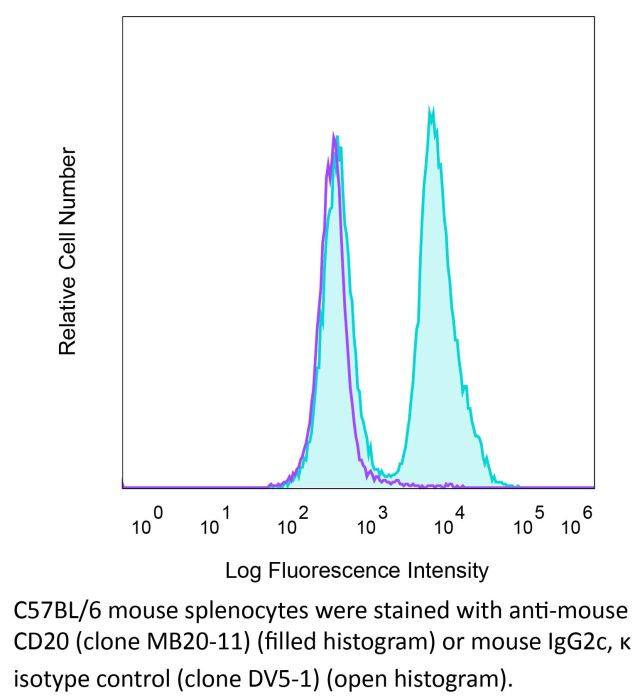
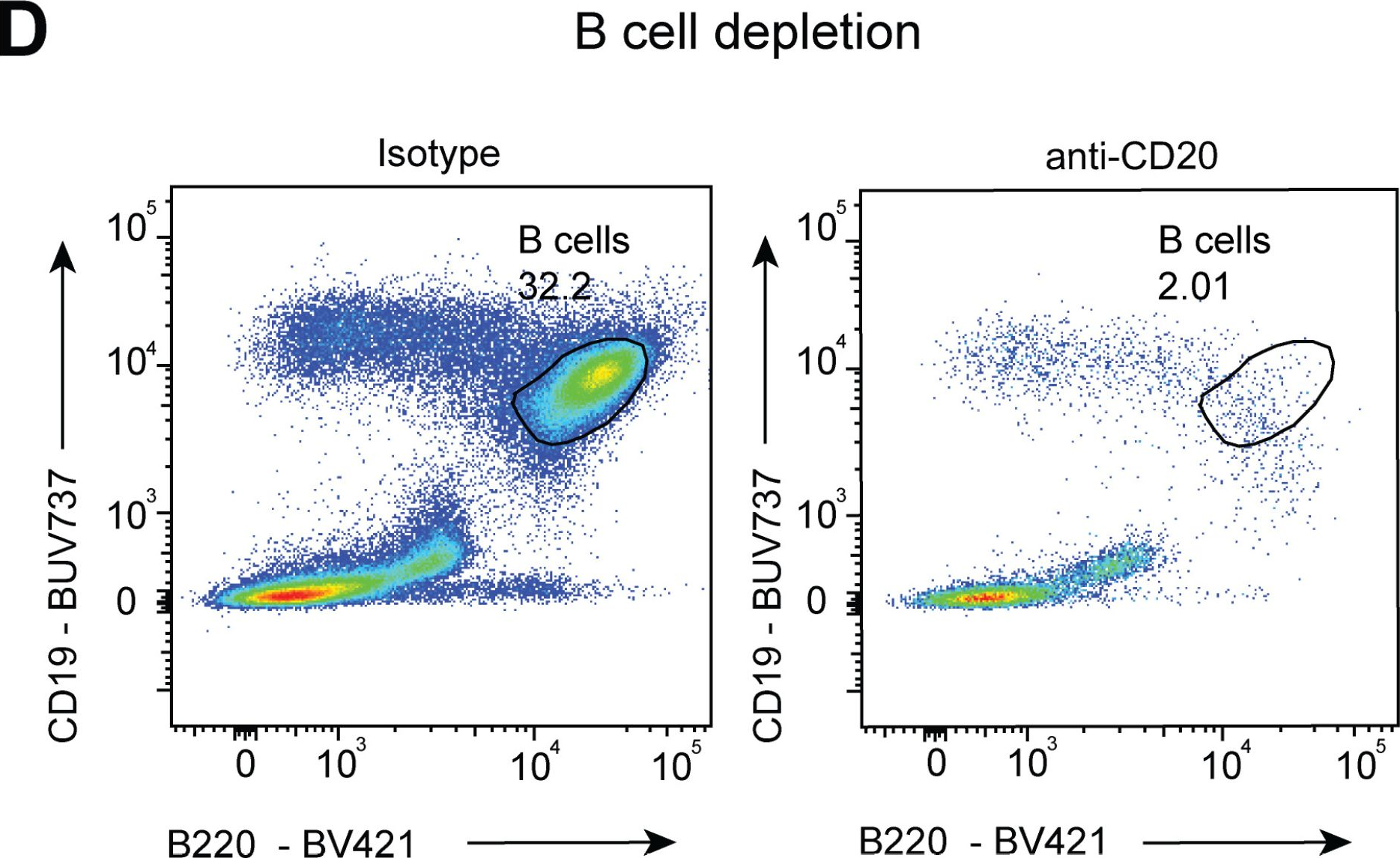
InVivoMAb anti-mouse CD20
Product Description
Bio X Cell is pleased to also offer recombinant MB20-11-CP062. This monoclonal antibody has variable domain sequences identical to MB20-11 but the constant region has been converted from mouse IgG2c to mouse IgG2a for use in mice with the Igh-1a allele. Additionally, the highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results over hybridoma derived antibodies.
1: Uchida, Junji et al. “The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy.” The Journal of experimental medicine vol. 199,12 (2004): 1659-69. doi:10.1084/jem.20040119
2: Xiu, Yan et al. “B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions.” Journal of immunology (Baltimore, Md. : 1950) vol. 180,5 (2008): 2863-75. doi:10.4049/jimmunol.180.5.2863
3: Hamaguchi, Yasuhito et al. “Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy.” The Journal of experimental medicine vol. 203,3 (2006): 743-53. doi:10.1084/jem.20052283
4: Zhang, Zhiping et al. “Possible allelic structure of IgG2a and IgG2c in mice.” Molecular immunology vol. 50,3 (2012): 169-71. doi:10.1016/j.molimm.2011.11.006
Specifications
| Isotype | Mouse IgG2c, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2c isotype control, anti-dengue virus |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CD20-GFP transfected 300.19 cells |
| Reported Applications |
in vivo B cell depletion Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894775 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo B cell depletion
Haas, K. M., et al (2010). "Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice" J Immunol 184(9): 4789-4800.
PubMed
Delineating the relative contributions of B lymphocytes during the course of autoimmune disease has been difficult. Therefore, the effects of depleting all mature B cells using a potent CD20 mAb, or of depleting circulating and marginal zone B cells using a ligand-blocking CD22 mAb, were compared in NZB/W F(1) mice, a model for human systemic lupus erythematosus. Single low-dose mAb treatments depleted B cells efficiently in both NZB/W F(1) and C57BL/6 mice. Prophylactic B cell depletion by repeated CD20 mAb treatments prolonged survival during pristane-accelerated lupus in NZB/W F(1) mice, whereas CD22 mAb had little effect. Despite effective B cell depletion, neither mAb treatment prevented autoantibody generation. In addition, CD20, CD22, and control mAb-treated NZB/W F(1) mice developed anti-mouse IgG autoantibodies in contrast to parental NZB and NZW strains, which may have reduced the effectiveness of B cell depletion. Despite this, low-dose CD20 mAb treatment initiated in 12-28-wk-old mice, and administered every 4 wk thereafter, significantly delayed spontaneous disease in NZB/W F(1) mice. By contrast, B cell depletion initiated in 4-wk-old mice hastened disease onset, which paralleled depletion of the IL-10-producing regulatory B cell subset called B10 cells. B10 cells were phenotypically similar in NZB/W F(1) and C57BL/6 mice, but were expanded significantly in young NZB/W F(1) mice. Thus, B cell depletion had significant effects on NZB/W F(1) mouse survival that were dependent on the timing of treatment initiation. Therefore, distinct B cell populations can have opposing protective and pathogenic roles during lupus progression.
in vivo B cell depletion
Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF (2008). "B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions
PubMed
NOD mice deficient for B lymphocytes from birth fail to develop autoimmune or type 1 diabetes. To assess whether B cell depletion influences type 1 diabetes in mice with an intact immune system, NOD female mice representing early and late preclinical stages of disease were treated with mouse anti-mouse CD20 mAbs. Short-term CD20 mAb treatment in 5-wk-old NOD female mice reduced B cell numbers by approximately 95%, decreased subsequent insulitis, and prevented diabetes in >60% of littermates. In addition, CD20 mAb treatment of 15-wk-old NOD female mice significantly delayed, but did not prevent, diabetes onset. Protection from diabetes did not result from altered T cell numbers or subset distributions, or regulatory/suppressor T cell generation. Rather, impaired CD4+ and CD8+ T cell activation in the lymph nodes of B cell-depleted NOD mice may delay diabetes onset. B cell depletion was achieved despite reduced sensitivity of NOD mice to CD20 mAbs compared with C57BL/6 mice. Decreased B cell depletion resulted from deficient FcgammaRI binding of IgG2a/c CD20 mAbs and 60% reduced spleen monocyte numbers, which in combination reduced Ab-dependent cellular cytotoxicity. With high-dose CD20 mAb treatment (250 microg) in NOD mice, FcgammaRIII and FcgammaRIV compensated for inadequate FcgammaRI function and mediated B cell depletion. Thereby, NOD mice provide a model for human FcgammaR polymorphisms that reduce therapeutic mAb efficacy in vivo. Moreover, this study defines a new, clinically relevant approach whereby B cell depletion early in the course of disease development may prevent diabetes or delay progression of disease.
in vivo B cell depletion
Hamaguchi, Y., et al (2006). "Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy" J Exp Med 203(3): 743-753.
PubMed
CD20 monoclonal antibody (mAb) immunotherapy is effective for lymphoma and autoimmune disease. In a mouse model of immunotherapy using mouse anti-mouse CD20 mAbs, the innate monocyte network depletes B cells through immunoglobulin (Ig)G Fc receptor (FcgammaR)-dependent pathways with a hierarchy of IgG2a/c>IgG1/IgG2b>IgG3. To understand the molecular basis for these CD20 mAb subclass differences, B cell depletion was assessed in mice deficient or blocked for stimulatory FcgammaRI, FcgammaRIII, FcgammaRIV, or FcR common gamma chain, or inhibitory FcgammaRIIB. IgG1 CD20 mAbs induced B cell depletion through preferential, if not exclusive, interactions with low-affinity FcgammaRIII. IgG2b CD20 mAbs interacted preferentially with intermediate affinity FcgammaRIV. The potency of IgG2a/c CD20 mAbs resulted from FcgammaRIV interactions, with potential contributions from high-affinity FcgammaRI. Regardless, FcgammaRIV could mediate IgG2a/b/c CD20 mAb-induced depletion in the absence of FcgammaRI and FcgammaRIII. In contrast, inhibitory FcgammaRIIB deficiency significantly increased CD20 mAb-induced B cell depletion by enhancing monocyte function. Although FcgammaR-dependent pathways regulated B cell depletion from lymphoid tissues, both FcgammaR-dependent and -independent pathways contributed to mature bone marrow and circulating B cell clearance by CD20 mAbs. Thus, isotype-specific mAb interactions with distinct FcgammaRs contribute significantly to the effectiveness of CD20 mAbs in vivo, which may have important clinical implications for CD20 and other mAb-based therapies.
in vivo B cell depletion
Uchida, J., et al (2004). "The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy" J Exp Med 199(12): 1659-1669.
PubMed
Anti-CD20 antibody immunotherapy effectively treats non-Hodgkin’s lymphoma and autoimmune disease. However, the cellular and molecular pathways for B cell depletion remain undefined because human mechanistic studies are limited. Proposed mechanisms include antibody-, effector cell-, and complement-dependent cytotoxicity, the disruption of CD20 signaling pathways, and the induction of apoptosis. To identify the mechanisms for B cell depletion in vivo, a new mouse model for anti-CD20 immunotherapy was developed using a panel of twelve mouse anti-mouse CD20 monoclonal antibodies representing all four immunoglobulin G isotypes. Anti-CD20 antibodies rapidly depleted the vast majority of circulating and tissue B cells in an isotype-restricted manner that was completely dependent on effector cell Fc receptor expression. B cell depletion used both FcgammaRI- and FcgammaRIII-dependent pathways, whereas B cells were not eliminated in FcR common gamma chain-deficient mice. Monocytes were the dominant effector cells for B cell depletion, with no demonstrable role for T or natural killer cells. Although most anti-CD20 antibodies activated complement in vitro, B cell depletion was completely effective in mice with genetic deficiencies in C3, C4, or C1q complement components. That the innate monocyte network depletes B cells through FcgammaR-dependent pathways during anti-CD20 immunotherapy has important clinical implications for anti-CD20 and other antibody-based therapies.
in vivo B cell depletion
Uchida, J., et al (2004). "Mouse CD20 expression and function" Int Immunol 16(1): 119-129.
PubMed
CD20 plays a role in human B cell proliferation and is an effective target for immunotherapy. In this study, mouse CD20 expression and biochemistry were assessed for the first time using a new panel of CD20-specific mAb, with CD20 function assessed using CD20-deficient (CD20(-/-)) mice. CD20 expression was B cell restricted and was initiated during late pre-B cell development. The frequency and density of CD20 expression increased during B cell maturation in the bone marrow, with a subpopulation of transitional IgM(hi) B cells expressing higher CD20 levels than the majority of mature recirculating B cells. Transitional T1 B cells in the spleen also expressed high CD20 levels, providing a useful new marker for this B cell subset. In CD20(-/-) mice, immature and mature B cell IgM expression was approximately 20-30% lower relative to B cells from wild-type littermates. In addition, CD19-induced intracellular calcium responses were significantly reduced in CD20(-/-) B cells, with a less dramatic effect on IgM-induced responses. These results reveal a role for CD20 in transmembrane Ca(2+) movement in mouse primary B cells that complements previous results obtained using human CD20 cDNA-transfected cell lines. Otherwise, B cell development, tissue localization, signal transduction, proliferation, T cell-dependent antibody responses and affinity maturation were normal in CD20(-/-) mice. Thus, mouse and human CD20 share similar patterns of expression and function. These studies thereby provide an animal model for studying CD20 function in vivo and the molecular mechanisms that influence anti-CD20 immunotherapy.
Product Citations
-
-
Immunology and Microbiology
Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity.
In Cell Rep Med on 19 August 2025 by Ahn, J. J., Dudics, S., et al.
PubMed
Wild-type interleukin (IL)-2 induces anti-tumor immunity and toxicity, predominated by vascular leak syndrome (VLS) leading to edema, hypotension, organ toxicity, and regulatory T cell (Treg) expansion. Efforts to uncouple IL-2 toxicity from its potency have failed in the clinic. We hypothesize that IL-2 toxicity is driven by cytokine release syndrome (CRS) followed by VLS and that coupling IL-2 with IL-10 will ameliorate toxicity. Our data, generated using human primary cells, mouse models, and non-human primates, suggest that coupling of these cytokines prevents toxicity while retaining cytotoxic T cell activation and limiting Treg expansion. In syngeneic murine tumor models, DK210 epidermal growth factor receptor (EGFR), an IL-2/IL-10 fusion molecule targeted to EGFR via an anti-EGFR single-chain variable fragment (scFV), potently activates T cells and natural killer (NK) cells and elicits interferon (IFN)γ-dependent anti-tumor function without peripheral inflammatory toxicity or Treg accumulation. Therefore, combining IL-2 with IL-10 uncouples toxicity from immune activation, leading to a balanced and pleiotropic anti-tumor immune response.
-
-
-
Immunology and Microbiology
Hypoimmune CD19 CAR T cells treat allogeneic mice with features of spontaneous systemic lupus erythematosus.
In iScience on 18 July 2025 by Hu, X., White, K., et al.
PubMed
Hypoimmune (HIP) MHC class I- and II-deficient and CD47-overexpressing CD19 CAR T cells were generated and tested in an allogeneic NZB/W mouse model of spontaneous systemic lupus erythematosus with established disease. HIP CAR T cells showed persistent engraftment, achieved lasting deep tissue B cell depletion, diminished antibody levels and systemic pro-inflammatory cytokine levels, mitigated proteinuria and glucosuria, alleviated structural kidney injury, and improved survival after 21 weeks. HIP CAR T cells did not induce any immune activation in this fully allogeneic model and thus completely escaped allorejection. In contrast, MHC-replete, non-HIP-edited wild-type (WT) CD19 CAR T cells induced a strong adaptive immune response and vanished quickly without inducing meaningful B cell depletion and without improving disease markers or survival. Conditioning of NZB/W mice with irradiation did not enhance the HIP CAR T cell efficacy and might hint at their potency for autoimmune patients without prior lymphodepletion.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
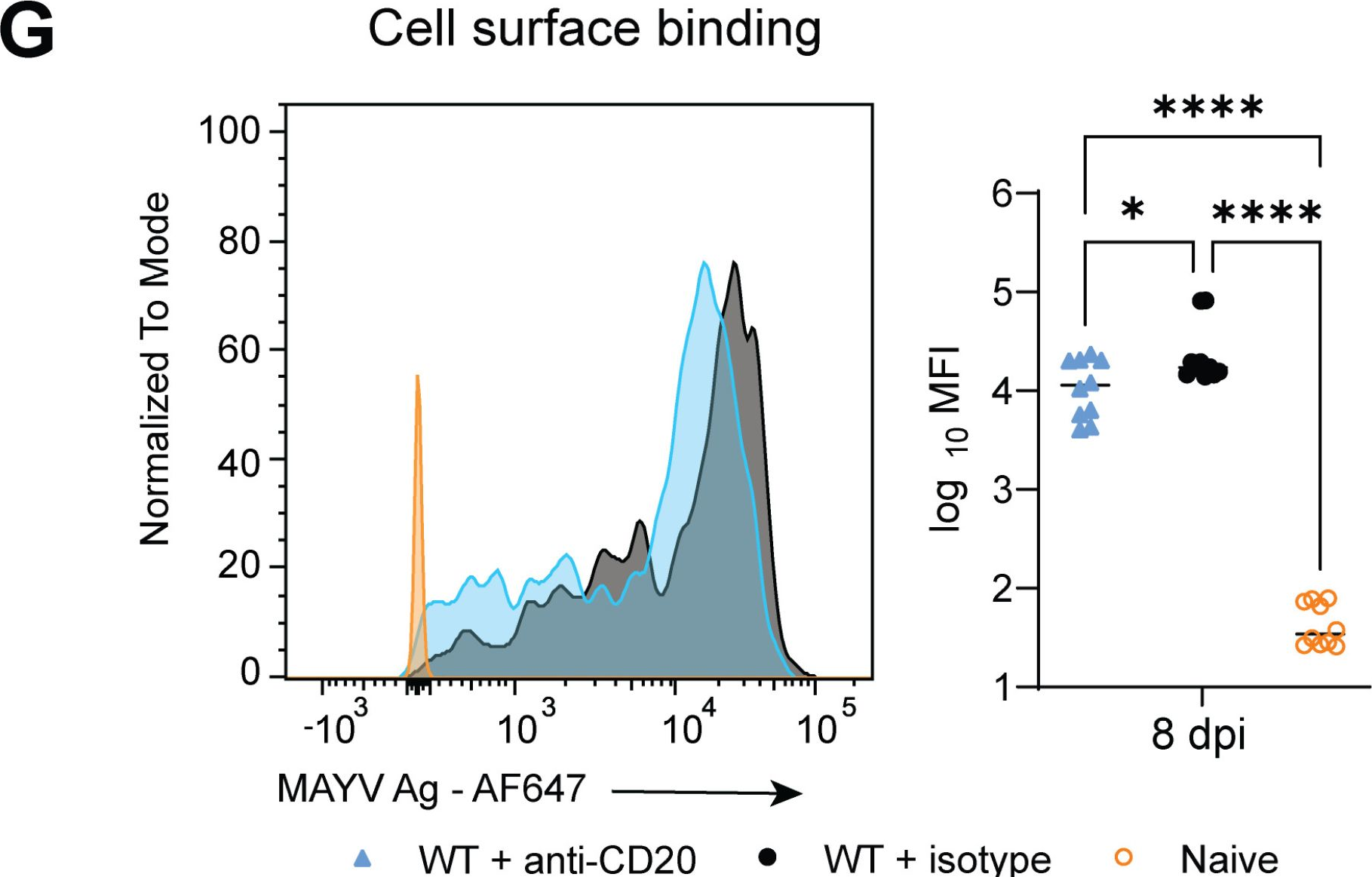
Interaction of the endogenous antibody response with activating FcγRs enhance control of Mayaro virus through monocytes.
In PLoS Pathog on 1 February 2025 by Dunagan, M. M., Dábilla, N., et al.
PubMed
Mayaro virus (MAYV) is an emerging arbovirus. Previous studies have shown antibody Fc effector functions are critical for optimal monoclonal antibody-mediated protection against alphaviruses; however, the requirement of Fc gamma receptors (FcγRs) for protection during natural infection has not been evaluated. Here, we showed mice lacking activating FcγRs (FcRγ-/-) developed prolonged clinical disease with increased MAYV in joint-associated tissues. Viral reduction was associated with anti-MAYV cell surface binding antibodies rather than neutralizing antibodies. Lack of Fc-FcγR engagement increased the number of monocytes present in the joint-associated tissue through chronic timepoints. Single-cell RNA sequencing showed elevated levels of pro-inflammatory monocytes in joint-associated tissue with increased MAYV RNA present in FcRγ-/- monocytes and macrophages. Transfer of FcRγ-/- monocytes into wild type animals was sufficient to increase virus in joint-associated tissue. Overall, this study suggests that engagement of antibody Fc with activating FcγRs promotes protective responses during MAYV infection and prevents a pro-viral role for monocytes.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Blockade of TGF-β and PD-L1 by bintrafusp alfa promotes survival in preclinical ovarian cancer models by promoting T effector and NK cell responses.
In Br J Cancer on 1 June 2024 by Kment, J., Newsted, D., et al.
PubMed
Failure of immunotherapy in high-grade serous ovarian cancer (HGSC) may be due to high levels of transforming growth factor-β (TGF-β) in ascites or tumour immune microenvironment (TIME). Here, we test whether coordinated blockade of TGF-β and PD-L1 with bintrafusp alfa (BA) can provoke anti-tumour immune responses in preclinical HGSC models.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Interleukin-21 receptor signaling promotes metabolic dysfunction-associated steatohepatitis-driven hepatocellular carcinoma by inducing immunosuppressive IgA+ B cells.
In Mol Cancer on 8 May 2024 by Xie, Y., Huang, Y., et al.
PubMed
Dysregulation of immune surveillance is tightly linked to the development of metabolic dysfunction-associated steatohepatitis (MASH)-driven hepatocellular carcinoma (HCC); however, its underlying mechanisms remain unclear. Herein, we aimed to determine the role of interleukin-21 receptor (IL-21R) in MASH-driven HCC.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
Stroke and myocardial infarction induce neutrophil extracellular trap release disrupting lymphoid organ structure and immunoglobulin secretion.
In Nat Cardiovasc Res on 1 May 2024 by Tuz, A. A., Ghosh, S., et al.
PubMed
Post-injury dysfunction of humoral immunity accounts for infections and poor outcomes in cardiovascular diseases. Among immunoglobulins (Ig), IgA, the most abundant mucosal antibody, is produced by plasma B cells in intestinal Peyer's patches (PP) and lamina propria. Here we show that patients with stroke and myocardial ischemia (MI) had strongly reduced IgA blood levels. This was phenocopied in experimental mouse models where decreased plasma and fecal IgA were accompanied by rapid loss of IgA-producing plasma cells in PP and lamina propria. Reduced plasma IgG was detectable in patients and experimental mice 3-10 d after injury. Stroke/MI triggered the release of neutrophil extracellular traps (NETs). Depletion of neutrophils, NET degradation or blockade of NET release inhibited the loss of IgA+ cells and circulating IgA in experimental stroke and MI and in patients with stroke. Our results unveil how tissue-injury-triggered systemic NET release disrupts physiological Ig secretion and how this can be inhibited in patients.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
An immune cell map of human lung adenocarcinoma development reveals an anti-tumoral role of the Tfh-dependent tertiary lymphoid structure.
In Cell Rep Med on 19 March 2024 by Liu, W., You, W., et al.
PubMed
The immune responses during the initiation and invasion stages of human lung adenocarcinoma (LUAD) development are largely unknown. Here, we generated a single-cell RNA sequencing map to decipher the immune dynamics during human LUAD development. We found that T follicular helper (Tfh)-like cells, germinal center B cells, and dysfunctional CD8+ T cells increase during tumor initiation/invasion and form a tertiary lymphoid structure (TLS) inside the tumor. This TLS starts with an aggregation of CD4+ T cells and the generation of CXCL13-expressing Tfh-like cells, followed by an accumulation of B cells, and then forms a CD4+ T and B cell aggregate. TLS and its associated cells are correlated with better patient survival. Inhibiting TLS formation by Tfh or B cell depletion promotes tumor growth in mouse models. The anti-tumoral effect of the Tfh-dependent TLS is mediated through interleukin-21 (IL-21)-IL-21 receptor signaling. Our study establishes an anti-tumoral role of the Tfh-dependent TLS in the development of LUAD.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Natural antibodies drive type 2 immunity in response to damage-associated molecular patterns.
In JCI Insight on 12 March 2024 by Mara, A. B., Rawat, K., et al.
PubMed
Allergic airway disease (AAD) is an example of type 2 inflammation that leads to chronic airway eosinophilia controlled by CD4 Th2 cells. Inflammation is reinforced by mast cells and basophils armed with allergen-specific IgE made by allergen-specific B2 B cells of the adaptive immune system. Little is known about how AAD is affected by innate B1 cells, which produce natural antibodies (NAbs) that facilitate apoptotic cell clearance and detect damage- and pathogen-associated molecular patterns (DAMPS and PAMPS). We used transgenic mice lacking either B cells or NAbs in distinct mouse models of AAD that require either DAMPS or PAMPS as the initial trigger for type 2 immunity. In a DAMP-induced allergic model, driven by alum and uric acid, mouse strains lacking B cells (CD19DTA), NAbs (IgHEL MD4), or all secreted antibodies (sIgm-/-Aid-/-) displayed a significant reduction in both eosinophilia and Th2 priming compared with WT or Aid-/- mice lacking only germinal center-dependent high-affinity class-switched antibodies. Replenishing B cell-deficient mice with either unimmunized B1 B cells or NAbs during sensitization restored eosinophilia, suggesting that NAbs are required for licensing antigen-presenting cells to prime type 2 immunity. Conversely, PAMP-dependent type 2 priming to house dust mite or Aspergillus was not dependent on NAbs. This study reveals an underappreciated role of B1 B cell-generated NAbs in selectively driving DAMP-induced type 2 immunity.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
CMTM6 maintains B cell-intrinsic CD40 expression to regulate anti-tumor immunity
In bioRxiv on 10 March 2024 by Long, Y., Chen, R., et al.
-
-
-
Mus musculus (Mouse)
Dnmt3amutations limit normal and autoreactive Tfh differentiation
In bioRxiv on 21 February 2024 by Shen, Y., Li, Z., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Glycosylation-modified antigens as a tolerance-inducing vaccine platform prevent anaphylaxis in a pre-clinical model of food allergy.
In Cell Rep Med on 16 January 2024 by Cao, S., Maulloo, C. D., et al.
PubMed
The only FDA-approved oral immunotherapy for a food allergy provides protection against accidental exposure to peanuts. However, this therapy often causes discomfort or side effects and requires long-term commitment. Better preventive and therapeutic solutions are urgently needed. We develop a tolerance-inducing vaccine technology that utilizes glycosylation-modified antigens to induce antigen-specific non-responsiveness. The glycosylation-modified antigens are administered intravenously (i.v.) or subcutaneously (s.c.) and traffic to the liver or lymph nodes, respectively, leading to preferential internalization by antigen-presenting cells, educating the immune system to respond in an innocuous way. In a mouse model of cow's milk allergy, treatment with glycosylation-modified β-lactoglobulin (BLG) is effective in preventing the onset of allergy. In addition, s.c. administration of glycosylation-modified BLG shows superior safety and potential in treating existing allergies in combination with anti-CD20 co-therapy. This platform provides an antigen-specific immunomodulatory strategy to prevent and treat food allergies.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Engineer a double team of short-lived and glucose-sensing bacteria for cancer eradication.
In Cell Rep Med on 20 June 2023 by Jin, Y., Fu, L., et al.
PubMed
Rationally designed and engineered bacteria represent an emerging unique approach for cancer treatment. Here, we engineer a short-lived bacterium, mp105, that is effective against diverse cancer types and safe for intravenous administration. We reveal that mp105 combats cancer by direct oncolysis, depletion of tumor-associated macrophages, and elicitation of CD4+ T cell immunity. We further engineer a glucose-sensing bacterium named m6001 that selectively colonizes solid tumors. When intratumorally injected, m6001 clears tumors more efficiently than mp105 due to its post-delivery replication in tumors and potent oncolytic capacity. Finally, we combine intravenous injection of mp105 and intratumoral injection of m6001, forming a double team against cancer. The double team enhances cancer therapy compared with single treatment for subjects carrying both intratumorally injectable and uninjectable tumors. The two anticancer bacteria and their combination are applicable to different scenarios, turning bacterial therapy for cancer into a feasible solution.
-
-
-
Cancer Research
Profiling of syngeneic mouse HCC tumor models as a framework to understand anti-PD-1 sensitive tumor microenvironments.
In Hepatology on 1 May 2023 by Zabransky, D. J., Danilova, L., et al.
PubMed
The treatment of hepatocellular carcinoma (HCC) has been transformed by the use of immune checkpoint inhibitors. However, most patients with HCC do not benefit from treatment with immunotherapy. There is an urgent need to understand the mechanisms that underlie response or resistance to immunotherapy for patients with HCC. The use of syngeneic mouse models that closely recapitulate the heterogeneity of human HCC will provide opportunities to examine the complex interactions between cancer cells and nonmalignant cells in the tumor microenvironment.
-
-
-
Cancer Research
-
Immunology and Microbiology
Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity.
In Cell Rep Med on 20 September 2022 by Mirlekar, B., Wang, Y., et al.
PubMed
Plasma cell responses are associated with anti-tumor immunity and favorable response to immunotherapy. B cells can amplify anti-tumor immune responses through antibody production; yet B cells in patients and tumor-bearing mice often fail to support this effector function. We identify dysregulated transcriptional program in B cells that disrupts differentiation of naive B cells into anti-tumor plasma cells. The signaling network contributing to this dysfunction is driven by interleukin (IL) 35 stimulation of a STAT3-PAX5 complex that upregulates the transcriptional regulator BCL6 in naive B cells. Transient inhibition of BCL6 in tumor-educated naive B cells is sufficient to reverse the dysfunction in B cell differentiation, stimulating the intra-tumoral accumulation of plasma cells and effector T cells and rendering pancreatic tumors sensitive to anti-programmed cell death protein 1 (PD-1) blockade. Our findings argue that B cell effector dysfunction in cancer can be due to an active systemic suppression program that can be targeted to synergize with T cell-directed immunotherapy.
-