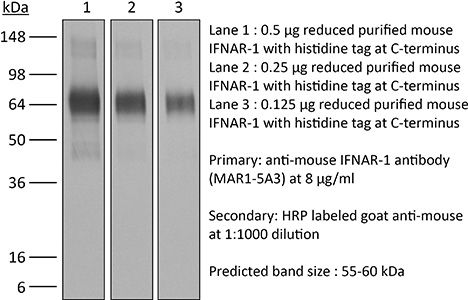
InVivoMAb anti-mouse IFNAR-1
Product Description
Bio X Cell is pleased to also offer MAR1-5A3-CP056. This monoclonal antibody is a recombinant, chimeric version of the original MAR1-5A3 antibody. The variable domain sequences are identical to clone MAR1-5A3, but the constant region has been converted from mouse IgG1 to mouse IgG2a. MAR1-5A3-CP056 also contains Fc silencing mutations rendering it unable to bind to endogenous Fcγ receptors, similar to therapeutic anti-IFNAR-1 antibodies such as Anifrolumab. These mutations prevent Fc-effector functions like antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). The highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results over hybridoma derived antibodies.
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Extracellular domain of mouse IFNAR-1 |
| Reported Applications |
in vivo IFNAR-1 blockade in vitro IFNAR-1 blockade Western blot Flow Cytometry ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687723 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro IFNAR-1 blockade
Falahat R, Berglund A, Perez-Villarroel P, Putney RM, Hamaidi I, Kim S, Pilon-Thomas S, Barber GN, Mulé JJ (2023). "Epigenetic state determines the in vivo efficacy of STING agonist therapy" Nat Commun 14(1):1573.
PubMed
While STING-activating agents have shown limited efficacy in early-phase clinical trials, multiple lines of evidence suggest the importance of tumor cell-intrinsic STING function in mediating antitumor immune responses. Although STING signaling is impaired in human melanoma, its restoration through epigenetic reprogramming can augment its antigenicity and T cell recognition. In this study, we show that reversal of methylation silencing of STING in murine melanoma cell lines using a clinically available DNA methylation inhibitor can improve agonist-induced STING activation and type-I IFN induction, which, in tumor-bearing mice, can induce tumor regression through a CD8+ T cell-dependent immune response. These findings not only provide mechanistic insight into how STING signaling dysfunction in tumor cells can contribute to impaired responses to STING agonist therapy, but also suggest that pharmacological restoration of STING signaling through epigenetic reprogramming might improve the therapeutic efficacy of STING agonists.
in vitro IFNAR-1 blockade
Hosseini S, Michaelsen-Preusse K, Grigoryan G, Chhatbar C, Kalinke U, Korte M (2020). "Type I Interferon Receptor Signaling in Astrocytes Regulates Hippocampal Synaptic Plasticity and Cognitive Function of the Healthy CNS" Cell Rep 31(7):107666.
PubMed
Type I interferon receptor (IFNAR) signaling is a hallmark of viral control and host protection. Here, we show that, in the hippocampus of healthy IFNAR-deficient mice, synapse number and synaptic plasticity, as well as spatial learning, are impaired. This is also the case for IFN-β-deficient animals. Moreover, antibody-mediated IFNAR blocking acutely interferes with neuronal plasticity, whereas a low-dose application of IFN-β has a positive effect on dendritic spine structure. Interfering with IFNAR signaling in different cell types shows a role for cognitive function and synaptic plasticity specifically mediated by astrocytes. Intriguingly, levels of the astrocytic glutamate-aspartate transporter (GLAST) are reduced significantly upon IFN-β treatment and increase following inhibition of IFNAR signaling. These results indicate that, besides the prominent role for host defense, IFNAR is important for synaptic plasticity as well as cognitive function. Astrocytes are at the center stage of this so-far-unknown signaling cascade.
in vitro IFNAR-1 blockade
Hosseini S, Michaelsen-Preusse K, Grigoryan G, Chhatbar C, Kalinke U, Korte M (2020). "Type I Interferon Receptor Signaling in Astrocytes Regulates Hippocampal Synaptic Plasticity and Cognitive Function of the Healthy CNS" Cell Rep 31(7):107666.
PubMed
Type I interferon receptor (IFNAR) signaling is a hallmark of viral control and host protection. Here, we show that, in the hippocampus of healthy IFNAR-deficient mice, synapse number and synaptic plasticity, as well as spatial learning, are impaired. This is also the case for IFN-β-deficient animals. Moreover, antibody-mediated IFNAR blocking acutely interferes with neuronal plasticity, whereas a low-dose application of IFN-β has a positive effect on dendritic spine structure. Interfering with IFNAR signaling in different cell types shows a role for cognitive function and synaptic plasticity specifically mediated by astrocytes. Intriguingly, levels of the astrocytic glutamate-aspartate transporter (GLAST) are reduced significantly upon IFN-β treatment and increase following inhibition of IFNAR signaling. These results indicate that, besides the prominent role for host defense, IFNAR is important for synaptic plasticity as well as cognitive function. Astrocytes are at the center stage of this so-far-unknown signaling cascade.
ELISA
Lomakova YD, Londregan J, Maslanka J, Goldman N, Somerville J, Riggs JE (2019). "PHA eludes macrophage suppression to activate CD8+ T cells" Immunobiology 224(1):94-101.
PubMed
Tumors may include a high proportion of immune modulatory cells and molecules that restrain the anti-cancer response. Activation of T cells to eliminate cancer cells within the immune-suppressive tumor microenvironment remains a challenge. We have shown that C57BL/6 J peritoneal cell culture models features of macrophage-dense tumors as TCR ligation fails to activate T cells unless IFNγ is neutralized or iNOS is inhibited. We tested other forms of T cell activation and found phytohemagglutinin (PHA) distinctive in the ability to markedly expand CD8 T cells in this model. IFNγ or iNOS inhibition was not necessary for this response. PHA triggered less IFNγ production and inhibitory PD-L1 expression than TCR ligation. Macrophages and CD44hi T cells bound PHA. Spleen T cell responses to PHA were markedly enhanced by the addition of peritoneal cells revealing that macrophages enhance T cell expansion. That PHA increases CD8 T cell responses within macrophage-dense culture suggests this mitogen might enhance anti-tumor immunity.
in vivo IFNAR-1 blockade
Macal, M., et al (2018). "Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection" Immunity 48(4): 730-744 e735.
PubMed
Although characterization of T cell exhaustion has unlocked powerful immunotherapies, the mechanisms sustaining adaptations of short-lived innate cells to chronic inflammatory settings remain unknown. During murine chronic viral infection, we found that concerted events in bone marrow and spleen mediated by type I interferon (IFN-I) and Toll-like receptor 7 (TLR7) maintained a pool of functionally exhausted plasmacytoid dendritic cells (pDCs). In the bone marrow, IFN-I compromised the number and the developmental capacity of pDC progenitors, which generated dysfunctional pDCs. Concurrently, exhausted pDCs in the periphery were maintained by self-renewal via IFN-I- and TLR7-induced proliferation of CD4(-) subsets. On the other hand, pDC functional loss was mediated by TLR7, leading to compromised IFN-I production and resistance to secondary infection. These findings unveil the mechanisms sustaining a self-perpetuating pool of functionally exhausted pDCs and provide a framework for deciphering long-term exhaustion of other short-lived innate cells during chronic inflammation.
Flow Cytometry
Troegeler A, Mercier I, Cougoule C, Pietretti D, Colom A, Duval C, Vu Manh TP, Capilla F, Poincloux R, Pingris K, Nigou J, Rademann J, Dalod M, Verreck FA, Al Saati T, Lugo-Villarino G, Lepenies B, Hudrisier D, Neyrolles O (2017). "C-type lectin rece
PubMed
Immune response against pathogens is a tightly regulated process that must ensure microbial control while preserving integrity of the infected organs. Tuberculosis (TB) is a paramount example of a chronic infection in which antimicrobial immunity is protective in the vast majority of infected individuals but can become detrimental if not finely tuned. Here, we report that C-type lectin dendritic cell (DC) immunoreceptor (DCIR), a key component in DC homeostasis, is required to modulate lung inflammation and bacterial burden in TB. DCIR is abundantly expressed in pulmonary lesions in Mycobacterium tuberculosis-infected nonhuman primates during both latent and active disease. In mice, we found that DCIR deficiency impairs STAT1-mediated type I IFN signaling in DCs, leading to increased production of IL-12 and increased differentiation of T lymphocytes toward Th1 during infection. As a consequence, DCIR-deficient mice control M. tuberculosis better than WT animals but also develop more inflammation characterized by an increased production of TNF and inducible NOS (iNOS) in the lungs. Altogether, our results reveal a pathway by which a C-type lectin modulates the equilibrium between infection-driven inflammation and pathogen's control through sustaining type I IFN signaling in DCs.
in vivo IFNAR-1 blockade
Liu, X., et al (2015). "CD47 blockade triggers T cell-mediated destruction of immunogenic tumors" Nat Med 21(10): 1209-1215.
PubMed
Macrophage phagocytosis of tumor cells mediated by CD47-specific blocking antibodies has been proposed to be the major effector mechanism in xenograft models. Here, using syngeneic immunocompetent mouse tumor models, we reveal that the therapeutic effects of CD47 blockade depend on dendritic cell but not macrophage cross-priming of T cell responses. The therapeutic effects of anti-CD47 antibody therapy were abrogated in T cell-deficient mice. In addition, the antitumor effects of CD47 blockade required expression of the cytosolic DNA sensor STING, but neither MyD88 nor TRIF, in CD11c(+) cells, suggesting that cytosolic sensing of DNA from tumor cells is enhanced by anti-CD47 treatment, further bridging the innate and adaptive responses. Notably, the timing of administration of standard chemotherapy markedly impacted the induction of antitumor T cell responses by CD47 blockade. Together, our findings indicate that CD47 blockade drives T cell-mediated elimination of immunogenic tumors.
in vivo IFNAR-1 blockade
Welten, S. P., et al (2015). "The viral context instructs the redundancy of costimulatory pathways in driving CD8(+) T cell expansion" Elife 4. doi : 10.7554/eLife.07486.
PubMed
Signals delivered by costimulatory molecules are implicated in driving T cell expansion. The requirements for these signals, however, vary from dispensable to essential in different infections. We examined the underlying mechanisms of this differential T cell costimulation dependence and found that the viral context determined the dependence on CD28/B7-mediated costimulation for expansion of naive and memory CD8(+) T cells, indicating that the requirement for costimulatory signals is not imprinted. Notably, related to the high-level costimulatory molecule expression induced by lymphocytic choriomeningitis virus (LCMV), CD28/B7-mediated costimulation was dispensable for accumulation of LCMV-specific CD8(+) T cells because of redundancy with the costimulatory pathways induced by TNF receptor family members (i.e., CD27, OX40, and 4-1BB). Type I IFN signaling in viral-specific CD8(+) T cells is slightly redundant with costimulatory signals. These results highlight that pathogen-specific conditions differentially and uniquely dictate the utilization of costimulatory pathways allowing shaping of effector and memory antigen-specific CD8(+) T cell responses.
in vitro IFNAR-1 blockade
Schliehe, C., et al (2015). "The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection" Nat Immunol 16(1): 67-74.
PubMed
Immune responses are tightly regulated to ensure efficient pathogen clearance while avoiding tissue damage. Here we report that Setdb2 was the only protein lysine methyltransferase induced during infection with influenza virus. Setdb2 expression depended on signaling via type I interferons, and Setdb2 repressed expression of the gene encoding the neutrophil attractant CXCL1 and other genes that are targets of the transcription factor NF-kappaB. This coincided with occupancy by Setdb2 at the Cxcl1 promoter, which in the absence of Setdb2 displayed diminished trimethylation of histone H3 Lys9 (H3K9me3). Mice with a hypomorphic gene-trap construct of Setdb2 exhibited increased infiltration of neutrophils during sterile lung inflammation and were less sensitive to bacterial superinfection after infection with influenza virus. This suggested that a Setdb2-mediated regulatory crosstalk between the type I interferons and NF-kappaB pathways represents an important mechanism for virus-induced susceptibility to bacterial superinfection.
in vivo IFNAR-1 blockade
Yang, H., et al (2015). "STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells" Cancer Res 75(18): 3812-3822.
PubMed
STAT3 is an oncogenic transcription factor with potent immunosuppressive functions. We found that pharmacologic inhibition of STAT3 or its selective knockout in cancer cells improved the tumor growth-inhibitory efficacy of anthracycline-based chemotherapies. This combined effect of STAT3 inhibition/depletion and anthracyclines was only found in tumors growing on immunocompetent (not in immunodeficient) mice. As compared with Stat3-sufficient control tumors, Stat3(-/-) cancer cells exhibited an increased infiltration by dendritic cells and cytotoxic T lymphocytes after chemotherapy. Anthracyclines are known to induce several stress pathways that enhance the immunogenicity of dying and dead cancer cells, thereby stimulating a dendritic cell-dependent and T lymphocyte-mediated anticancer immune response. Among these therapy-relevant stress pathways, Stat3(-/-) cancer cells manifested one significant improvement, namely an increase in the expression of multiple type-1 interferon-responsive genes, including that of the chemokines Cxcl9 and Cxcl10. This enhanced type-1 interferon response could be suppressed by reintroducing wild-type Stat3 (but not a transactivation-deficient mutant Stat3(Y705F)) into the tumor cells. This maneuver also abolished the improved chemotherapeutic response of Stat3(-/-) cancers. Finally, the neutralization of the common type-1 interferon receptor or that of the chemokine receptor CXCR3 (which binds CXCL9 and CXCL10) abolished the difference in the chemotherapeutic response between Stat3(-/-) and control tumors. Altogether, these results suggest that STAT3 inhibitors may improve the outcome of chemotherapy by enhancing the type-1 interferon response of cancer cells.
in vivo IFNAR-1 blockade
Beug, S. T., et al (2014). "Smac mimetics and innate immune stimuli synergize to promote tumor death" Nat Biotechnol 32(2): 182-190.
PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
in vivo IFNAR-1 blockade
Calame, D. G., et al (2014). "The C5a anaphylatoxin receptor (C5aR1) protects against Listeria monocytogenes infection by inhibiting type 1 IFN expression" J Immunol 193(10): 5099-5107.
PubMed
Listeria monocytogenes is a major cause of mortality resulting from food poisoning in the United States. In mice, C5 has been genetically linked to host resistance to listeriosis. Despite this genetic association, it remains poorly understood how C5 and its activation products, C5a and C5b, confer host protection to this Gram-positive intracellular bacterium. In this article, we show in a systemic infection model that the major receptor for C5a, C5aR1, is required for a normal robust host immune response against L. monocytogenes. In comparison with wild-type mice, C5aR1(-/-) mice had reduced survival and increased bacterial burden in their livers and spleens. Infected C5aR1(-/-) mice exhibited a dramatic reduction in all major subsets of splenocytes, which was associated with elevated caspase-3 activity and increased TUNEL staining. Because type 1 IFN has been reported to impede the host response to L. monocytogenes through the promotion of splenocyte death, we examined the effect of C5aR1 on type 1 IFN expression in vivo. Indeed, serum levels of IFN-alpha and IFN-beta were significantly elevated in L. monocytogenes-infected C5aR1(-/-) mice. Similarly, the expression of TRAIL, a type 1 IFN target gene and a proapoptotic factor, was elevated in NK cells isolated from infected C5aR1(-/-) mice. Treatment of C5aR1(-/-) mice with a type 1 IFNR blocking Ab resulted in near-complete rescue of L. monocytogenes-induced mortality. Thus, these findings reveal a critical role for C5aR1 in host defense against L. monocytogenes through the suppression of type 1 IFN expression.
in vivo IFNAR-1 blockade
Ma, Y., et al (2014). "Borrelia burgdorferi arthritis-associated locus Bbaa1 regulates Lyme arthritis and K/BxN serum transfer arthritis through intrinsic control of type I IFN production" J Immunol 193(12): 6050-6060.
PubMed
Localized upregulation of type I IFN was previously implicated in development of Borrelia burgdorferi-induced arthritis in C3H mice, and was remarkable due to its absence in the mildly arthritic C57BL/6 (B6) mice. Independently, forward genetics analysis identified a quantitative trait locus on Chr4, termed B. burgdorferi-associated locus 1 (Bbaa1), that regulates Lyme arthritis severity and includes the 15 type I IFN genes. Involvement of Bbaa1 in arthritis development was confirmed in B6 mice congenic for the C3H allele of Bbaa1 (B6.C3-Bbaa1), which developed more severe Lyme arthritis and K/BxN model of rheumatoid arthritis (RA) than did parental B6 mice. Administration of a type I IFN receptor blocking mAb reduced the severity of both Lyme arthritis and RA in B6.C3-Bbaa1 mice, formally linking genetic elements within Bbaa1 to pathological production of type I IFN. Bone marrow-derived macrophages from Bbaa1 congenic mice implicated this locus as a regulator of type I IFN induction and downstream target gene expression. Bbaa1-mediated regulation of IFN-inducible genes was upstream of IFN receptor-dependent amplification; however, the overall magnitude of the response was dependent on autocrine/paracrine responses to IFN-beta. In addition, the Bbaa1 locus modulated the functional phenotype ascribed to bone marrow-derived macrophages: the B6 allele promoted expression of M2 markers, whereas the C3H allele promoted induction of M1 responses. This report identifies a genetic locus physically and functionally linked to type I IFN that contributes to the pathogenesis of both Lyme and RA.
in vivo IFNAR-1 blockade
Stock, A. T., et al (2014). "Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection" J Exp Med 211(5): 751-759.
PubMed
Infection induces the expression of inflammatory chemokines that recruit immune cells to the site of inflammation. Whereas tissues such as the intestine and skin express unique chemokines during homeostasis, whether different tissues express distinct chemokine profiles during inflammation remains unclear. With this in mind, we performed a comprehensive screen of the chemokines expressed by two tissues (skin and sensory ganglia) infected with a common viral pathogen (herpes simplex virus type 1). After infection, the skin and ganglia showed marked differences in their expression of the family of Cxcr2 chemokine ligands. Specifically, Cxcl1/2/3, which in turn controlled neutrophil recruitment, was up-regulated in the skin but absent from the ganglia. Within the ganglia, Cxcl2 expression and subsequent neutrophil recruitment was inhibited by type I interferon (IFN). Using a combination of bone marrow chimeras and intracellular chemokine staining, we show that type I IFN acted by directly suppressing Cxcl2 expression by monocytes, abrogating their ability to recruit neutrophils to the ganglia. Overall, our findings describe a novel role for IFN in the direct, and selective, inhibition of Cxcr2 chemokine ligands, which results in the inhibition of neutrophil recruitment to neuronal tissue.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (House mouse)
Bortezomib Induces Anti-Multiple Myeloma Immune Response Mediated by cGAS/STING Pathway Activation.
In Cancer Discovery on 23 April 2021 by Gullà, A., Morelli, E., et al.
PubMed
The proteasome inhibitor bortezomib induces apoptosis in multiple myeloma cells and has transformed patient outcome. Using in vitro as well as in vivo immunodeficient and immunocompetent murine multiple myeloma models, we here show that bortezomib also triggers immunogenic cell death (ICD), characterized by exposure of calreticulin on dying multiple myeloma cells, phagocytosis of tumor cells by dendritic cells, and induction of multiple myeloma-specific immunity. We identify a bortezomib-triggered specific ICD gene signature associated with better outcome in two independent cohorts of patients with multiple myeloma. Importantly, bortezomib stimulates multiple myeloma cell immunogenicity via activation of the cGAS/STING pathway and production of type I IFNs, and STING agonists significantly potentiate bortezomib-induced ICD. Our study therefore delineates mechanisms whereby bortezomib exerts immunotherapeutic activity and provides the framework for clinical trials of STING agonists with bortezomib to induce potent tumor-specific immunity and improve patient outcome in multiple myeloma. SIGNIFICANCE: Our study demonstrates that cGAS/STING-dependent immunostimulatory activity mediates bortezomib anti-myeloma activity in experimental models and associates with clinical response to bortezomib in patients with multiple myeloma. These findings provide the rationale for clinical evaluation of STING agonists to further potentiate anti-multiple myeloma immune response.See related commentary by Zitvogel and Kroemer. ©2021 American Association for Cancer Research.
-
-
-
Immunology and Microbiology
-
Genetics
-
COVID-19
-
Cancer Research
SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade.
In Nature on 1 November 2025 by Grippin, A., Marconi, C., et al.
PubMed
Immune checkpoint inhibitors (ICIs) extend survival in many patients with cancer but are ineffective in patients without pre-existing immunity1-9. Although personalized mRNA cancer vaccines sensitize tumours to ICIs by directing immune attacks against preselected antigens, personalized vaccines are limited by complex and time-intensive manufacturing processes10-14. Here we show that mRNA vaccines targeting SARS-CoV-2 also sensitize tumours to ICIs. In preclinical models, SARS-CoV-2 mRNA vaccines led to a substantial increase in type I interferon, enabling innate immune cells to prime CD8+ T cells that target tumour-associated antigens. Concomitant ICI treatment is required for maximal efficacy in immunologically cold tumours, which respond by increasing PD-L1 expression. Similar correlates of vaccination response are found in humans, including increases in type I interferon, myeloid-lymphoid activation in healthy volunteers and PD-L1 expression on tumours. Moreover, receipt of SARS-CoV-2 mRNA vaccines within 100 days of initiating ICI is associated with significantly improved median and three-year overall survival in multiple large retrospective cohorts. This benefit is similar among patients with immunologically cold tumours. Together, these results demonstrate that clinically available mRNA vaccines targeting non-tumour-related antigens are potent immune modulators capable of sensitizing tumours to ICIs.
-
-
-
Immunology and Microbiology
Condensate nanovaccine adjuvants augment CD8+ T-Cell-dependent antitumor immunity through mtDNA leakage-triggered cGAS-STING axis activation.
In Signal Transduct Target Ther on 21 October 2025 by Tang, Y., Luo, Z., et al.
PubMed
The variety and functionality of current clinical vaccine adjuvants remain limited. Conventional aluminum-based adjuvants predominantly induce Th2-biased humoral immunity but exhibit a limited capacity to elicit Th1-mediated cellular immune responses, particularly tumor antigen-specific cytotoxic CD8+ T lymphocytes (CTLs), which are essential for effective cancer vaccine performance. Inspired by natural biomolecular condensates, we developed a versatile noncovalent protein self-assembly strategy distinct from traditional approaches requiring structural domain modifications or bifunctional crosslinkers. Our methodology employs amphiphilic molecules (sodium myristate/SMA and sodium dodecyl thiolate/SDT) as molecular bridges to mediate protein‒protein interactions through hydrophobic forces and disulfide bond formation. This process generates nanoscale protein condensate (PCD) vaccines with exceptional stability. As a novel adjuvant system, these synthetic condensates significantly enhance antigen cross-presentation by optimizing key parameters: antigen loading capacity, lymph node targeting, cytosolic delivery, and lysosomal escape. Consequently, they induce robust antigen-specific CTL responses and humoral immunity, demonstrating potent antitumor efficacy. Importantly, we found that the synthetic protein condensate (PCD) alone can act as a nanoadjuvant. By increasing mitochondrial membrane permeability, PCD induces mitochondrial DNA leakage into the cytosol, activating the cGAS‒STING pathway and promoting DC maturation. This safe and scalable platform eliminates the need for complex covalent modifications or genetic engineering, and it facilitates the design of diverse modular antigens, including neoantigens and viral antigens. Given its straightforward manufacturing process and superior immunogenicity, this synthetic PCD vaccine adjuvant has significant potential for clinical application and translation.
-
-
-
Immunology and Microbiology
EP300 compromises antitumor immunity by increasing SOCS1 expression.
In J Immunother Cancer on 15 October 2025 by Zeng, Y., Zhou, Y., et al.
PubMed
Beyond supporting cancer cell proliferation, tumor growth relies on the ability of cancer cells to evade immune surveillance. Identifying novel molecules that promote tumor immune escape may help develop more effective immunotherapeutic strategies. The histone acetyltransferase E1A-binding protein p300 (EP300) is a key epigenetic regulator that modulates gene transcription through chromatin remodeling and acetylation of histones and transcription factors. However, its role in regulating immune evasion remains incompletely understood. This study investigates the impact of EP300 on tumor immune escape and suggests its potential as an immunotherapeutic target.
-
-
Macrophages orchestrate elimination of Shigella from the intestinal epithelial cell niche via TLR-induced IL-12 and IFN-γ.
In Cell Host Microbe on 10 September 2025 by Eislmayr, K. D., Nichols, C. A., et al.
PubMed
Bacteria of the genus Shigella replicate in intestinal epithelial cells and cause shigellosis, a severe diarrheal disease that resolves spontaneously in most healthy individuals. During shigellosis, neutrophils are abundantly recruited to the gut and have long been thought to be central to Shigella control and pathogenesis. However, how shigellosis resolves remains poorly understood due to the longstanding lack of a tractable and physiological animal model. Here, using our newly developed Nlrc4-/-Casp11-/- mouse model of shigellosis, we unexpectedly find no major role for neutrophils in limiting Shigella or in disease pathogenesis. Instead, we uncover an essential role for macrophages in the host control of Shigella. Macrophages respond to Shigella via Toll-like receptors (TLRs) to produce IL-12, which then induces IFN-γ, a cytokine that is essential to control Shigella replication in intestinal epithelial cells. Collectively, our findings reshape our understanding of the innate immune response to Shigella.
-
-
Immunology and Microbiology
Oropouche virus disrupts neurodevelopment and is vertically transmitted
In Research Square on 9 September 2025 by Jurado, K., Bannerman, C., et al.
-
-
-
Immunology and Microbiology
Engineered bacteria launch and control an oncolytic virus.
In Nat Biomed Eng on 15 August 2025 by Singer, Z. S., Pabon, J., et al.
PubMed
The ability of bacteria and viruses to selectively replicate in tumours has led to synthetic engineering of new microbial therapies. Here we design a cooperative strategy whereby Salmonella typhimurium bacteria transcribe and deliver the Senecavirus A RNA genome inside host cells, launching a potent oncolytic viral infection. 'Encapsidated' by bacteria, the viral genome can further bypass circulating antiviral antibodies to reach the tumour and initiate replication and spread within immune mice. Finally, we engineer the virus to require a bacterially delivered protease to achieve virion maturation, demonstrating bacterial control over the virus. Together, we refer to this platform as 'CAPPSID' for Coordinated Activity of Prokaryote and Picornavirus for Safe Intracellular Delivery. This work extends bacterially delivered therapeutics to viral genomes, and shows how a consortium of microbes can achieve a cooperative aim.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Metformin as antiviral therapy protects hyperglycemic and diabetic patients.
In MBio on 11 June 2025 by Wang, X., Zheng, X., et al.
PubMed
Viral infections disrupt glucose metabolism; however, their impact on disease prognosis in highly pathogenic viruses remains largely unknown. There is an additional need to investigate the antiviral mechanisms of glucose-lowering therapeutics. Here, our multicenter clinical study shows that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients infected with severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging and highly pathogenic bunyavirus. SFTSV infection triggers gluconeogenesis, which, in turn, inhibits AMPK activity and subsequent interferon I (IFN-I) responses, thereby facilitating viral replication. In vitro and animal studies further reveal that metformin inhibits SFTSV replication by suppressing autophagy through the AMPK-mTOR pathway, contributing to protection against lethal SFTSV infection in mice. Importantly, our large cohort study demonstrates that metformin reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, contrasting with the disadvantageous effect of insulin. These findings highlight the promising therapeutic potential of metformin in treating viral infections, particularly among individuals with hyperglycemia or diabetes.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Metformin as antiviral therapy protects hyperglycemic and diabetic patients.
In MBio on 11 June 2025 by Wang, X., Zheng, X., et al.
PubMed
Viral infections disrupt glucose metabolism; however, their impact on disease prognosis in highly pathogenic viruses remains largely unknown. There is an additional need to investigate the antiviral mechanisms of glucose-lowering therapeutics. Here, our multicenter clinical study shows that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients infected with severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging and highly pathogenic bunyavirus. SFTSV infection triggers gluconeogenesis, which, in turn, inhibits AMPK activity and subsequent interferon I (IFN-I) responses, thereby facilitating viral replication. In vitro and animal studies further reveal that metformin inhibits SFTSV replication by suppressing autophagy through the AMPK-mTOR pathway, contributing to protection against lethal SFTSV infection in mice. Importantly, our large cohort study demonstrates that metformin reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, contrasting with the disadvantageous effect of insulin. These findings highlight the promising therapeutic potential of metformin in treating viral infections, particularly among individuals with hyperglycemia or diabetes.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Metformin as antiviral therapy protects hyperglycemic and diabetic patients.
In MBio on 11 June 2025 by Wang, X., Zheng, X., et al.
PubMed
Viral infections disrupt glucose metabolism; however, their impact on disease prognosis in highly pathogenic viruses remains largely unknown. There is an additional need to investigate the antiviral mechanisms of glucose-lowering therapeutics. Here, our multicenter clinical study shows that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients infected with severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging and highly pathogenic bunyavirus. SFTSV infection triggers gluconeogenesis, which, in turn, inhibits AMPK activity and subsequent interferon I (IFN-I) responses, thereby facilitating viral replication. In vitro and animal studies further reveal that metformin inhibits SFTSV replication by suppressing autophagy through the AMPK-mTOR pathway, contributing to protection against lethal SFTSV infection in mice. Importantly, our large cohort study demonstrates that metformin reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, contrasting with the disadvantageous effect of insulin. These findings highlight the promising therapeutic potential of metformin in treating viral infections, particularly among individuals with hyperglycemia or diabetes.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Metformin as antiviral therapy protects hyperglycemic and diabetic patients.
In MBio on 11 June 2025 by Wang, X., Zheng, X., et al.
PubMed
Viral infections disrupt glucose metabolism; however, their impact on disease prognosis in highly pathogenic viruses remains largely unknown. There is an additional need to investigate the antiviral mechanisms of glucose-lowering therapeutics. Here, our multicenter clinical study shows that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients infected with severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging and highly pathogenic bunyavirus. SFTSV infection triggers gluconeogenesis, which, in turn, inhibits AMPK activity and subsequent interferon I (IFN-I) responses, thereby facilitating viral replication. In vitro and animal studies further reveal that metformin inhibits SFTSV replication by suppressing autophagy through the AMPK-mTOR pathway, contributing to protection against lethal SFTSV infection in mice. Importantly, our large cohort study demonstrates that metformin reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, contrasting with the disadvantageous effect of insulin. These findings highlight the promising therapeutic potential of metformin in treating viral infections, particularly among individuals with hyperglycemia or diabetes.
-
-
-
Immunology and Microbiology
Viral hijacking of host DDX60 promotes Crimean-Congo haemorrhagic fever virus replication via G-quadruplex unwinding.
In PLoS Pathog on 1 June 2025 by Sui, Y., Xu, Q., et al.
PubMed
Crimean-Congo haemorrhagic fever virus (CCHFV) is the most prevalent tick-borne zoonotic bunyavirus, causing severe hemorrhagic fever and fatality in humans. Currently, the absence of approved vaccines or therapeutics for CCHFV infection necessitates the development of innovative therapeutic strategies. Here, we identify a guanine (G)-rich sequence located within the mRNA of the glycoprotein precursor in the medium (M) segment of the CCHFV genome, designated as M-PQS-1664(+). M-PQS-1664(+) can form stable G-quadruplex (G4) structure and functions as a negative regulatory element for viral replication. Host DDX60 is up-regulated in response to CCHFV infection, thereby it is hijacked to unwind M-PQS-1664(+) G4 for facilitating viral replication. The FDA-approved drug Cepharanthine (CEP), which competes with DDX60 to specifically stabilize M-PQS-1664(+) G4 without a global induction of host cellular G4s formation, exhibits remarkable antiviral activity in vitro and in vivo. More importantly, CEP possesses antiviral activity (50% inhibitory concentration ~ 0.2 μM) that having ~ 88 × the potency of ribavirin. Our findings underscore the CCHFV G4s as a promising target for drug development and highlight the significant potential of CEP in combating CCHFV.
-
-
-
Immunology and Microbiology
-
COVID-19
Toll-like receptor 7 (TLR7)-mediated antiviral response protects mice from lethal SARS-CoV-2 infection.
In J Virol on 20 May 2025 by Ghimire, R., Shrestha, R., et al.
PubMed
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced impaired antiviral immunity and excessive inflammatory responses cause lethal pneumonia. However, the in vivo roles of key pattern recognition receptors that elicit protective antiviral and fatal inflammatory responses, specifically in the lungs, are not well described. Coronaviruses possess single-stranded RNA genome that activates TLR7/8 to induce an antiviral interferon (IFN) and robust inflammatory cytokine response. Here, using wild-type and TLR7-deficient (TLR7-/-) mice infected with mouse-adapted SARS-CoV-2 (MA-CoV-2), we examined the role of TLR7 in the lung antiviral and inflammatory response and severe pneumonia. We showed that TLR7 deficiency significantly increased lung virus loads and morbidity/mortality, which correlated with reduced levels of type I IFNs (Ifna/b), type III IFNs (Ifnl), and IFN-stimulated genes (ISGs) in the lungs. A detailed evaluation of MA-CoV-2-infected lungs revealed increased neutrophil accumulation and lung pathology in TLR7-/- mice. We further showed that blocking type I IFN receptor (IFNAR) signaling enhanced SARS-CoV-2 replication in the lungs and caused severe lung pathology, leading to 100% mortality compared to infected control mice. Moreover, immunohistochemical assessment of the lungs revealed increased numbers of SARS-CoV-2 antigen-positive macrophages, pneumocytes, and bronchial epithelial cells in TLR7-/- and IFNAR-deficient mice compared to control mice. In summary, we conclusively demonstrated that despite TLR7-induced robust lung inflammation, TLR7-induced IFN/ISG responses suppress lung virus replication and pathology and provide protection against SARS-CoV-2-induced fatal pneumonia. Additionally, given the similar disease outcomes in control, TLR7-/-, and IFNAR-deficient MA-CoV-2-infected mice and coronavirus disease 2019 (COVID-19) patients, we propose that MA-CoV-2-infected mice constitute an excellent model for studying COVID-19.IMPORTANCESevere coronavirus disease 2019 (COVID-19) is caused by a delicate balance between a strong antiviral and an exuberant inflammatory response. A robust antiviral immunity and regulated inflammation are protective, while a weak antiviral response and excessive inflammation are detrimental. However, the key host immune sensors that elicit protective antiviral and inflammatory responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) challenge are poorly defined. Here, we examined the role of viral RNA-mediated TLR7 activation in the lung antiviral and inflammatory responses in SARS-CoV-2-infected mice. We demonstrate that TLR7 deficiency led to a high rate of morbidity and mortality, which correlated with an impaired antiviral interferon (IFN)-I/III response, enhanced lung virus replication, and severe lung pathology. Furthermore, we show that blocking IFN-I signaling using anti-IFN receptor antibody promoted SARS-CoV-2 replication in the lungs and caused severe disease. These results provide conclusive evidence that TLR7 and IFN-I receptor deficiencies lead to severe disease in mice, replicating clinical features observed in COVID-19 patients.
-
-
-
Flow cytometry/Cell sorting
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
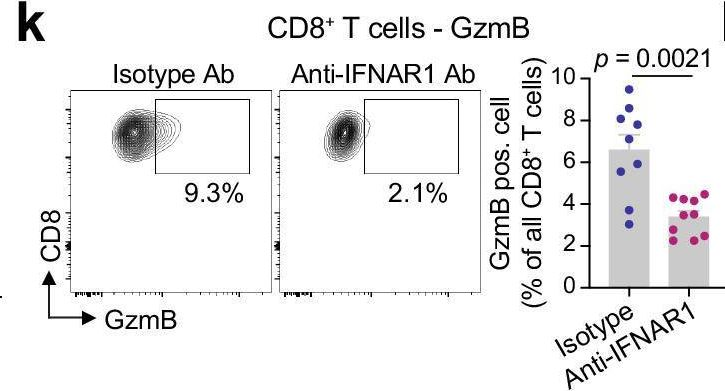
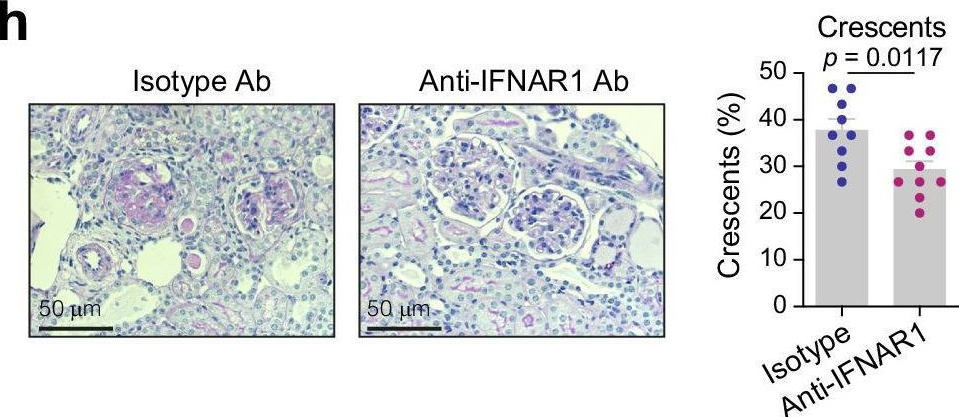
Type I interferon drives T cell cytotoxicity by upregulation of interferon regulatory factor 7 in autoimmune kidney diseases in mice.
In Nat Commun on 20 May 2025 by Wang, H., Engesser, J., et al.
PubMed
In anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) and systemic lupus erythematosus (SLE), glomerulonephritis is a severe kidney complication driven by immune cells, including T cells. However, the mechanisms underlying T cell activation in these contexts remain elusive. Here we report that in patients with AAV and SLE, type I interferon (IFN-I) induces T cell differentiation into interferon-stimulated genes-expressing T (ISG-T) cells, which are characterized by an elevated IFN-I signature, an immature phenotype, and cytotoxicity in inflamed tissue. Mechanistically, IFN-I stimulates the expression of interferon regulatory factor 7 (IRF7) in T cells, which in turn induces granzyme B production. In mice, blocking IFN-I signaling reduces IRF7 and granzyme B expression in T cells, thus ameliorating glomerulonephritis. In parallel, spatial transcriptomic analyses of kidney biopsies from patients with AAV or SLE reveal an elevated ISG signature and the presence of ISG-T cells in close proximity to plasmacytoid dendritic cells, the primary producers of IFN-I. Our results from both patients and animal models thus suggest that IFN-I production in inflamed tissue may drive ISG-T cell differentiation to expand the pool of cytotoxic T cells in autoimmune diseases.
-
-
-
Cell Biology
-
Immunology and Microbiology
Caspase-11 drives macrophage hyperinflammation in models of Polg-related mitochondrial disease.
In Nat Commun on 20 May 2025 by VanPortfliet, J. J., Lei, Y., et al.
PubMed
Mitochondrial diseases (MtD) represent a significant public health challenge due to their heterogenous clinical presentation, often severe and progressive symptoms, and lack of effective therapies. Environmental exposures, such bacterial and viral infection, can further compromise mitochondrial function and exacerbate the progression of MtD. However, the underlying immune alterations that enhance immunopathology in MtD remain unclear. Here we employ in vitro and in vivo approaches to clarify the molecular and cellular basis for innate immune hyperactivity in models of polymerase gamma (Polg)-related MtD. We reveal that type I interferon (IFN-I)-mediated upregulation of caspase-11 and guanylate-binding proteins (GBP) increase macrophage sensing of the opportunistic microbe Pseudomonas aeruginosa (PA) in Polg mutant mice. Furthermore, we show that excessive cytokine secretion and activation of pyroptotic cell death pathways contribute to lung inflammation and morbidity after infection with PA. Our work provides a mechanistic framework for understanding innate immune dysregulation in MtD and reveals potential targets for limiting infection- and inflammation-related complications in Polg-related MtD.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Genetics
-
Immunology and Microbiology
A bivalent self-amplifying RNA vaccine against yellow fever and Zika viruses.
In Front Immunol on 14 May 2025 by Battisti, P., Ykema, M. R., et al.
PubMed
Yellow fever (YFV) and Zika (ZIKV) viruses cause significant morbidity and mortality, despite the existence of an approved YFV vaccine and the development of multiple ZIKV vaccine candidates to date. New technologies may improve access to vaccines against these pathogens. We previously described a nanostructured lipid carrier (NLC)-delivered self-amplifying RNA (saRNA) vaccine platform with excellent thermostability and immunogenicity, appropriate for prevention of tropical infectious diseases.
-
-
-
Immunology and Microbiology
Lymphotropic Virotherapy Engages DC and High Endothelial Venule Inflammation to Mediate CancerIn SituVaccination
In medRxiv on 25 April 2025 by Ludwig, A. L., McKay, Z. P., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
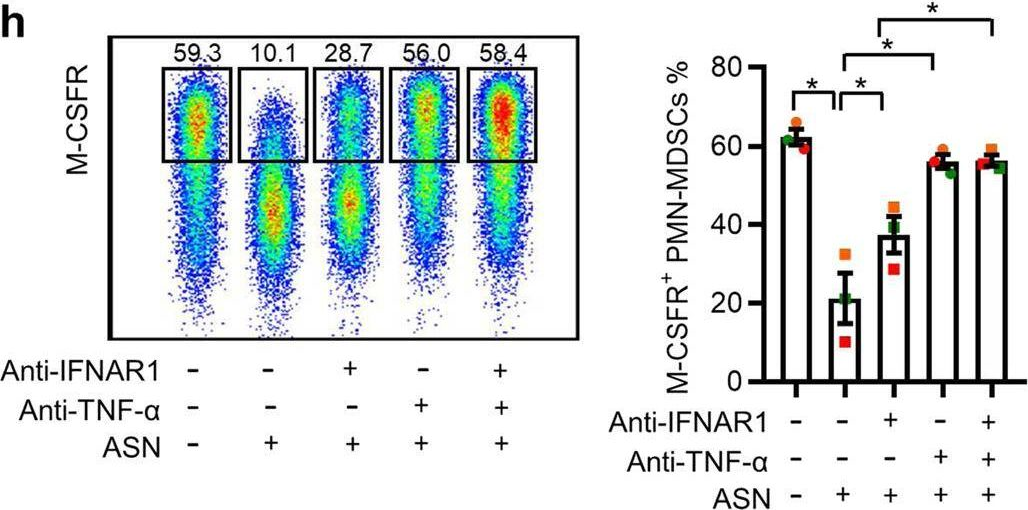
Asparagine drives immune evasion in bladder cancer via RIG-I stability and type I IFN signaling.
In J Clin Invest on 15 April 2025 by Wei, W., Li, H., et al.
PubMed
Tumor cells often employ many ways to restrain type I IFN signaling to evade immune surveillance. However, whether cellular amino acid metabolism regulates this process remains unclear, and its effects on antitumor immunity are relatively unexplored. Here, we found that asparagine inhibited IFN-I signaling and promoted immune escape in bladder cancer. Depletion of asparagine synthetase (ASNS) strongly limited in vivo tumor growth in a CD8+ T cell-dependent manner and boosted immunotherapy efficacy. Moreover, clinically approved L-asparaginase (ASNase),synergized with anti-PD-1 therapy in suppressing tumor growth. Mechanistically, asparagine can directly bind to RIG-I and facilitate CBL-mediated RIG-I degradation, thereby suppressing IFN signaling and antitumor immune responses. Clinically, tumors with higher ASNS expression show decreased responsiveness to immune checkpoint inhibitor therapy. Together, our findings uncover asparagine as a natural metabolite to modulate RIG-I-mediated IFN-I signaling, providing the basis for developing the combinatorial use of ASNase and anti-PD-1 for bladder cancer.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Metabolic deficiencies underlie reduced plasmacytoid dendritic cell IFN-I production following viral infection.
In Nat Commun on 7 February 2025 by Greene, T. T., Jo, Y., et al.
PubMed
Type I Interferons (IFN-I) are central to host protection against viral infections, with plasmacytoid dendritic cells (pDC) being the most significant source, yet pDCs lose their IFN-I production capacity following an initial burst of IFN-I, resulting in susceptibility to secondary infections. The underlying mechanisms of these dynamics are not well understood. Here we find that viral infection reduces the capacity of pDCs to engage both oxidative and glycolytic metabolism. Mechanistically, we identify lactate dehydrogenase B (LDHB) as a positive regulator of pDC IFN-I production in mice and humans; meanwhile, LDHB deficiency is associated with suppressed IFN-I production, pDC metabolic capacity, and viral control following infection. In addition, preservation of LDHB expression is sufficient to partially retain the function of otherwise exhausted pDCs, both in vitro and in vivo. Furthermore, restoring LDHB in vivo in pDCs from infected mice increases IFNAR-dependent, infection-associated pathology. Our work thus identifies a mechanism for balancing immunity and pathology during viral infections, while also providing insight into the highly preserved infection-driven pDC inhibition.
-
-
-
Genetics
-
Immunology and Microbiology
A bivalent self-amplifying RNA vaccine against yellow fever and Zika viruses
In bioRxiv on 4 February 2025 by Battisti, P., Ykema, M. R., et al.
-