InVivoMAb anti-mouse VEGFR-2
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse VEGFR-2-SEAPs soluble receptor |
| Reported Applications |
in vivo blocking of VEGF/VEGFR-2 signaling in vitro blocking of VEGFR signaling Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107766 |
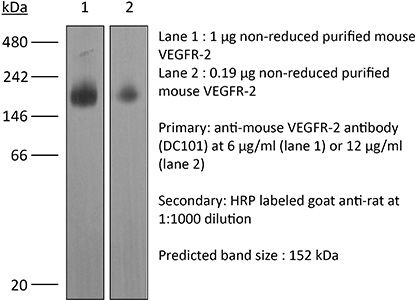
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of VEGF/VEGFR-2 signaling
Arulanandam, R., et al (2015). "VEGF-Mediated Induction of PRD1-BF1/Blimp1 Expression Sensitizes Tumor Vasculature to Oncolytic Virus Infection" Cancer Cell 28(2): 210-224.
PubMed
Oncolytic viruses designed to attack malignant cells can in addition infect and destroy tumor vascular endothelial cells. We show here that this expanded tropism of oncolytic vaccinia virus to the endothelial compartment is a consequence of VEGF-mediated suppression of the intrinsic antiviral response. VEGF/VEGFR2 signaling through Erk1/2 and Stat3 leads to upregulation, nuclear localization, and activation of the transcription repressor PRD1-BF1/Blimp1. PRD1-BF1 does not contribute to the mitogenic effects of VEGF, but directly represses genes involved in type I interferon (IFN)-mediated antiviral signaling. In vivo suppression of VEGF signaling diminishes PRD1-BF1/Blimp1 expression in tumor vasculature and inhibits intravenously administered oncolytic vaccinia delivery to and consequent spread within the tumor.
in vivo blocking of VEGF/VEGFR-2 signaling
Ding, X., et al (2015). "Distinct functions of epidermal and myeloid-derived VEGF-A in skin tumorigenesis mediated by HPV8" Cancer Res 75(2): 330-343.
PubMed
Beta human papillomaviruses (HPV) have been suspected to be carcinogenic in nonmelanoma skin cancers (NMSC), but the basis for potential viral contributions to these cancers is poorly understood. In particular, it is unresolved how HPV-infected keratinocytes escape cell-cycle control and whether their cross-talk with immune cells is critical for tumorigenesis. In nonviral preclinical models, the angiogenic cytokine VEGF-A has been identified as a critical regulator of NMSC. In this study, we dissected the contribution of epidermal versus myeloid cell-derived VEGF-A in HPV-mediated skin cancer by interbreeding an HPV8 transgenic mouse model with a conditional disruption of VEGF-A restricted to either epidermal or myeloid cells. Although only epidermal-derived VEGF-A was essential for initiation of skin tumor development, both spontaneously and UV-light triggered, both epidermal and myeloid cell-derived VEGF-A contributed to regeneration-induced tumorigenesis upon HPV8 overexpression, partly not only through a paracrine effect on endothelial cells, but also most probably through an additional autocrine effect on epidermal cells. Our findings offer new mechanistic insights into distinct functions of epidermal versus myeloid cell-derived VEGF-A during HPV-mediated tumorigenesis, with possible implications for preventing this disease.
in vivo blocking of VEGF/VEGFR-2 signaling
Lee, H. J., et al (2015). "Inhibition of vascular endothelial growth factor A and hypoxia-inducible factor 1alpha maximizes the effects of radiation in sarcoma mouse models through destruction of tumor vasculature" Int J Radiat Oncol Biol Phys 91(3):
PubMed
PURPOSE: To examine the addition of genetic or pharmacologic inhibition of hypoxia-inducible factor 1alpha (HIF-1alpha) to radiation therapy (RT) and vascular endothelial growth factor A (VEGF-A) inhibition (ie trimodality therapy) for soft-tissue sarcoma. METHODS AND MATERIALS: Hypoxia-inducible factor 1alpha was inhibited using short hairpin RNA or low metronomic doses of doxorubicin, which blocks HIF-1alpha binding to DNA. Trimodality therapy was examined in a mouse xenograft model and a genetically engineered mouse model of sarcoma, as well as in vitro in tumor endothelial cells (ECs) and 4 sarcoma cell lines. RESULTS: In both mouse models, any monotherapy or bimodality therapy resulted in tumor growth beyond 250 mm(3) within the 12-day treatment period, but trimodality therapy with RT, VEGF-A inhibition, and HIF-1alpha inhibition kept tumors at <250 mm(3) for up to 30 days. Trimodality therapy on tumors reduced HIF-1alpha activity as measured by expression of nuclear HIF-1alpha by 87% to 95% compared with RT alone, and cytoplasmic carbonic anhydrase 9 by 79% to 82%. Trimodality therapy also increased EC-specific apoptosis 2- to 4-fold more than RT alone and reduced microvessel density by 75% to 82%. When tumor ECs were treated in vitro with trimodality therapy under hypoxia, there were significant decreases in proliferation and colony formation and increases in DNA damage (as measured by Comet assay and gammaH2AX expression) and apoptosis (as measured by cleaved caspase 3 expression). Trimodality therapy had much less pronounced effects when 4 sarcoma cell lines were examined in these same assays. CONCLUSIONS: Inhibition of HIF-1alpha is highly effective when combined with RT and VEGF-A inhibition in blocking sarcoma growth by maximizing DNA damage and apoptosis in tumor ECs, leading to loss of tumor vasculature.
in vivo blocking of VEGF/VEGFR-2 signaling
Kizhatil, K., et al (2014). "Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process" PLoS Biol 12(7): e1001912.
PubMed
Schlemm’s canal (SC) plays central roles in ocular physiology. These roles depend on the molecular phenotypes of SC endothelial cells (SECs). Both the specific phenotype of SECs and development of SC remain poorly defined. To allow a modern and extensive analysis of SC and its origins, we developed a new whole-mount procedure to visualize its development in the context of surrounding tissues. We then applied genetic lineage tracing, specific-fluorescent reporter genes, immunofluorescence, high-resolution confocal microscopy, and three-dimensional (3D) rendering to study SC. Using these techniques, we show that SECs have a unique phenotype that is a blend of both blood and lymphatic endothelial cell phenotypes. By analyzing whole mounts of postnatal mouse eyes progressively to adulthood, we show that SC develops from blood vessels through a newly discovered process that we name “canalogenesis.” Functional inhibition of KDR (VEGFR2), a critical receptor in initiating angiogenesis, shows that this receptor is required during canalogenesis. Unlike angiogenesis and similar to stages of vasculogenesis, during canalogenesis tip cells divide and form branched chains prior to vessel formation. Differing from both angiogenesis and vasculogenesis, during canalogenesis SECs express Prox1, a master regulator of lymphangiogenesis and lymphatic phenotypes. Thus, SC development resembles a blend of vascular developmental programs. These advances define SC as a unique vessel with a combination of blood vascular and lymphatic phenotypes. They are important for dissecting its functions that are essential for ocular health and normal vision.
in vivo blocking of VEGF/VEGFR-2 signaling
in vitro blocking of VEGFR signaling
Larrayoz, M., et al (2014). "Contrasting responses of non-small cell lung cancer to antiangiogenic therapies depend on histological subtype" EMBO Mol Med 6(4): 539-550.
PubMed
The vascular endothelial growth factor (VEGF) pathway is a clinically validated antiangiogenic target for non-small cell lung cancer (NSCLC). However, some contradictory results have been reported on the biological effects of antiangiogenic drugs. In order to evaluate the efficacy of these drugs in NSCLC histological subtypes, we analyzed the anticancer effect of two anti-VEGFR2 therapies (sunitinib and DC101) in chemically induced mouse models and tumorgrafts of lung adenocarcinoma (ADC) and squamous cell carcinoma (SCC). Antiangiogenic treatments induced vascular trimming in both histological subtypes. In ADC tumors, vascular trimming was accompanied by tumor stabilization. In contrast, in SCC tumors, antiangiogenic therapy was associated with disease progression and induction of tumor proliferation. Moreover, in SCC, anti-VEGFR2 therapies increased the expression of stem cell markers such as aldehyde dehydrogenase 1A1, CD133, and CD15, independently of intratumoral hypoxia. In vitro studies with ADC cell lines revealed that antiangiogenic treatments reduced pAKT and pERK signaling and inhibited proliferation, while in SCC-derived cell lines the same treatments increased pAKT and pERK, and induced survival. In conclusion, this study evaluates for the first time the effect of antiangiogenic drugs in lung SCC murine models in vivo and sheds light on the contradictory results of antiangiogenic therapies in NSCLC.
in vivo blocking of VEGF/VEGFR-2 signaling
Chatterjee, S., et al (2013). "Junctional adhesion molecule-A regulates vascular endothelial growth factor receptor-2 signaling-dependent mouse corneal wound healing" PLoS One 8(5): e63674.
PubMed
Inflammation and angiogenesis are integral parts of wound healing. However, excessive and persistent wound-induced inflammation and angiogenesis in an avascular tissue such as the cornea may be associated with scarring and visual impairment. Junctional adhesion molecule A (Jam-A) is a tight junction protein that regulates leukocyte transmigration as well as fibroblast growth factor-2 (FGF-2)-induced angiogenesis. However its function in wound-induced inflammation and angiogenesis is still unknown. In this study, we report spontaneous corneal opacity in Jam-A deficient mice associated with inflammation, angiogenesis and the presence of myofibroblasts. Since wounds and/or corneal infections cause corneal opacities, we tested the role of Jam-A in wound-induced inflammation, angiogenesis and scarring by subjecting Jam-A deficient mice to full thickness corneal wounding. Analysis of these wounds demonstrated increased inflammation, angiogenesis, and increased number of myofibroblasts thereby indicating that Jam-A regulates the wound-healing response by controlling wound-induced inflammation, angiogenesis and scarring in the cornea. These effects were not due to inflammation alone since the inflammation-induced wound-healing response in Jam-A deficient mice was similar to wild type mice. In order to determine the molecular mechanism associated with the observed aberrant corneal wound healing in Jam-A deficient mice, we assessed the expression of the components of vascular endothelial growth factor A (VEGF-A)/vascular endothelial growth factor receptor- 2(VEGFR-2) signaling pathway. Interestingly, we observed increased levels of VEGF-A mRNA in Jam-A deficient eyes. We also observed nuclear localization of phosphorylated SMAD3 (pSMAD3) indicative of TGFbeta pathway activation in the Jam-A deficient eyes. Furthermore the increased wound-induced corneal inflammation, angiogenesis, and scarring in Jam-A deficient mice was attenuated by treatment with DC101, an anti-vascular endothelial growth factor receptor-2 (VEGFR-2) antibody. Our results suggest that in the absence of Jam-A, the VEGF-A/VEGFR-2 pathway is upregulated, thereby augmenting wound induced corneal inflammation, angiogenesis, and myofibroblast accumulation leading to scarring.
in vivo blocking of VEGF/VEGFR-2 signaling
Kim, Y. J., et al (2013). "Overcoming evasive resistance from vascular endothelial growth factor a inhibition in sarcomas by genetic or pharmacologic targeting of hypoxia-inducible factor 1alpha" Int J Cancer 132(1): 29-41.
PubMed
Increased levels of hypoxia and hypoxia-inducible factor 1alpha (HIF-1alpha) in human sarcomas correlate with tumor progression and radiation resistance. Prolonged antiangiogenic therapy of tumors not only delays tumor growth but may also increase hypoxia and HIF-1alpha activity. In our recent clinical trial, treatment with the vascular endothelial growth factor A (VEGF-A) antibody, bevacizumab, followed by a combination of bevacizumab and radiation led to near complete necrosis in nearly half of sarcomas. Gene Set Enrichment Analysis of microarrays from pretreatment biopsies found that the Gene Ontology category “Response to hypoxia” was upregulated in poor responders and that the hierarchical clustering based on 140 hypoxia-responsive genes reliably separated poor responders from good responders. The most commonly used chemotherapeutic drug for sarcomas, doxorubicin (Dox), was recently found to block HIF-1alpha binding to DNA at low metronomic doses. In four sarcoma cell lines, HIF-1alpha shRNA or Dox at low concentrations blocked HIF-1alpha induction of VEGF-A by 84-97% and carbonic anhydrase 9 by 83-93%. HT1080 sarcoma xenografts had increased hypoxia and/or HIF-1alpha activity with increasing tumor size and with anti-VEGF receptor antibody (DC101) treatment. Combining DC101 with HIF-1alpha shRNA or metronomic Dox had a synergistic effect in suppressing growth of HT1080 xenografts, at least in part via induction of tumor endothelial cell apoptosis. In conclusion, sarcomas respond to increased hypoxia by expressing HIF-1alpha target genes that may promote resistance to antiangiogenic and other therapies. HIF-1alpha inhibition blocks this evasive resistance and augments destruction of the tumor vasculature.
in vivo blocking of VEGF/VEGFR-2 signaling
Villalta, S. A., et al (2013). "Inhibition of VEGFR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function" Diabetes 62(8): 2870-2878.
PubMed
The dysregulation of receptor tyrosine kinases (RTKs) in multiple cell types during chronic inflammation is indicative of their pathogenic role in autoimmune diseases. Among the many RTKs, vascular endothelial growth factor receptor (VEGFR) stands out for its multiple effects on immunity, vascularization, and cell migration. Herein, we examined whether VEGFR participated in the pathogenesis of type 1 diabetes (T1D) in nonobese diabetic (NOD) mice. We found that RTK inhibitors (RTKIs) and VEGF or VEGFR-2 antibodies reversed diabetes when administered at the onset of hyperglycemia. Increased VEGF expression promoted islet vascular remodeling in NOD mice, and inhibition of VEGFR activity with RTKIs abrogated the increase in islet vascularity, impairing T-cell migration into the islet and improving glucose control. Metabolic studies confirmed that RTKIs worked by preserving islet function, as treated mice had improved glucose tolerance without affecting insulin sensitivity. Finally, examination of human pancreata from patients with T1D revealed that VEGFR-2 was confined to the islet vascularity, which was increased in inflamed islets. Collectively, this work reveals a previously unappreciated role for VEGFR-2 signaling in the pathogenesis of T1D by controlling T-cell accessibility to the pancreatic islets and highlights a novel application of VEGFR-2 antagonists for the therapeutic treatment of T1D.
in vivo blocking of VEGF/VEGFR-2 signaling
Kumar, V., et al (2010). "Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway" Blood 115(23): 4725-4733.
PubMed
Adaptive immune responses are characterized by substantial restructuring of secondary lymphoid organs. The molecular and cellular factors responsible for virus-induced lymphoid remodeling are not well known to date. Here we applied optical projection tomography, a mesoscopic imaging technique, for a global analysis of the entire 3-dimensional structure of mouse peripheral lymph nodes (PLNs), focusing on B-cell areas and high endothelial venule (HEV) networks. Structural homeostasis of PLNs was characterized by a strict correlation between total PLN volume, B-cell volume, B-cell follicle number, and HEV length. After infection with lymphocytic choriomeningitis virus, we observed a substantial, lymphotoxin (LT) beta-receptor-dependent reorganization of the PLN microarchitecture, in which an initial B-cell influx was followed by 3-fold increases in PLN volume and HEV network length on day 8 after infection. Adoptive transfer experiments revealed that virus-induced PLN and HEV network remodeling required LTalpha(1)beta(2)-expressing B cells, whereas the inhibition of vascular endothelial growth factor-A signaling pathways had no significant effect on PLN expansion. In summary, lymphocytic choriomeningitis virus-induced PLN growth depends on a vascular endothelial growth factor-A-independent, LT- and B cell-dependent morphogenic pathway, as revealed by an in-depth mesoscopic analysis of the global PLN structure.
in vivo blocking of VEGF/VEGFR-2 signaling
Kilarski, W. W., et al (2009). "Biomechanical regulation of blood vessel growth during tissue vascularization" Nat Med 15(6): 657-664.
PubMed
Formation of new vessels in granulation tissue during wound healing has been assumed to occur solely through sprouting angiogenesis. In contrast, we show here that neovascularization can be accomplished by nonangiogenic expansion of preexisting vessels. Using neovascularization models based on the chick chorioallantoic membrane and the healing mouse cornea, we found that tissue tension generated by activated fibroblasts or myofibroblasts during wound contraction mediated and directed translocation of the vasculature. These mechanical forces pulled vessels from the preexisting vascular bed as vascular loops with functional circulation that expanded as an integral part of the growing granulation tissue through vessel enlargement and elongation. Blockade of vascular endothelial growth factor receptor-2 confirmed that biomechanical forces were sufficient to mediate the initial vascular growth independently of endothelial sprouting or proliferation. The neovascular network was further remodeled by splitting, sprouting and regression of individual vessels. This model explains the rapid appearance of large functional vessels in granulation tissue during wound healing.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
VEGFR2 blockade converts thermally ablative focused ultrasound into a potent driver of T cell-dependent anti-tumor immunity
In bioRxiv on 24 October 2025 by Schwartz, M. R., Anwar, N. Z., et al.
-
-
VEGFD/VEGFR2 axis induces the dedifferentiation of high endothelial venules and impairs lymphocyte homing.
In JCI Insight on 22 July 2025 by Yang, W., Wu, J., et al.
PubMed
High endothelial venules (HEVs) are important structures in lymph nodes (LNs) that mediate lymphocyte homing, and their dedifferentiation is a necessary step before LN metastasis. Whether vascular endothelial growth factor-related (VEGF-related) signaling, which plays an important role in LN metastasis, is involved in the dedifferentiation of HEVs remains unclear. Here, we confirmed increased expression of VEGFA, VEGFC, and VEGFD; HEV dedifferentiation; and impaired lymphocyte homing function in tumor-draining LNs (TDLNs). Furthermore, we demonstrated that tumor-secreted VEGFA induced lymphangiogenesis in TDLNs to promote premetastatic niche (PMN) formation; VEGFC promoted HEV proliferation but did not affect its lymphocyte homing function. Notably, we showed that VEGFD induced the dedifferentiation of HEVs by binding to VEGFR2 on the endothelial surface of HEVs and further impaired the lymphocyte homing function of TDLNs. Overall, we revealed that tumor-secreted VEGFD interacted with VEGFR2, induced HEV dedifferentiation, and reduced lymphocyte homing, providing potential insights for the prevention and treatment of LN metastasis.
-
-
Cancer Research
Sequential treatment of anti-PD-L1 therapy prior to anti-VEGFR2 therapy contributes to more significant clinical benefits in non-small cell lung cancer.
In Neoplasia on 1 January 2025 by Lin, Q. X., Song, W. W., et al.
PubMed
Anti-angiogenic therapy and immune checkpoint blockade therapy are currently important treatments for non-small cell lung cancer. However, the combined use of the two therapies is controversial, and few studies have investigated the effects of different time sequences of the two therapies on treatment outcomes.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
FLT1 activation in cancer cells promotes PARP-inhibitor resistance in breast cancer.
In EMBO Mol Med on 1 August 2024 by Tai, Y., Chow, A., et al.
PubMed
Acquired resistance to PARP inhibitors (PARPi) remains a treatment challenge for BRCA1/2-mutant breast cancer that drastically shortens patient survival. Although several resistance mechanisms have been identified, none have been successfully targeted in the clinic. Using new PARPi-resistance models of Brca1- and Bard1-mutant breast cancer generated in-vivo, we identified FLT1 (VEGFR1) as a driver of resistance. Unlike the known role of VEGF signaling in angiogenesis, we demonstrate a novel, non-canonical role for FLT1 signaling that protects cancer cells from PARPi in-vivo through a combination of cell-intrinsic and cell-extrinsic pathways. We demonstrate that FLT1 blockade suppresses AKT activation, increases tumor infiltration of CD8+ T cells, and causes dramatic regression of PARPi-resistant breast tumors in a T-cell-dependent manner. Moreover, PARPi-resistant tumor cells can be readily re-sensitized to PARPi by targeting Flt1 either genetically (Flt1-suppression) or pharmacologically (axitinib). Importantly, a retrospective series of breast cancer patients treated with PARPi demonstrated shorter progression-free survival in cases with FLT1 activation at pre-treatment. Our study therefore identifies FLT1 as a potential therapeutic target in PARPi-resistant, BRCA1/2-mutant breast cancer.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
Macrophages preserve endothelial cell specialization in the adrenal gland to modulate aldosterone secretion and blood pressure.
In Cell Rep on 23 July 2024 by Fan, Z., Karakone, M., et al.
PubMed
Macrophages play crucial roles in organ-specific functions and homeostasis. In the adrenal gland, macrophages closely associate with sinusoidal capillaries in the aldosterone-producing zona glomerulosa. We demonstrate that macrophages preserve capillary specialization and modulate aldosterone secretion. Using macrophage-specific deletion of VEGF-A, single-cell transcriptomics, and functional phenotyping, we found that the loss of VEGF-A depletes PLVAP+ fenestrated endothelial cells in the zona glomerulosa, leading to increased basement membrane collagen IV deposition and subendothelial fibrosis. This results in increased aldosterone secretion, called "haptosecretagogue" signaling. Human aldosterone-producing adenomas also show capillary rarefaction and basement membrane thickening. Mice with myeloid cell-specific VEGF-A deletion exhibit elevated serum aldosterone, hypokalemia, and hypertension, mimicking primary aldosteronism. These findings underscore macrophage-to-endothelial cell signaling as essential for endothelial cell specialization, adrenal gland function, and blood pressure regulation, with broader implications for other endocrine organs.
-
-
-
Immunology and Microbiology
Langerhans cells regulate immunity in adulthood by regulating postnatal dermal lymphatic development
In bioRxiv on 16 July 2024 by Sim, J. H., Bell, R., et al.
-
-
-
Cancer Research
Antioxidants stimulate BACH1-dependent tumor angiogenesis.
In J Clin Invest on 16 October 2023 by Wang, T., Dong, Y., et al.
PubMed
Lung cancer progression relies on angiogenesis, which is a response to hypoxia typically coordinated by hypoxia-inducible transcription factors (HIFs), but growing evidence indicates that transcriptional programs beyond HIFs control tumor angiogenesis. Here, we show that the redox-sensitive transcription factor BTB and CNC homology 1 (BACH1) controls the transcription of a broad range of angiogenesis genes. BACH1 is stabilized by lowering ROS levels; consequently, angiogenesis gene expression in lung cancer cells, tumor organoids, and xenograft tumors increased substantially following administration of vitamins C and E and N-acetylcysteine in a BACH1-dependent fashion under normoxia. Moreover, angiogenesis gene expression increased in endogenous BACH1-overexpressing cells and decreased in BACH1-knockout cells in the absence of antioxidants. BACH1 levels also increased upon hypoxia and following administration of prolyl hydroxylase inhibitors in both HIF1A-knockout and WT cells. BACH1 was found to be a transcriptional target of HIF1α, but BACH1's ability to stimulate angiogenesis gene expression was HIF1α independent. Antioxidants increased tumor vascularity in vivo in a BACH1-dependent fashion, and overexpressing BACH1 rendered tumors sensitive to antiangiogenesis therapy. BACH1 expression in tumor sections from patients with lung cancer correlated with angiogenesis gene and protein expression. We conclude that BACH1 is an oxygen- and redox-sensitive angiogenesis transcription factor.
-
-
-
Cancer Research
-
In vivo experiments
-
Mus musculus (Mouse)
THBS1-producing tumor-infiltrating monocyte-like cells contribute to immunosuppression and metastasis in colorectal cancer.
In Nat Commun on 25 September 2023 by Omatsu, M., Nakanishi, Y., et al.
PubMed
Mesenchymal activation, characterized by dense stromal infiltration of immune and mesenchymal cells, fuels the aggressiveness of colorectal cancers (CRC), driving progression and metastasis. Targetable molecules in the tumor microenvironment (TME) need to be identified to improve the outcome in CRC patients with this aggressive phenotype. This study reports a positive link between high thrombospondin-1 (THBS1) expression and mesenchymal characteristics, immunosuppression, and unfavorable CRC prognosis. Bone marrow-derived monocyte-like cells recruited by CXCL12 are the primary source of THBS1, which contributes to the development of metastasis by inducing cytotoxic T-cell exhaustion and impairing vascularization. Furthermore, in orthotopically generated CRC models in male mice, THBS1 loss in the TME renders tumors partially sensitive to immune checkpoint inhibitors and anti-cancer drugs. Our study establishes THBS1 as a potential biomarker for identifying mesenchymal CRC and as a critical suppressor of antitumor immunity that contributes to the progression of this malignancy with a poor prognosis.
-
-
-
Cancer Research
-
Cardiovascular biology
-
Mus musculus (Mouse)
-
Immunohistochemistry
Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment.
In Nat Commun on 15 August 2023 by Cai, Q., Li, X., et al.
PubMed
The treatment of glioblastoma has limited clinical progress over the past decade, partly due to the lack of effective drug delivery strategies across the blood-brain-tumor barrier. Moreover, discrepancies between preclinical and clinical outcomes demand a reliable translational platform that can precisely recapitulate the characteristics of human glioblastoma. Here we analyze the intratumoral blood-brain-tumor barrier heterogeneity in human glioblastoma and characterize two genetically engineered models in female mice that recapitulate two important glioma phenotypes, including the diffusely infiltrative tumor margin and angiogenic core. We show that pulsed laser excitation of vascular-targeted gold nanoparticles non-invasively and reversibly modulates the blood-brain-tumor barrier permeability (optoBBTB) and enhances the delivery of paclitaxel in these two models. The treatment reduces the tumor volume by 6 and 2.4-fold and prolongs the survival by 50% and 33%, respectively. Since paclitaxel does not penetrate the blood-brain-tumor barrier and is abandoned for glioblastoma treatment following its failure in early-phase clinical trials, our results raise the possibility of reevaluating a number of potent anticancer drugs by combining them with strategies to increase blood-brain-tumor barrier permeability. Our study reveals that optoBBTB significantly improves therapeutic delivery and has the potential to facilitate future drug evaluation for cancers in the central nervous system.
-
-
-
Mus musculus (Mouse)
Mechanisms of skin vascular maturation and maintenance captured by longitudinal imaging of live mice.
In Cell on 25 May 2023 by Kam, C. Y., Singh, I. D., et al.
PubMed
A functional network of blood vessels is essential for organ growth and homeostasis, yet how the vasculature matures and maintains homeostasis remains elusive in live mice. By longitudinally tracking the same neonatal endothelial cells (ECs) over days to weeks, we found that capillary plexus expansion is driven by vessel regression to optimize network perfusion. Neonatal ECs rearrange positions to evenly distribute throughout the developing plexus and become positionally stable in adulthood. Upon local ablation, adult ECs survive through a plasmalemmal self-repair response, while neonatal ECs are predisposed to die. Furthermore, adult ECs reactivate migration to assist vessel repair. Global ablation reveals coordinated maintenance of the adult vascular architecture that allows for eventual network recovery. Lastly, neonatal remodeling and adult maintenance of the skin vascular plexus are orchestrated by temporally restricted, neonatal VEGFR2 signaling. Our work sheds light on fundamental mechanisms that underlie both vascular maturation and adult homeostasis in vivo.
-
-
-
Cancer Research
-
Immunology and Microbiology
Angiogenic inhibitor pre-administration improves the therapeutic effects of immunotherapy.
In Cancer Med on 1 April 2023 by Sato, M., Maishi, N., et al.
PubMed
In lung cancer, immune checkpoint inhibitors (ICIs) are often inadequate for tumor growth inhibition. Angiogenic inhibitors (AIs) are required to normalize tumor vasculature for improved immune cell infiltration. However, in clinical practice, ICIs and cytotoxic antineoplastic agents are simultaneously administered with an AI when tumor vessels are abnormal. Therefore, we examined the effects of pre-administering an AI for lung cancer immunotherapy in a mouse lung cancer model. Using DC101, an anti-vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody, a murine subcutaneous Lewis lung cancer (LLC) model was used to determine the timing of vascular normalization. Microvessel density (MVD), pericyte coverage, tissue hypoxia, and CD8-positive cell infiltration were analyzed. The effects of an ICI and paclitaxel after DC101 pre-administration were investigated. On Day 3, increased pericyte coverage and alleviated tumor hypoxia represented the highest vascular normalization. CD8+ T-cell infiltration was also highest on Day 3. When combined with an ICI, DC101 pre-administration significantly reduced PD-L1 expression. When combined with an ICI and paclitaxel, only DC101 pre-administration significantly inhibited tumor growth, but simultaneous administration did not. AI pre-administration, and not simultaneous administration, may increase the therapeutic effects of ICIs due to improved immune cell infiltration.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy.
In Cell Metab on 7 March 2023 by Zhang, D., Li, A. M., et al.
PubMed
The efficacy of immunotherapy is limited by the paucity of T cells delivered and infiltrated into the tumors through aberrant tumor vasculature. Here, we report that phosphoglycerate dehydrogenase (PHGDH)-mediated endothelial cell (EC) metabolism fuels the formation of a hypoxic and immune-hostile vascular microenvironment, driving glioblastoma (GBM) resistance to chimeric antigen receptor (CAR)-T cell immunotherapy. Our metabolome and transcriptome analyses of human and mouse GBM tumors identify that PHGDH expression and serine metabolism are preferentially altered in tumor ECs. Tumor microenvironmental cues induce ATF4-mediated PHGDH expression in ECs, triggering a redox-dependent mechanism that regulates endothelial glycolysis and leads to EC overgrowth. Genetic PHGDH ablation in ECs prunes over-sprouting vasculature, abrogates intratumoral hypoxia, and improves T cell infiltration into the tumors. PHGDH inhibition activates anti-tumor T cell immunity and sensitizes GBM to CAR T therapy. Thus, reprogramming endothelial metabolism by targeting PHGDH may offer a unique opportunity to improve T cell-based immunotherapy.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Mus musculus (Mouse)
“Tumor Explants Elucidate a Cascade of Paracrine SHH, WNT, and VEGF Signals Driving Pancreatic Cancer Angiosuppression”
In bioRxiv on 2 March 2023 by Hasselluhn, M. C., Decker, A. R., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Targeting tumor vasculature to improve antitumor activity of T cells armed ex vivo with T cell engaging bispecific antibody.
In J Immunother Cancer on 1 March 2023 by Park, J. A., Espinosa-Cotton, M., et al.
PubMed
Success of T cell immunotherapy hinges on the tumor microenvironment (TME), and abnormal tumor vasculature is a hallmark of most solid tumors and associated with immune evasion. The efficacy of T cell engaging bispecific antibody (BsAb) treatment relies on the successful trafficking and cytolytic activity of T cells in solid tumors. Normalization of tumor vasculature using vascular endothelial growth factor (VEGF) blockades could improve efficacy of BsAb-based T cell immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches through a feed-forward loop.
In Cancer Cell on 12 December 2022 by Hua, Y., Vella, G., et al.
PubMed
The lack of T cell infiltrates is a major obstacle to effective immunotherapy in cancer. Conversely, the formation of tumor-associated tertiary-lymphoid-like structures (TA-TLLSs), which are the local site of humoral and cellular immune responses against cancers, is associated with good prognosis, and they have recently been detected in immune checkpoint blockade (ICB)-responding patients. However, how these lymphoid aggregates develop remains poorly understood. By employing single-cell transcriptomics, endothelial fate mapping, and functional multiplex immune profiling, we demonstrate that antiangiogenic immune-modulating therapies evoke transdifferentiation of postcapillary venules into inflamed high-endothelial venules (HEVs) via lymphotoxin/lymphotoxin beta receptor (LT/LTβR) signaling. In turn, tumor HEVs boost intratumoral lymphocyte influx and foster permissive lymphocyte niches for PD1- and PD1+TCF1+ CD8 T cell progenitors that differentiate into GrzB+PD1+ CD8 T effector cells. Tumor-HEVs require continuous CD8 and NK cell-derived signals revealing that tumor HEV maintenance is actively sculpted by the adaptive immune system through a feed-forward loop.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Low-dose anti-VEGFR2 therapy promotes anti-tumor immunity in lung adenocarcinoma by down-regulating the expression of layilin on tumor-infiltrating CD8+T cells.
In Cell Oncol (Dordr) on 1 December 2022 by Yang, B., Deng, B., et al.
PubMed
Our study intended to explore how low-dose anti-angiogenic drugs affected anti-tumor immunity of tumor-infiltrating exhausted CD8+T cells and achieved better clinical response when combined with immunotherapy. We set out to find potential targets or predictive biomarker on CD8+T cells for immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity.
In Cancer Cell on 10 October 2022 by Chryplewicz, A., Scotton, J., et al.
PubMed
Glioblastoma (GBM) is poorly responsive to therapy and invariably lethal. One conceivable strategy to circumvent this intractability is to co-target distinctive mechanistic components of the disease, aiming to concomitantly disrupt multiple capabilities required for tumor progression and therapeutic resistance. We assessed this concept by combining vascular endothelial growth factor (VEGF) pathway inhibitors that remodel the tumor vasculature with the tricyclic antidepressant imipramine, which enhances autophagy in GBM cancer cells and unexpectedly reprograms immunosuppressive tumor-associated macrophages via inhibition of histamine receptor signaling to become immunostimulatory. While neither drug is efficacious as monotherapy, the combination of imipramine with VEGF pathway inhibitors orchestrates the infiltration and activation of CD8 and CD4 T cells, producing significant therapeutic benefit in several GBM mouse models. Inclusion up front of immune-checkpoint blockade with anti-programmed death-ligand 1 (PD-L1) in eventually relapsing tumors markedly extends survival benefit. The results illustrate the potential of mechanism-guided therapeutic co-targeting of disparate biological vulnerabilities in the tumor microenvironment.
-
-
-
Cancer Research
Nucleolin Therapeutic Targeting Decreases Pancreatic Cancer Immunosuppression.
In Cancers (Basel) on 31 August 2022 by Ponzo, M., Debesset, A., et al.
PubMed
Background: The pancreatic ductal adenocarcinoma (PDAC) microenvironment is highly fibrotic and hypoxic, with poor immune cell infiltration. Recently, we showed that nucleolin (NCL) inhibition normalizes tumour vessels and impairs PDAC growth. Methods: Immunocompetent mouse models of PDAC were treated by the pseudopeptide N6L, which selectively inhibits NCL. Tumour-infiltrating immune cells and changes in the tumour microenvironment were analysed. Results: N6L reduced the proportion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and increased tumour-infiltrated T lymphocytes (TILs) with an activated phenotype. Low-dose anti-VEGFR2 treatment normalized PDAC vessels but did not modulate the immune suppressive microenvironment. RNAseq analysis of N6L-treated PDAC tumours revealed a reduction of cancer-associated fibroblast (CAF) expansion in vivo and in vitro. Notably, N6L treatment decreased IL-6 levels both in tumour tissues and in serum. Treating mPDAC by an antibody blocking IL-6 reduced the proportion of Tregs and MDSCs and increased the amount of TILs, thus mimicking the effects of N6L. Conclusions: These results demonstrate that NCL inhibition blocks the amplification of lymphoid and myeloid immunosuppressive cells and promotes T cell activation in PDAC through a new mechanism of action dependent on the direct inhibition of the tumoral stroma.
-
-
-
Cancer Research
-
Immunology and Microbiology
Low-dose anti-VEGFR2 therapy promotes anti-tumor immunity in lung adenocarcinoma by down-regulating the expression of layilin on tumor-infiltrating CD8+T cells
In Research Square on 18 May 2022 by Yang, B., Deng, B., et al.
-
-
-
Cancer Research
-
Cell Biology
-
Mus musculus (Mouse)
Increased alveolar epithelial TRAF6 via autophagy-dependent TRIM37 degradation mediates particulate matter-induced lung metastasis.
In Autophagy on 1 May 2022 by Liu, J., Li, S., et al.
PubMed
Epidemiological and clinical studies have shown that exposure to particulate matter (PM) is associated with an increased incidence of lung cancer and metastasis. However, the underlying mechanism remains unclear. Here, we demonstrated the central role of PM-induced neutrophil recruitment in promoting lung cancer metastasis. We found that reactive oxygen species (ROS)-mediated alveolar epithelial macroautophagy/autophagy was essential for initiating neutrophil chemotaxis and pre-metastatic niche formation in the lungs in response to PM exposure. During PM-induced autophagy, the E3 ubiquitin ligase TRIM37 was degraded and protected TRAF6 from proteasomal degradation in lung epithelial cells, which promoted the NFKB-dependent production of chemokines to recruit neutrophils. Importantly, ROS blockade, autophagy inhibition or TRAF6 knockdown abolished PM-induced neutrophil recruitment and lung metastasis enhancement. Our study indicates that host lung epithelial cells and neutrophils coordinate to promote cancer metastasis to the lungs in response to PM exposure and provides ideal therapeutic targets for metastatic progression.Abbreviations: ACTA2/α-SMA: actin alpha 2, smooth muscle, aorta; ATII: alveolar type II; Cho-Traf6 siRNA: 5'-cholesterol-Traf6 siRNA; EMT: epithelial-mesenchymal transition; HBE: human bronchial epithelial; HCQ: hydroxychloroquine; MAPK: mitogen-activated protein kinase; NAC: N-acetyl-L-cysteine; NFKB: nuclear factor of kappa light polypeptide gene enhancer in B cells; NS: normal saline; PM: particulate matter; ROS: reactive oxygen species; TRAF6: TNF receptor-associated factor 6; TRIM37: tripartite motif-containing 37.
-