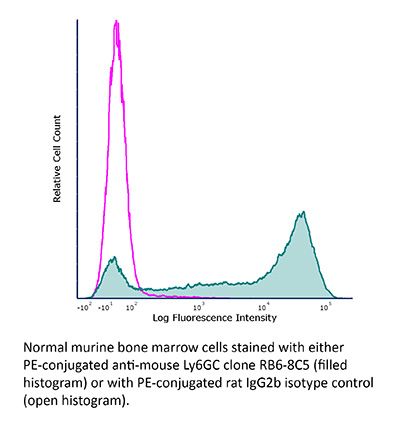
FlowMAb PE anti-mouse Ly6G/Ly6C (Gr-1)
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | FlowMAb PE rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Conjugation | PE |
| Excitation Source | Yellow-Green 488 nm, 532 nm, 561 nm |
| Excitation Max | 496 nm, 566 nm |
| Emission Max | 576 nm |
| Immunogen | Mouse granulocytes |
| Reported Applications |
Flow cytometry Immunohistochemistry (paraffin) Immunohistochemistry (frozen) |
| Protocol Information | It is recommended that the reagent be carefully titrated for optimal performance in the assay of interest. |
| Concentration | 0.2 mg/ml |
| Formulation |
PBS, pH 7.0 Contains 0.09% Sodium Azide |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G. Conjugated with R-phycoerythrin under optimal conditions. |
| Storage | The antibody solution should be stored at the stock concentration at 4°C and protected from prolonged exposure to light. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
Wang, H., et al. (2015). "P2RX7 sensitizes Mac-1/ICAM-1-dependent leukocyte-endothelial adhesion and promotes neurovascular injury during septic encephalopathy" Cell Res 25(6): 674-690.
PubMed
Septic encephalopathy (SE) is a critical factor determining sepsis mortality. Vascular inflammation is known to be involved in SE, but the molecular events that lead to the development of encephalopathy remain unclear. Using time-lapse in vivo two-photon laser scanning microscopy, we provide the first direct evidence that cecal ligation and puncture in septic mice induces microglial trafficking to sites adjacent to leukocyte adhesion on inflamed cerebral microvessels. Our data further demonstrate that septic injury increased the chemokine CXCL1 level in brain endothelial cells by activating endothelial P2RX7 and eventually enhanced the binding of Mac-1 (CD11b/CD18)-expressing leukocytes to endothelial ICAM-1. In turn, leukocyte adhesion upregulated endothelial CX3CL1, thereby triggering microglia trafficking to the injured site. The sepsis-induced increase in endothelial CX3CL1 was abolished in CD18 hypomorphic mutant mice. Inhibition of the P2RX7 pathway not only decreased endothelial ICAM-1 expression and leukocyte adhesion but also prevented microglia overactivation, reduced brain injury, and consequently doubled the early survival of septic mice. These results demonstrate the role of the P2RX7 pathway in linking neurovascular inflammation to brain damage in vivo and provide a rationale for targeting endothelial P2RX7 for neurovascular protection during SE.
Flow Cytometry
Bodogai, M., et al. (2015). "Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells" Cancer Res 75(17): 3456-3465.
PubMed
Myeloid-derived suppressive cells (MDSC) have been reported to promote metastasis, but the loss of cancer-induced B cells/B regulatory cells (tBreg) can block metastasis despite MDSC expansion in cancer. Here, using multiple murine tumor models and human MDSC, we show that MDSC populations that expand in cancer have only partially primed regulatory function and limited prometastatic activity unless they are fully educated by tBregs. Cancer-induced tBregs directly activate the regulatory function of both the monocyte and granulocyte subpopulations of MDSC, relying, in part, on TgfbetaR1/TgfbetaR2 signaling. MDSC fully educated in this manner exhibit an increased production of reactive oxygen species and NO and more efficiently suppress CD4(+) and CD8(+) T cells, thereby promoting tumor growth and metastasis. Thus, loss of tBregs or TgfbetaR deficiency in MDSC is sufficient to disable their suppressive function and to block metastasis. Overall, our data indicate that cancer-induced B cells/B regulatory cells are important regulators of the immunosuppressive and prometastatic functions of MDSC.
Flow Cytometry
Bryant, J., et al. (2014). "Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection" J Immunol 192(12): 6092-6101.
PubMed
We have previously shown that preemptive infusion of apoptotic donor splenocytes treated with the chemical cross-linker ethylcarbodiimide (ECDI-SPs) induces long-term allograft survival in full MHC-mismatched models of allogeneic islet and cardiac transplantation. The role of myeloid-derived suppressor cells (MDSCs) in the graft protection provided by ECDI-SPs is unclear. In this study, we demonstrate that infusions of ECDI-SPs increase two populations of CD11b(+) cells in the spleen that phenotypically resemble monocytic-like (CD11b(+)Ly6C(high)) and granulocytic-like (CD11b(+)Gr1(high)) MDSCs. Both populations suppress T cell proliferation in vitro and traffic to the cardiac allografts in vivo to mediate their protection via inhibition of local CD8 T cell accumulation and potentially also via induction and homing of regulatory T cells. Importantly, repeated treatments with ECDI-SPs induce the CD11b(+)Gr1(high) cells to produce a high level of IFN-gamma and to exhibit an enhanced responsiveness to IFN-gamma by expressing higher levels of downstream effector molecules ido and nos2. Consequently, neutralization of IFN-gamma completely abolishes the suppressive capacity of this population. We conclude that donor ECDI-SPs induce the expansion of two populations of MDSCs important for allograft protection mediated in part by intrinsic IFN-gamma-dependent mechanisms. This form of preemptive donor apoptotic cell infusions has significant potential for the therapeutic manipulation of MDSCs for transplant tolerance induction.
Flow Cytometry
Khmaladze, I., et al. (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
Flow Cytometry
Schulze, F. S., et al. (2014). "Fcgamma receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid" Am J Pathol 184(8): 2185-2196.
PubMed
Bullous pemphigoid (BP) and epidermolysis bullosa acquisita are subepidermal autoimmune blistering diseases mediated by autoantibodies against type XVII collagen (Col17) and Col7, respectively. For blister formation, Fc-mediated events, such as infiltration of inflammatory cells in the skin, complement activation, and release of proteases at the dermal-epidermal junction, are essential. Although in the neonatal passive transfer mouse model of BP, tissue destruction is mediated by Fcgamma receptors (FcgammaRs) I and III, the passive transfer model of epidermolysis bullosa acquisita completely depends on FcgammaRIV. To clarify this discrepancy, we developed a novel experimental model for BP using adult mice. Lesion formation was Fc mediated because gamma-chain-deficient mice and mice treated with anti-Col17 IgG, depleted from its sugar moiety at the Fc portion, were resistant to disease induction. By the use of various FcgammaR-deficient mouse strains, tissue destruction was shown to be mediated by FcgammaRIV, FcgammaRIII, and FcgammaRIIB, whereas FcgammaRI was not essential. Furthermore, anti-inflammatory mediators in already clinically diseased mice can be explored in the novel BP model, because the pharmacological inhibition of FcgammaRIV and depletion of granulocytes abolished skin blisters. Herein, we extended our knowledge about the importance of FcgammaRs in experimental BP and established a novel BP mouse model suitable to study disease development over a longer time period and explore novel treatment strategies in a quasi-therapeutic setting.
Flow Cytometry
van der Merwe, M., et al. (2013). "Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplantation" J Immunol 191(11): 5764-5776.
PubMed
We showed previously that nonmyeloablative total lymphoid irradiation/rabbit anti-thymocyte serum (TLI/ATS) conditioning facilitates potent donor-recipient immune tolerance following bone marrow transplantation (BMT) across MHC barriers via recipient invariant NKT (iNKT) cell-derived IL-4-dependent expansion of donor Foxp3(+) naturally occurring regulatory T cells (nTregs). In this study, we report a more specific mechanism. Wild-type (WT) BALB/c (H-2(d)) hosts were administered TLI/ATS and BMT from WT or STAT6(-/-) C57BL/6 (H-2(b)) donors. Following STAT6(-/-) BMT, donor nTregs demonstrated no loss of proliferation in vivo, indicating that an IL-4-responsive population in the recipient, rather than the donor, drives donor nTreg proliferation. In graft-versus-host disease (GVHD) target organs, three recipient CD11b(+) cell subsets (Gr-1(high)CD11c(-), Gr-1(int)CD11c(-), and Gr-1(low)CD11c(+)) were enriched early after TLI/ATS + BMT versus total body irradiation/ATS + BMT. Gr-1(low)CD11c(+) cells induced potent H-2K(b+)CD4(+)Foxp3(+) nTreg proliferation in vitro in 72-h MLRs. Gr-1(low)CD11c(+) cells were reduced significantly in STAT6(-/-) and iNKT cell-deficient Jalpha18(-/-) BALB/c recipients after TLI/ATS + BMT. Depletion of CD11b(+) cells resulted in severe acute GVHD, and adoptive transfer of WT Gr-1(low)CD11c(+) cells to Jalpha18(-/-) BALB/c recipients of TLI/ATS + BMT restored day-6 donor Foxp3(+) nTreg proliferation and protection from CD8 effector T cell-mediated GVHD. Blockade of programmed death ligand 1 and 2, but not CD40, TGF-beta signaling, arginase 1, or iNOS, inhibited nTreg proliferation in cocultures of recipient-derived Gr-1(low)CD11c(+) cells with donor nTregs. Through iNKT-dependent Th2 polarization, myeloid-derived immunomodulatory dendritic cells are expanded after nonmyeloablative TLI/ATS conditioning and allogeneic BMT, induce PD-1 ligand-dependent donor nTreg proliferation, and maintain potent graft-versus-host immune tolerance.
Flow Cytometry
Norris, B. A., et al. (2013). "Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity" Immunity 38(2): 309-321.
PubMed
Resolution of acute and chronic viral infections requires activation of innate cells to initiate and maintain adaptive immune responses. Here we report that infection with acute Armstrong (ARM) or chronic Clone 13 (C13) strains of lymphocytic choriomeningitis virus (LCMV) led to two distinct phases of innate immune response. During the first 72 hr of infection, dendritic cells upregulated activation markers and stimulated antiviral CD8(+) T cells, independent of viral strain. Seven days after infection, there was an increase in Ly6C(hi) monocytic and Gr-1(hi) neutrophilic cells in lymphoid organs and blood. This expansion in cell numbers was enhanced and sustained in C13 infection, whereas it occurred only transiently with ARM infection. These cells resembled myeloid-derived suppressor cells and potently suppressed T cell proliferation. The reduction of monocytic cells in Ccr2(-/-) mice or after Gr-1 antibody depletion enhanced antiviral T cell function. Thus, innate cells have an important immunomodulatory role throughout chronic infection.
Immunohistochemistry (paraffin)
Li, M., et al. (2006). "Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis" Proc Natl Acad Sci U S A 103(31): 11736-11741.
PubMed
We have demonstrated that cytokine thymic stromal lymphopoietin (TSLP), whose expression is rapidly induced upon keratinocyte-selective ablation of retinoid X receptors (RXRs) -alpha and -beta in the mouse (RXRalphabeta(ep-/-) mice), plays a key role in initiating a skin and systemic atopic dermatitis-like phenotype. We show here that topical application of the physiologically active ligand [1alpha,25-(OH)(2)D(3); calcitriol] of the vitamin D receptor, or of its low-calcemic analog MC903 (calcipotriol; Dovonex), induces TSLP expression in epidermal keratinocytes, which results in an atopic dermatitis-like syndrome mimicking that seen in RXRalphabeta(ep-/-) mutants and transgenic mice overexpressing TSLP in keratinocytes. Furthermore, topical application of retinoic acid receptor RARgamma-selective agonist BMS961 also induces TSLP expression either on its own or synergistically with 1alpha,25-(OH)(2)D(3). Our data demonstrate that RXR/vitamin D receptor and RXR/retinoic acid receptor-gamma heterodimers and their ligands cell-autonomously control the expression of TSLP in epidermal keratinocytes of the mouse. We propose molecular mechanisms through which vitamin D3 and retinoic acid signalings could be involved in the pathogenesis of atopic diseases.
Immunohistochemistry (frozen)
Brown, C. R., et al. (2004). "Treatment of mice with the neutrophil-depleting antibody RB6-8C5 results in early development of experimental lyme arthritis via the recruitment of Gr-1- polymorphonuclear leukocyte-like cells" Infect Immun 72(9): 4956-4965.
PubMed
Recently, we demonstrated that blocking the entry of neutrophils into Borrelia burgdorferi-infected joints in mice deficient in the chemokine receptor CXCR2 prevented the development of experimental Lyme arthritis. Neutrophils were marginalized in blood vessels at the site of infection but could not enter the joint tissue. In the present study, we treated both genetically arthritis-resistant DBA/2J (DBA) and arthritis-susceptible C3H/HeJ (C3H) mice with the neutrophil-depleting monoclonal antibody RB6-8C5 (RB6) to determine the effect on arthritis development. Surprisingly, both DBA and C3H mice treated with RB6 developed arthritis at 1 week postinfection, approximately 1 week earlier than the control-treated C3H mice. The early development of arthritis in the RB6-treated mice was accompanied by an influx into the joints of cells with ring-shaped polymorphonuclear leukocyte (PMN) cell morphology that were negative for the Gr-1 neutrophil maturation marker. RB6 treatment of mice also resulted in increased numbers of B. burgdorferi cells in the joints at 7 days postinfection and earlier expression of the chemokines KC and monocyte chemoattractant protein 1 in the joints compared to control-treated animals. Together, these results suggest that recruitment of neutrophils or PMN-like cells into an infected joint is a key requirement for Lyme arthritis development and that altered recruitment of these cells into the joints of arthritis-resistant mice can exacerbate the development of pathology.