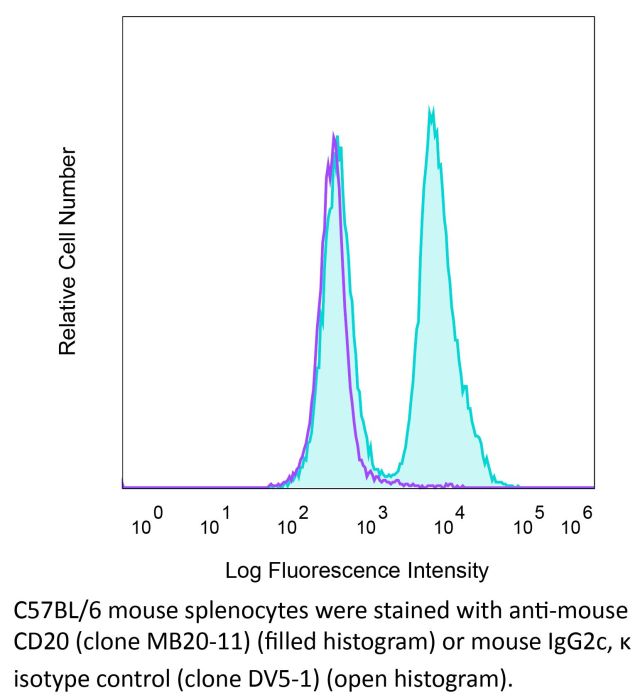
InVivoPlus anti-mouse CD20
Product Description
Bio X Cell is pleased to also offer recombinant MB20-11-CP062. This monoclonal antibody has variable domain sequences identical to MB20-11 but the constant region has been converted from mouse IgG2c to mouse IgG2a for use in mice with the Igh-1a allele. Additionally, the highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results over hybridoma derived antibodies.
1: Uchida, Junji et al. “The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy.” The Journal of experimental medicine vol. 199,12 (2004): 1659-69. doi:10.1084/jem.20040119
2: Xiu, Yan et al. “B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions.” Journal of immunology (Baltimore, Md. : 1950) vol. 180,5 (2008): 2863-75. doi:10.4049/jimmunol.180.5.2863
3: Hamaguchi, Yasuhito et al. “Antibody isotype-specific engagement of Fc gamma receptors regulates B lymphocyte depletion during CD20 immunotherapy.” The Journal of experimental medicine vol. 203,3 (2006): 743-53. doi:10.1084/jem.20052283
4: Zhang, Zhiping et al. “Possible allelic structure of IgG2a and IgG2c in mice.” Molecular immunology vol. 50,3 (2012): 169-71. doi:10.1016/j.molimm.2011.11.006
Specifications
| Isotype | Mouse IgG2c, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus mouse IgG2c isotype control, anti-dengue virus |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CD20-GFP transfected 300.19 cells |
| Reported Applications |
in vivo B cell depletion Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894775 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo B cell depletion
Haas, K. M., et al (2010). "Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice" J Immunol 184(9): 4789-4800.
PubMed
Delineating the relative contributions of B lymphocytes during the course of autoimmune disease has been difficult. Therefore, the effects of depleting all mature B cells using a potent CD20 mAb, or of depleting circulating and marginal zone B cells using a ligand-blocking CD22 mAb, were compared in NZB/W F(1) mice, a model for human systemic lupus erythematosus. Single low-dose mAb treatments depleted B cells efficiently in both NZB/W F(1) and C57BL/6 mice. Prophylactic B cell depletion by repeated CD20 mAb treatments prolonged survival during pristane-accelerated lupus in NZB/W F(1) mice, whereas CD22 mAb had little effect. Despite effective B cell depletion, neither mAb treatment prevented autoantibody generation. In addition, CD20, CD22, and control mAb-treated NZB/W F(1) mice developed anti-mouse IgG autoantibodies in contrast to parental NZB and NZW strains, which may have reduced the effectiveness of B cell depletion. Despite this, low-dose CD20 mAb treatment initiated in 12-28-wk-old mice, and administered every 4 wk thereafter, significantly delayed spontaneous disease in NZB/W F(1) mice. By contrast, B cell depletion initiated in 4-wk-old mice hastened disease onset, which paralleled depletion of the IL-10-producing regulatory B cell subset called B10 cells. B10 cells were phenotypically similar in NZB/W F(1) and C57BL/6 mice, but were expanded significantly in young NZB/W F(1) mice. Thus, B cell depletion had significant effects on NZB/W F(1) mouse survival that were dependent on the timing of treatment initiation. Therefore, distinct B cell populations can have opposing protective and pathogenic roles during lupus progression.
in vivo B cell depletion
Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF (2008). "B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions
PubMed
NOD mice deficient for B lymphocytes from birth fail to develop autoimmune or type 1 diabetes. To assess whether B cell depletion influences type 1 diabetes in mice with an intact immune system, NOD female mice representing early and late preclinical stages of disease were treated with mouse anti-mouse CD20 mAbs. Short-term CD20 mAb treatment in 5-wk-old NOD female mice reduced B cell numbers by approximately 95%, decreased subsequent insulitis, and prevented diabetes in >60% of littermates. In addition, CD20 mAb treatment of 15-wk-old NOD female mice significantly delayed, but did not prevent, diabetes onset. Protection from diabetes did not result from altered T cell numbers or subset distributions, or regulatory/suppressor T cell generation. Rather, impaired CD4+ and CD8+ T cell activation in the lymph nodes of B cell-depleted NOD mice may delay diabetes onset. B cell depletion was achieved despite reduced sensitivity of NOD mice to CD20 mAbs compared with C57BL/6 mice. Decreased B cell depletion resulted from deficient FcgammaRI binding of IgG2a/c CD20 mAbs and 60% reduced spleen monocyte numbers, which in combination reduced Ab-dependent cellular cytotoxicity. With high-dose CD20 mAb treatment (250 microg) in NOD mice, FcgammaRIII and FcgammaRIV compensated for inadequate FcgammaRI function and mediated B cell depletion. Thereby, NOD mice provide a model for human FcgammaR polymorphisms that reduce therapeutic mAb efficacy in vivo. Moreover, this study defines a new, clinically relevant approach whereby B cell depletion early in the course of disease development may prevent diabetes or delay progression of disease.
in vivo B cell depletion
Hamaguchi, Y., et al (2006). "Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy" J Exp Med 203(3): 743-753.
PubMed
CD20 monoclonal antibody (mAb) immunotherapy is effective for lymphoma and autoimmune disease. In a mouse model of immunotherapy using mouse anti-mouse CD20 mAbs, the innate monocyte network depletes B cells through immunoglobulin (Ig)G Fc receptor (FcgammaR)-dependent pathways with a hierarchy of IgG2a/c>IgG1/IgG2b>IgG3. To understand the molecular basis for these CD20 mAb subclass differences, B cell depletion was assessed in mice deficient or blocked for stimulatory FcgammaRI, FcgammaRIII, FcgammaRIV, or FcR common gamma chain, or inhibitory FcgammaRIIB. IgG1 CD20 mAbs induced B cell depletion through preferential, if not exclusive, interactions with low-affinity FcgammaRIII. IgG2b CD20 mAbs interacted preferentially with intermediate affinity FcgammaRIV. The potency of IgG2a/c CD20 mAbs resulted from FcgammaRIV interactions, with potential contributions from high-affinity FcgammaRI. Regardless, FcgammaRIV could mediate IgG2a/b/c CD20 mAb-induced depletion in the absence of FcgammaRI and FcgammaRIII. In contrast, inhibitory FcgammaRIIB deficiency significantly increased CD20 mAb-induced B cell depletion by enhancing monocyte function. Although FcgammaR-dependent pathways regulated B cell depletion from lymphoid tissues, both FcgammaR-dependent and -independent pathways contributed to mature bone marrow and circulating B cell clearance by CD20 mAbs. Thus, isotype-specific mAb interactions with distinct FcgammaRs contribute significantly to the effectiveness of CD20 mAbs in vivo, which may have important clinical implications for CD20 and other mAb-based therapies.
in vivo B cell depletion
Uchida, J., et al (2004). "The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy" J Exp Med 199(12): 1659-1669.
PubMed
Anti-CD20 antibody immunotherapy effectively treats non-Hodgkin’s lymphoma and autoimmune disease. However, the cellular and molecular pathways for B cell depletion remain undefined because human mechanistic studies are limited. Proposed mechanisms include antibody-, effector cell-, and complement-dependent cytotoxicity, the disruption of CD20 signaling pathways, and the induction of apoptosis. To identify the mechanisms for B cell depletion in vivo, a new mouse model for anti-CD20 immunotherapy was developed using a panel of twelve mouse anti-mouse CD20 monoclonal antibodies representing all four immunoglobulin G isotypes. Anti-CD20 antibodies rapidly depleted the vast majority of circulating and tissue B cells in an isotype-restricted manner that was completely dependent on effector cell Fc receptor expression. B cell depletion used both FcgammaRI- and FcgammaRIII-dependent pathways, whereas B cells were not eliminated in FcR common gamma chain-deficient mice. Monocytes were the dominant effector cells for B cell depletion, with no demonstrable role for T or natural killer cells. Although most anti-CD20 antibodies activated complement in vitro, B cell depletion was completely effective in mice with genetic deficiencies in C3, C4, or C1q complement components. That the innate monocyte network depletes B cells through FcgammaR-dependent pathways during anti-CD20 immunotherapy has important clinical implications for anti-CD20 and other antibody-based therapies.
in vivo B cell depletion
Uchida, J., et al (2004). "Mouse CD20 expression and function" Int Immunol 16(1): 119-129.
PubMed
CD20 plays a role in human B cell proliferation and is an effective target for immunotherapy. In this study, mouse CD20 expression and biochemistry were assessed for the first time using a new panel of CD20-specific mAb, with CD20 function assessed using CD20-deficient (CD20(-/-)) mice. CD20 expression was B cell restricted and was initiated during late pre-B cell development. The frequency and density of CD20 expression increased during B cell maturation in the bone marrow, with a subpopulation of transitional IgM(hi) B cells expressing higher CD20 levels than the majority of mature recirculating B cells. Transitional T1 B cells in the spleen also expressed high CD20 levels, providing a useful new marker for this B cell subset. In CD20(-/-) mice, immature and mature B cell IgM expression was approximately 20-30% lower relative to B cells from wild-type littermates. In addition, CD19-induced intracellular calcium responses were significantly reduced in CD20(-/-) B cells, with a less dramatic effect on IgM-induced responses. These results reveal a role for CD20 in transmembrane Ca(2+) movement in mouse primary B cells that complements previous results obtained using human CD20 cDNA-transfected cell lines. Otherwise, B cell development, tissue localization, signal transduction, proliferation, T cell-dependent antibody responses and affinity maturation were normal in CD20(-/-) mice. Thus, mouse and human CD20 share similar patterns of expression and function. These studies thereby provide an animal model for studying CD20 function in vivo and the molecular mechanisms that influence anti-CD20 immunotherapy.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Tumor neoantigens as key drivers of significant anti - tumor immunity in triple - negative breast cancer mouse models.
In Neoplasia on 1 September 2025 by Her, Y., Kim, J. Y., et al.
PubMed
Recent studies have highlighted the therapeutic potential of targeting tumor neoantigens in solid tumors; however, its efficacy in breast cancer remains unclear. Here, we evaluate the impact of tumor neoantigen-targeted strategies in a syngeneic mouse mammary carcinoma model. Mice previously exposed to 4T1 tumor cells (PETCs) or treated with tumor cell-derived lysates (TdLs) exhibited robust antitumor immunity, leading to reduced tumor growth and metastasis through tumor immune microenvironment remodeling. TdL administration in mice harboring orthotopic tumors significantly enhanced the efficacy of immune checkpoint blockade, suggesting its potential as an immunotherapeutic adjuvant. To further optimize neoantigen-based approaches, we developed a lipid nanoparticle (LNP)-based delivery system for neoantigen peptides, which effectively suppressed tumor progression and metastasis in vivo. Mechanistically, this strategy promoted antigen-specific T cell activation and reshaped the tumor immune landscape, enhancing immune-mediated tumor rejection. These findings underscore the therapeutic promise of personalized tumor neoantigen-targeted immunotherapy in breast cancer and support its further evaluation in clinical settings.
-
-
N153-linked glycans on envelope protein protect orthoflaviviruses from antibody-mediated clearance
In bioRxiv on 23 February 2025 by Ting, D. H. R., Marzinek, J. K., et al.
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Zinc finger protein 296 promotes hepatocellular carcinoma progression via intervening interaction between macrophages and B cells.
In Chin J Cancer Res on 30 October 2024 by Xu, N., Xiang, X., et al.
PubMed
Hepatocellular carcinoma (HCC) is a prevalent malignancy with poor survival. Different cell types in the tumor microenvironment participate in the tumorigenesis and progression of HCC. This study aimed to analyze the immune microenvironment of HCC and its relationship with clinical outcomes.
-
-
-
Immunology and Microbiology
Vaccination with O-linked Mannans Protects against Systemic Candidiasis through Innate Lymphocyte Populations.
In J Immunol on 15 September 2024 by Taira, C. L., Dos Santos Dias, L., et al.
PubMed
Candida spp. are the fourth leading cause of bloodstream infections in hospitalized patients and the most common cause of invasive fungal infection. No vaccine against Candida spp. or other fungal pathogens of humans is available. We recently discovered the Blastomyces Dectin-2 ligand endoglucanase 2 that harbors antigenic and adjuvant functions and can function as a protective vaccine against that fungus. We also reported that the adjuvant activity, which is mediated by O-mannans decorating the C terminus of Blastomyces Dectin-2 ligand endoglucanase 2, can augment peptide Ag-induced vaccine immunity against heterologous agents, including Cryptococcus, Candida, and influenza. In this article, we report that the O-linked mannans alone, in the absence of any antigenic peptide, can also protect against systemic candidiasis, reducing kidney fungal load and increasing survival in a Dectin-2-dependent manner. We found that this long-term glycan-induced protection is mediated by innate lymphocyte populations including TCR-γδ+ T cells, innate lymphoid cells, and NK cells that subsequently activate and release reactive oxygen species from neutrophils and monocytes. Our findings suggest that Blastomyces O-mannan displayed by Eng2 induces a form of protective trained immunity mediated by innate lymphocyte populations.
-