed
Clonally expanded CD8+ T cells may contribute to Alzheimer's disease (AD) pathology through interactions with brain-resident cells. However, the functional impact of AD-specific T cell receptor (TCR) clonotypes remains unclear. Here, we demonstrate that CD8+ T cells undergo clonal expansion in early-stage AD mouse models, AppNL-G-F and 5xFAD, and that their depletion reduces amyloid plaque accumulation. Expanded TCR-expressing CD8+ T cells preferentially infiltrate the brain, exacerbating plaque deposition. Moreover, brain-infiltrating CD8+ T cells impair microglial transition into disease-associated states, suppressing amyloid clearance via CCL5-CCR5 signaling. Pharmacological blockade of CCL5 attenuates amyloid deposition, whereas CCL5 administration aggravates pathology. Notably, T cell depletion at later disease stages exacerbates amyloid pathology, suggesting a temporal shift in their function. Early-stage CD8+ T cells exhibit cytotoxic and effector profiles, whereas late-stage cells acquire tissue-resident and exhausted phenotypes. This temporal switch-from pathogenic to protective roles-highlights the stage-specific contribution of CD8+ T cells to AD and their potential as therapeutic targets.
-
-
-
Immunology and Microbiology
Limited nasal IFN production contributes to delayed respiratory virus clearance and suboptimal vaccine responses.
In JCI Insight on 22 October 2025 by Sojati, J., Parks, O. B., et al.
PubMed
Acute lower respiratory infections are the primary cause of global mortality in postneonatal children. Most respiratory viruses primarily involve upper airway infection and inflammation, yet nasal responses are poorly characterized. Using a mouse model of human metapneumovirus (HMPV), we found viral burden was higher in nasal airways and exhibited delayed clearance. Despite high burden, there was low nasal expression of type I and III interferon (IFN). Single-cell RNA-sequencing (scRNA-Seq) from HMPV-infected mice showed lower nasal IFN-stimulated gene (ISG) expression and nasal enrichment of genes negatively regulating IFN. scRNA-Seq of patients with COVID-19 verified lower ISG expression in upper airways. HMPV infection downregulated nasal expression of IFN regulatory factor 3, suggesting a mechanism for limited response. To rescue the quiescent environment, we administered type I or III IFN to upper airways early postinfection, leading to lower nasal HMPV titer and virus-specific CD8+ T cell upregulation. Intranasal immunization adjuvanted with type I or III IFN improved immune response, reduced clinical disease, and enhanced viral clearance in HMPV and influenza infection. IFN adjuvant increased recruitment of dendritic cells, recruitment of resident memory T cells, and neutralizing antibodies. These findings reveal locally suppressed IFN production contributes to a quiescent nasal immune landscape that delays viral clearance and impairs mucosal vaccine responses.
-
-
-
Immunology and Microbiology
-
Cancer Research
Viral-Directed Augmentation of Kupffer Cell Cross-Presentation Provokes Antitumor Immunity Against Liver Metastasis.
In Adv Sci (Weinh) on 1 October 2025 by Chen, C., Zhang, Q., et al.
PubMed
Liver metastasis is associated with poor prognosis and resistance to immune checkpoint inhibitors. Functional modulation of Kupffer cells (KCs) holds promise as an alternative immunotherapeutic approach. Leveraging their capacity to capture circulating virions, an oncolytic virus-based KC-targeting strategy is developed that demonstrated efficacy and safety in treating multifocal liver metastasis. A single intravenous infusion of the M51R mutant vesicular stomatitis virus (VSV-M51R), but not wild-type (WT) VSV, induced significant tumor regression in mouse models of forced liver metastasis, independent of direct oncolysis. The ineffectiveness of VSV-WT is attributed to its induction of massive KC apoptosis, whereas VSV-M51R replicated transiently within KCs without compromising viability. Instead, VSV-M51R promoted KC proliferation in tumor-adjacent areas, enhancing their access to tumor foci and cross-presentation of tumor antigens. This led to robust activation of hepatic anti-tumor CD8+ T-cell responses, which required mitochondrial antiviral signaling protein-dependent type I interferon triggering in KCs. Depletion of KCs abolished the T cell stimulating and anti-tumor effects of VSV-M51R. Furthermore, simultaneous blockade of programmed cell death-ligand 1(PD-L1) during VSV-M51R treatment achieved remarkable synergistic efficacy in treating monotherapy-resistant late-stage liver metastasis. These findings underscore the pivotal role of KCs in systemic oncolytic virotherapy and offer a potentially applicable strategy for treating advanced liver metastasis.
-
-
-
Immunology and Microbiology
Simultaneous STING and lymphotoxin-β receptor activation induces B cell responses in tertiary lymphoid structures to potentiate antitumor immunity.
In Nat Immunol on 1 October 2025 by Sawada, J., Kikuchi, Y., et al.
PubMed
B cell-rich tertiary lymphoid structures (TLS) are associated with favorable prognosis and positive response to immunotherapy in cancer. Here we show that simultaneous activation of innate immune effectors, STING and lymphotoxin-β receptor (LTβR), results in CD8+ T cell-dependent tumor suppression while inducing high endothelial venule development and germinal center-like B cell responses in tumors to generate functional TLS in a T cell-dependent manner. In a neoadjuvant setting, activation of STING and LTβR by their agonists effectively immunized mice against tumor recurrence, leading to long-term survival. STING activation alone was insufficient for inducing B cell-containing TLS or eliciting long-term therapeutic effects. However, when combined with LTβR activation, it improved the fitness of TLS with B cell expansion and maturation to IgG-producing long-lived plasma cells and memory cells, increased CD4+ T cell recruitment and memory CD8+ T cell expansion, and shifted the TH2/TH17 balance, resulting in the potentiation of humoral and cellular immunity against tumors. These findings suggest a therapeutic approach of simultaneously activating STING and lymphotoxin pathways.
-
-
-
Immunology and Microbiology
Zeaxanthin augments CD8+ effector T cell function and immunotherapy efficacy.
In Cell Rep Med on 16 September 2025 by Zhang, F. Q., Li, J., et al.
PubMed
The detailed mechanisms underlying the regulatory significance of dietary components in modulating anti-tumor immunity remain largely unknown. Here, we apply a co-culture-based screening approach using a blood nutrient compound library and identify zeaxanthin (ZEA), a dietary carotenoid pigment found in many fruits and vegetables and known for its role in eye health, as an immunomodulator that enhances the cytotoxicity of CD8+ T cells against tumor cells. Oral supplementation with ZEA, but not its structural isomer lutein (LUT), enhances anti-tumor immunity in vivo. Integrated multi-omics mechanistic studies reveal that ZEA promotes T cell receptor (TCR) stimulation on the CD8+ T cell surface, leading to improved intracellular TCR signaling for effector T cell function. Hence, ZEA treatment augments the efficacy of anti-PD1 immune checkpoint inhibitor in vivo and the cytotoxicity of human TCR gene-engineered CD8+ T cells in vitro. Our findings uncover a previously unknown immunoregulatory function of ZEA, which has translational potential as a dietary element in bolstering immunotherapy.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Immunology and Microbiology
An age-related decrease in leptin contributes to CD8+ T cell aging in the tumor microenvironment.
In Cell Rep Med on 16 September 2025 by Wang, F., Bao, R., et al.
PubMed
T cell dysfunction with age underlies an increased incidence of cancer in elderly individuals; however, how T cell aging is triggered in the tumor microenvironment is unclear. Here, we show that an age-associated reduction in adipocyte-derived leptin contributes to the accumulation of tumor-infiltrating senescent CD8+ T cells. Single-cell profiling of human and mouse cancer tissues reveals that the frequency of intratumoral senescent CD8+ T cells increases with age, leading to a weak antitumor effect. Moreover, decreased levels of adipocyte-derived leptin are an indispensable factor for CD8+ T cell aging. Leptin signaling prevents p38-dependent CD8+ T cell senescence. Furthermore, plasma leptin levels are negatively related to intratumoral CD8+ T cell senescence in cancer patients. Our findings identify an unappreciated interplay between metabolic perturbation and T cell aging and suggest that modulating adipocyte-derived leptin levels may be a promising therapeutic strategy for older cancer patients.
-
-
-
Cancer Research
Intrinsic Properties of the Lymph Node Render It Immunologically Susceptible to Metastasis.
In Cancer Discov on 4 September 2025 by Kahn, B., Ng, R. W. S., et al.
PubMed
Lymph nodes (LN) are the staging grounds for antitumor immunity; therefore, their high susceptibility to metastatic colonization is a paradox. Previous studies have suggested that extrinsic tumor-derived factors precondition the draining LN to enable tumor cell survival by promoting a state of immune suppression. In this study, we investigate whether properties of the LN itself may impede its ability to clear metastasizing tumor cells. Using multiple immunocompetent transplant models, we show that LNs possess intrinsic features, independent of preconditioning, which make them an advantageous site for tumor cells to evade T-cell control. Tumor growth in the LN is facilitated by regulatory T cells, which locally suppress the cytolytic capacity of tumor-specific CD8+ T cells by restricting IL2. These findings identify an intrinsic mechanism that contributes to the high rate of LN metastasis in solid tumors.
-
-
-
Immunology and Microbiology
Pre-existing YFV-17D immunity mediates T cell cross-protection against dengue virus serotype 2 infection in mice.
In Commun Biol on 2 September 2025 by Tonto, P. B., Gallon, S., et al.
PubMed
Widespread yellow fever virus (YFV) immunity in Sub-Saharan Africa may mitigate orthoflavivirus outbreaks. Here, we investigate whether pre-existing YFV-17D immunity confers cross-protection against dengue virus serotype 2 (DENV-2) infection in a murine model. IFNAR1-/- mice immunized with YFV-17D exhibited significantly reduced DENV-2 viremia, weight loss, and disease severity, with improved survival compared to naïve controls. Mechanistic studies revealed that cross-protection was mediated by heterologous T cell responses rather than cross-binding antibodies. Depletion of T cells in YFV-17D-immune mice prior to DENV-2 challenge resulted in increased viremia, weight loss, and disease severity, underscoring the protective role of YFV-17D-elicited T cell immunity. Furthermore, YFV-17D-specific T cells displayed cytotoxicity against DENV NS3- and NS5-pulsed cells, demonstrating their functional role in viral control. These findings highlight the critical contribution of heterologous T cell immunity in YFV-17D-mediated protection against DENV-2 and suggest that vaccines designed to elicit T cell responses could enhance cross-protection against orthoflavivirus infections.
-
-
-
Immunology and Microbiology
-
Cancer Research
Deconjugating taurocholic acid with Bifidobacterium to mitigate obesity-driven cancer progression by restoring CD8+ T-cell infiltration.
In NPJ Biofilms Microbiomes on 21 August 2025 by Yuan, R., Li, Y., et al.
PubMed
Obesity is linked to an increased cancer risk, and probiotics show promise in weight management. Here, we elucidate the precise mechanisms through which the probiotic Bifidobacterium breve (B. breve) modulates the immune response in obesity-associated tumours utilizing a Hepa1-6 cell-bearing hepatocellular carcinoma (HCC) model sensitive to high-fat diet (HFD)-induced obesity. HFD-induced obesity expedited HCC progression and fostered an immunosuppressive microenvironment. Treatment with B. breve enhanced cancer control by rescuing the local infiltration of antitumour immune cells. Elevated serum taurocholic acid (TCA) levels were negatively correlated with B. breve levels in obese HCC mice. TCA hindered the infiltration of CD8+ T cells into the tumour microenvironment and diminished their antitumour efficacy by blocking ERK phosphorylation. B. breve deconjugated TCA via its type 4 bile salt hydrolase (BSH), and this effect was diminished upon BSH inhibition by AAA-10. These results highlight the potential application of the probiotic B. breve in the multidisciplinary treatment of cancer in obese individuals.
-
-
-
Cancer Research
-
Immunology and Microbiology
TGF-β and IL-12 conversely orchestrate the formation of CD103+ CD8 tumor-resident memory T cells to regulate response to therapeutic cancer vaccine.
In iScience on 15 August 2025 by Corgnac, S., Damei, I., et al.
PubMed
The accumulation of CD103+ CD8 tumor-resident memory T (TRM) cells is predictive of response to cancer immunotherapy. Here, we show that a therapeutic peptide vaccine controls tumor growth in wild-type mice, but not in CD103-knockout mice. CD8 tumor-infiltrating T lymphocytes include two major TRM subpopulations expressing either CD49a or CD103 integrin, with a subset co-expressing CD103 and Tcf-1. Vaccination induces a decrease in the percentage of Tcf-1+CD103+ TRM-like cells and expansion of CD49a+ TRM displaying an effector/exhausted profile. Tcf-1+CD103+ TRM-cell density increases in tumors from transgenic mice constitutively expressing active TGF-β-type-2-receptor and wild-type mice challenged with neutralizing anti-IL-12 antibodies. The stimulation of CD8 T cells with recombinant (r)TGF-β combined with anti-CD3 antibodies results in an increase in the proportion of Tcf-1+CD103+ cells, whereas rIL-12 decreases CD103 expression. These results highlight the opposite effects of TGF-β and IL-12 in the regulation of TRM-cell development and suggest that targeting Tcf-1+CD103+ CD8 TRM may improve immunotherapy efficacy.
-
-
-
Immunology and Microbiology
Immune cells promote paralytic disease in mice infected with enterovirus D68.
In J Clin Invest on 1 August 2025 by Woods Acevedo, M. A., Lan, J., et al.
PubMed
Enterovirus D68 (EV-D68) is associated with acute flaccid myelitis (AFM), a poliomyelitis-like illness causing paralysis in young children. However, the mechanisms of paralysis are unclear, and antiviral therapies are lacking. To better understand EV-D68 disease, we inoculated newborn mice intracranially to assess viral tropism, virulence, and immune responses. WT mice inoculated intracranially with a neurovirulent strain of EV-D68 showed infection of spinal cord neurons and developed paralysis. Spinal tissue from infected mice revealed increased levels of chemokines, inflammatory monocytes, macrophages, and T cells relative to those in controls, suggesting that immune cell infiltration influences pathogenesis. To define the contribution of cytokine-mediated immune cell recruitment to disease, we inoculated mice lacking CCR2, a receptor for several EV-D68-upregulated cytokines, or RAG1, which is required for lymphocyte maturation. WT, Ccr2-/-, and Rag1-/- mice had comparable viral titers in spinal tissue. However, Ccr2-/- and Rag1-/- mice were significantly less likely to be paralyzed relative to WT mice. Consistent with impaired T cell recruitment to sites of infection in Ccr2-/- and Rag1-/- mice, antibody-mediated depletion of CD4+ or CD8+ T cells from WT mice diminished paralysis. These results indicate that immune cell recruitment to the spinal cord promotes EV-D68-associated paralysis and illuminate potential new targets for therapeutic intervention.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Mogat1 drives metabolic adaptations to evade immune surveillance.
In Nat Commun on 31 July 2025 by Wei, H., Niu, C., et al.
PubMed
Immune checkpoint blockade (ICB) therapies for solid tumors often fail due to resistance, necessitating new strategies. While efforts target IFNγ signaling or antigen presentation, other immune evasion mechanisms are unclear. Here, we identify Monoacylglycerol O-Acyltransferase 1 (Mogat1) as a critical modulator of tumor immune evasion using an in vivo transcriptomic screen in progressing tumors. We find that tumors exploit Mogat1 to sequester fatty acids into triglycerides, a metabolic adaptation that fuels growth and fosters an immunosuppressive microenvironment, enabling immune escape. Genetic inhibition of Mogat1 suppresses tumor growth by promoting T-cell infiltration and enhancing their tumor-killing ability. Importantly, Mogat1 loss sensitizes tumors to PD-1 blockade, overcoming resistance and suggesting reduced reliance on conventional antigen presentation. Our findings reveal a lipid metabolism-centered immune evasion mechanism and highlight Mogat1 as a potential target to improve cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Universal Fab-Fc masked cytokine prodrug platform: αPD-L1/IL-15 prodrug activates CD44+ CD8+ T cells in tumor-draining lymph nodes to enhance antitumor immunity.
In J Immunother Cancer on 31 July 2025 by Song, L., Qi, G., et al.
PubMed
Immunocytokines targeting immune checkpoints have shown great potential in overcoming resistance to immune checkpoint blockade (ICB), making them a major focus of development in recent years. However, severe dose-limiting toxicity hindered their clinical application. Therefore, it is vital to develop versatile strategies to improve safety and elucidate the underlying mechanisms of resistance reversal for advancing immunocytokine therapy.
-
-
-
Immunology and Microbiology
Photoconverted cells allow rapid assessment of vaccine adjuvant potency in mice.
In iScience on 18 July 2025 by Zhong, Y., Chen, M., et al.
PubMed
Identifying vaccine adjuvants that optimally enhance CD8+ T cell responses remain a challenge, often requiring time-consuming and resource-intensive methods. Here, we introduce a photoconversion-based approach using KikGR mice to track migratory dendritic cells (Mig DCs) within 48 h post-vaccination. This method enables real-time visualization of skin-derived Mig DCs migration to draining lymph nodes (dLNs), providing an early and reliable predictor of CD8+ T cell priming and anti-tumor efficacy. Unlike traditional techniques that demand extensive experimentation, this system allows for faster, cost-effective screening of adjuvants. We further demonstrate that blocking CCR7 significantly reduces Mig DCs migration, impairing CD8+ T cell responses. Our approach revolutionizes adjuvant evaluation, enabling swift and accurate immune assessments, particularly in therapeutic vaccine development.
-
-
Cardiolipin-mimic lipid nanoparticles without antibody modification delivered senolytic in vivo CAR-T therapy for inflamm-aging.
In Cell Rep Med on 15 July 2025 by Zhang, Z., Ma, B., et al.
PubMed
mRNA-based in vivo chimeric antigen receptor (CAR)-T cell engineering offers advantages over ex vivo therapies, including streamlined manufacturing and transient expression. However, current delivery methods require antibody-modified vehicles with manufacturing challenges. In this study, inspired by cardiolipin, we identify cardiolipin-like di-phosphoramide lipids that improve T cell transfection without targeting ligands, both in vitro and in vivo. The T cell-favored tropism is likely due to the lipid's packing, shape, and rigidity. Encapsulating circular RNA further prolongs mRNA expression in the spleen and T cells. Using PL40 lipid nanoparticles, we deliver mRNA encoding a CAR targeting the senolytic and inflammatory antigen urokinase-type plasminogen activator receptor (uPAR), alleviating uPAR-related liver fibrosis and rheumatoid arthritis (RA). Single-cell sequencing in humans confirms uPAR's relevance to senescence and inflammation in RA. To facilitate clinical translation, we screen and humanize single-chain variable fragments (scFvs) against uPAR, establishing a PL40 mRNA-encoded humanized uPAR CAR with potential for treating aging-inflamed disorders.
-
-
Immunology and Microbiology
Serotonin transporter inhibits antitumor immunity through regulating the intratumoral serotonin axis.
In Cell on 10 July 2025 by Li, B., Elsten-Brown, J., et al.
PubMed
Identifying additional immune checkpoints hindering antitumor T cell responses is key to the development of next-generation cancer immunotherapies. Here, we report the induction of serotonin transporter (SERT), a regulator of serotonin levels and physiological functions in the brain and peripheral tissues, in tumor-infiltrating CD8 T cells. Inhibition of SERT using selective serotonin reuptake inhibitors (SSRIs), the most widely prescribed antidepressants, significantly suppressed tumor growth and enhanced T cell antitumor immunity in various mouse syngeneic and human xenograft tumor models. Importantly, SSRI treatment exhibited significant therapeutic synergy with programmed cell death protein 1 (PD-1) blockade, and clinical data correlation studies negatively associated intratumoral SERT expression with patient survival in a range of cancers. Mechanistically, SERT functions as a negative-feedback regulator inhibiting CD8 T cell reactivities by depleting intratumoral T cell-autocrine serotonin. These findings highlight the significance of the intratumoral serotonin axis and identify SERT as an immune checkpoint, positioning SSRIs as promising candidates for cancer immunotherapy.
-