Catalog #CP062
RecombiMAb anti-mouse CD20
Clone
MB20-11-CP062
Reactivities
Mouse
Isotype
Mouse IgG2a
(switched from mouse IgG2c)
(switched from mouse IgG2c)
You may also be interested in:
Product Description
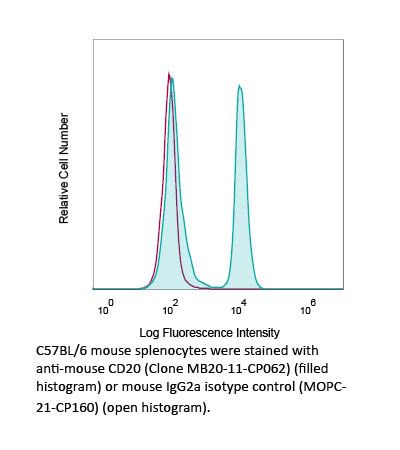
The MB20-11-CP062 monoclonal antibody is a recombinant, chimeric version of the original MB20-11 antibody. The recombinant MB20-11-CP062 antibody targets murine CD20. CD20 is expressed on the surface of immature and mature B cells and their malignant counterparts. MB20-11 rapidly and effectively depletes B cells via antibody-dependent cellular cytotoxicity (ADCC) through engaging Fcγ receptors on monocytes and other effector cells. Isotype-specific interactions contribute significantly to the effectiveness of CD20 mAbs in vivo, with IgG2a/c mAbs having greater potency than IgG1 or IgG2b. IgG2a and IgG2c isotypes are indistinguishable in their specificities to murine FcγR. The recombinant MB20-11-CP062 variable domain sequences are identical to clone MB20-11, but the constant region has been converted from mouse IgG2c to mouse IgG2a. Mouse strains such as C57Bl/6, C57Bl/10, SJL, and NOD mice possesses the Igh1-b allele resulting in only the expression of IgG2c. However, mouse strains such as BALB/c and Swiss Webster mice possess the Igh1-a allele which results in only the expression of IgG2a. It is important to consider matching the Ig-haplotype of the receiving mice to the isotype of the injected antibody to avoid eliciting an undesired immune response. Additionally, the highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results over hybridoma derived antibodies. B cell depleting antibodies are valuable tools for studying B cell biology and developing therapies for autoimmune diseases and malignancies.
References:
1: Uchida, Junji et al. “The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy.” The Journal of experimental medicine vol. 199,12 (2004): 1659-69. doi:10.1084/jem.20040119
2: Xiu, Yan et al. “B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions.” Journal of immunology (Baltimore, Md. : 1950) vol. 180,5 (2008): 2863-75. doi:10.4049/jimmunol.180.5.2863
3: Hamaguchi, Yasuhito et al. “Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy.” The Journal of experimental medicine vol. 203,3 (2006): 743-53. doi:10.1084/jem.20052283
4: Zhang, Zhiping et al. “Possible allelic structure of IgG2a and IgG2c in mice.” Molecular immunology vol. 50,3 (2012): 169-71. doi:10.1016/j.molimm.2011.11.006
References:
1: Uchida, Junji et al. “The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy.” The Journal of experimental medicine vol. 199,12 (2004): 1659-69. doi:10.1084/jem.20040119
2: Xiu, Yan et al. “B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions.” Journal of immunology (Baltimore, Md. : 1950) vol. 180,5 (2008): 2863-75. doi:10.4049/jimmunol.180.5.2863
3: Hamaguchi, Yasuhito et al. “Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy.” The Journal of experimental medicine vol. 203,3 (2006): 743-53. doi:10.1084/jem.20052283
4: Zhang, Zhiping et al. “Possible allelic structure of IgG2a and IgG2c in mice.” Molecular immunology vol. 50,3 (2012): 169-71. doi:10.1016/j.molimm.2011.11.006
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CD20-GFP transfected 300.19 cells |
| Reported Applications |
Western Blot Flow Cytometry *In vivo B cell depletion *Reported for the original MB20-11 antibody. For information on in vivo applications, please contact technicalservice@bioxcell.com |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from HEK293 cell supernatant in an animal-free facility |
| Purification | Protein G |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |