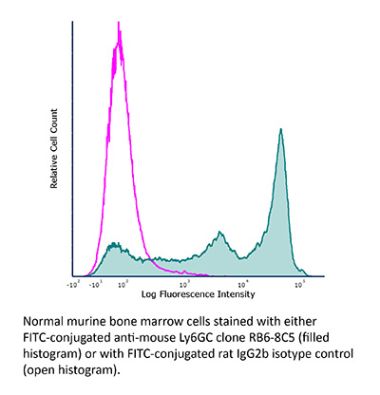
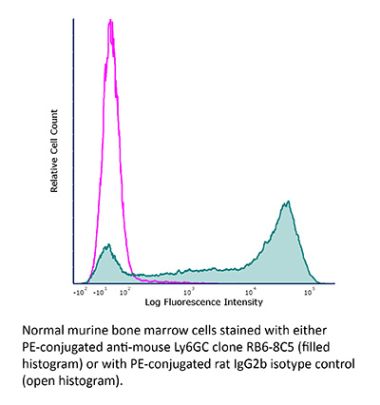
InVivoPlus anti-mouse Ly6G/Ly6C (Gr-1)
Product Details
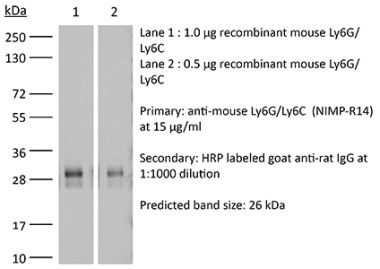
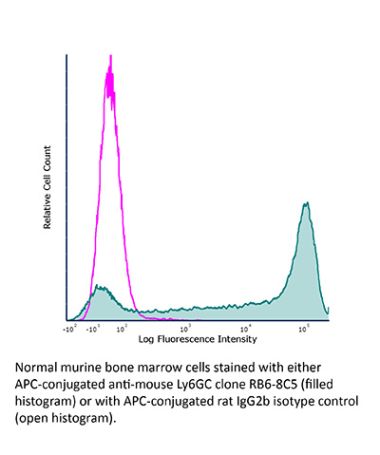
The RB6-8C5 monoclonal antibody reacts strongly with mouse Ly6G and weakly with mouse Ly6C previously referred to as GR-1. Ly6G is a 21-25 kDa member of the Ly-6 superfamily of GPI-anchored cell surface proteins with roles in cell signaling and cell adhesion. Ly6G is expressed differentially during development by cells in the myeloid lineage including monocytes macrophages granulocytes and neutrophils. Monocytes typically express Ly6G transiently during development while mature granulocytes and peripheral neutrophils retain expression making Ly6G a good cell surface marker for these populations.The RB6-8C5 antibody has been shown to inhibit the binding of the 1A8 antibody. The 1A8 monoclonal antibody reacts specifically with mouse Ly6G with no reported cross reactivity with Ly6C.Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse granulocytes |
| Reported Applications |
in vivo depletion of Gr-1+ myeloid cells Flow cytometry Immunohistochemistry (paraffin) Immunohistochemistry (frozen) |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Aggregation* |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10312146 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
in vivo depletion of Gr-1+ myeloid cells
Bansal, S., et al. (2018). "IL-1 Signaling Prevents Alveolar Macrophage Depletion during Influenza and Streptococcus pneumoniae Coinfection" J Immunol 200(4): 1425-1433. PubMed
Influenza and bacterial coinfection is a significant cause of hospitalization and death in humans during influenza epidemics and pandemics. However, the fundamental protective and pathogenic mechanisms involved in this complex virus-host-bacterium interaction remain incompletely understood. In this study, we have developed mild to lethal influenza and Streptococcus pneumoniae coinfection models for comparative analyses of disease pathogenesis. Specifically, wild-type and IL-1R type 1-deficient (Il1r1(-/-) ) mice were infected with influenza virus and then superchallenged with noninvasive S. pneumoniae serotype 14 (Spn14) or S. pneumoniae serotype 19A (Spn19A). The coinfections were followed by comparative analyses of inflammatory responses and animal protection. We found that resident alveolar macrophages are efficient in the clearance of both pneumococcal serotypes in the absence of influenza infection; in contrast, they are essential for airway control of Spn14 infection but not Spn19A infection. In agreement, TNF-alpha and neutrophils play a compensatory protective role in secondary bacterial infection associated with Spn19A; however, the essential requirement for alveolar macrophage-mediated clearance significantly enhances the virulence of Spn14 during postinfluenza pneumococcal infection. Furthermore, we show that, although IL-1 signaling is not required for host defense against pneumococcal infection alone, it is essential for sustaining antibacterial immunity during postinfluenza pneumococcal infection, as evidenced by significantly aggravated bacterial burden and animal mortality in Il1r1(-/-) mice. Mechanistically, we show that through preventing alveolar macrophage depletion, inflammatory cytokine IL-1 signaling is critically involved in host resistance to influenza and pneumococcal coinfection.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Bodogai, M., et al. (2015). "Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells" Cancer Res 75(17): 3456-3465. PubMed
Myeloid-derived suppressive cells (MDSC) have been reported to promote metastasis, but the loss of cancer-induced B cells/B regulatory cells (tBreg) can block metastasis despite MDSC expansion in cancer. Here, using multiple murine tumor models and human MDSC, we show that MDSC populations that expand in cancer have only partially primed regulatory function and limited prometastatic activity unless they are fully educated by tBregs. Cancer-induced tBregs directly activate the regulatory function of both the monocyte and granulocyte subpopulations of MDSC, relying, in part, on TgfbetaR1/TgfbetaR2 signaling. MDSC fully educated in this manner exhibit an increased production of reactive oxygen species and NO and more efficiently suppress CD4(+) and CD8(+) T cells, thereby promoting tumor growth and metastasis. Thus, loss of tBregs or TgfbetaR deficiency in MDSC is sufficient to disable their suppressive function and to block metastasis. Overall, our data indicate that cancer-induced B cells/B regulatory cells are important regulators of the immunosuppressive and prometastatic functions of MDSC.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Wang, H., et al. (2015). "P2RX7 sensitizes Mac-1/ICAM-1-dependent leukocyte-endothelial adhesion and promotes neurovascular injury during septic encephalopathy" Cell Res 25(6): 674-690. PubMed
Septic encephalopathy (SE) is a critical factor determining sepsis mortality. Vascular inflammation is known to be involved in SE, but the molecular events that lead to the development of encephalopathy remain unclear. Using time-lapse in vivo two-photon laser scanning microscopy, we provide the first direct evidence that cecal ligation and puncture in septic mice induces microglial trafficking to sites adjacent to leukocyte adhesion on inflamed cerebral microvessels. Our data further demonstrate that septic injury increased the chemokine CXCL1 level in brain endothelial cells by activating endothelial P2RX7 and eventually enhanced the binding of Mac-1 (CD11b/CD18)-expressing leukocytes to endothelial ICAM-1. In turn, leukocyte adhesion upregulated endothelial CX3CL1, thereby triggering microglia trafficking to the injured site. The sepsis-induced increase in endothelial CX3CL1 was abolished in CD18 hypomorphic mutant mice. Inhibition of the P2RX7 pathway not only decreased endothelial ICAM-1 expression and leukocyte adhesion but also prevented microglia overactivation, reduced brain injury, and consequently doubled the early survival of septic mice. These results demonstrate the role of the P2RX7 pathway in linking neurovascular inflammation to brain damage in vivo and provide a rationale for targeting endothelial P2RX7 for neurovascular protection during SE.
in vivo depletion of Gr-1+ myeloid cells
Dahlgren, M. W., et al. (2015). "T follicular helper, but not Th1, cell differentiation in the absence of conventional dendritic cells" J Immunol 194(11): 5187-5199. PubMed
Development of long-lived humoral immunity is dependent on CXCR5-expressing T follicular helper (Tfh) cells, which develop concomitantly to effector Th cells that support cellular immunity. Conventional dendritic cells (cDCs) are critical APCs for initial priming of naive CD4(+) T cells but, importantly, also provide accessory signals that govern effector Th cell commitment. To define the accessory role of cDCs during the concurrent development of Tfh and effector Th1 cells, we performed high-dose Ag immunization in conjunction with the Th1-biased adjuvant polyinosinic:polycytidylic acid (pI:C). In the absence of cDCs, pI:C failed to induce Th1 cell commitment and IgG2c production. However, cDC depletion did not impair Tfh cell differentiation or germinal center formation, and long-lived IgG1 responses of unaltered affinity developed in mice lacking cDCs at the time point for immunization. Thus, cDCs are required for the pI:C-driven Th1 cell fate commitment but have no crucial accessory function in relation to Tfh cell differentiation.
in vivo depletion of Gr-1+ myeloid cells
Condamine, T., et al. (2014). "ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis" J Clin Invest 124(6): 2626-2639. PubMed
Myeloid-derived suppressor cells (MDSCs) dampen the immune response thorough inhibition of T cell activation and proliferation and often are expanded in pathological conditions. Here, we studied the fate of MDSCs in cancer. Unexpectedly, MDSCs had lower viability and a shorter half-life in tumor-bearing mice compared with neutrophils and monocytes. The reduction of MDSC viability was due to increased apoptosis, which was mediated by increased expression of TNF-related apoptosis-induced ligand receptors (TRAIL-Rs) in these cells. Targeting TRAIL-Rs in naive mice did not affect myeloid cell populations, but it dramatically reduced the presence of MDSCs and improved immune responses in tumor-bearing mice. Treatment of myeloid cells with proinflammatory cytokines did not affect TRAIL-R expression; however, induction of ER stress in myeloid cells recapitulated changes in TRAIL-R expression observed in tumor-bearing hosts. The ER stress response was detected in MDSCs isolated from cancer patients and tumor-bearing mice, but not in control neutrophils or monocytes, and blockade of ER stress abrogated tumor-associated changes in TRAIL-Rs. Together, these data indicate that MDSC pathophysiology is linked to ER stress, which shortens the lifespan of these cells in the periphery and promotes expansion in BM. Furthermore, TRAIL-Rs can be considered as potential targets for selectively inhibiting MDSCs.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Schulze, F. S., et al. (2014). "Fcgamma receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid" Am J Pathol 184(8): 2185-2196. PubMed
Bullous pemphigoid (BP) and epidermolysis bullosa acquisita are subepidermal autoimmune blistering diseases mediated by autoantibodies against type XVII collagen (Col17) and Col7, respectively. For blister formation, Fc-mediated events, such as infiltration of inflammatory cells in the skin, complement activation, and release of proteases at the dermal-epidermal junction, are essential. Although in the neonatal passive transfer mouse model of BP, tissue destruction is mediated by Fcgamma receptors (FcgammaRs) I and III, the passive transfer model of epidermolysis bullosa acquisita completely depends on FcgammaRIV. To clarify this discrepancy, we developed a novel experimental model for BP using adult mice. Lesion formation was Fc mediated because gamma-chain-deficient mice and mice treated with anti-Col17 IgG, depleted from its sugar moiety at the Fc portion, were resistant to disease induction. By the use of various FcgammaR-deficient mouse strains, tissue destruction was shown to be mediated by FcgammaRIV, FcgammaRIII, and FcgammaRIIB, whereas FcgammaRI was not essential. Furthermore, anti-inflammatory mediators in already clinically diseased mice can be explored in the novel BP model, because the pharmacological inhibition of FcgammaRIV and depletion of granulocytes abolished skin blisters. Herein, we extended our knowledge about the importance of FcgammaRs in experimental BP and established a novel BP mouse model suitable to study disease development over a longer time period and explore novel treatment strategies in a quasi-therapeutic setting.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Khmaladze, I., et al. (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678. PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo depletion of Gr-1+ myeloid cells
Ermann, J., et al. (2014). "Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet-/-.Rag2-/- (TRUC) mice" Proc Natl Acad Sci U S A 111(25): E2559-2566. PubMed
T-bet(-/-).Rag2(-/-) (TRUC) mice spontaneously develop microbiota-driven, TNF-mediated large bowel inflammation that resembles human ulcerative colitis. We show here that IL-23 and IL-1-dependent secretion of IL-17A by innate lymphoid cells (ILCs; defined as CD45(+)lin(-)Thy1(hi)NKp46(-)) is a second critical pathway in this model. Using an in vitro coculture system of bone marrow-derived dendritic cells (DCs) and freshly isolated FACS-purified ILCs, we demonstrate that IL-23 and IL-1 secreted by DCs in response to microbial stimulation work together to induce IL-17A production by ILCs. TNF is not required for IL-17A secretion by ILCs in vitro but synergizes with IL-17A to induce the expression of neutrophil-attracting chemokines. Upstream, activation of the IL-23/IL-17A axis is regulated by nucleotide-binding oligomerization domain containing (Nod)/receptor-interacting serine-threonine kinase 2 (Ripk2) signals in DCs. Genetic ablation of the Nod/Ripk2 signaling pathway protects TRUC mice from developing colitis without affecting the colitogenicity of the intestinal microbiota. Our data provide insight into the complex network of interactions between IL-17A-secreting ILCs and other components of the innate immune system in the development of colitis.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Bryant, J., et al. (2014). "Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection" J Immunol 192(12): 6092-6101. PubMed
We have previously shown that preemptive infusion of apoptotic donor splenocytes treated with the chemical cross-linker ethylcarbodiimide (ECDI-SPs) induces long-term allograft survival in full MHC-mismatched models of allogeneic islet and cardiac transplantation. The role of myeloid-derived suppressor cells (MDSCs) in the graft protection provided by ECDI-SPs is unclear. In this study, we demonstrate that infusions of ECDI-SPs increase two populations of CD11b(+) cells in the spleen that phenotypically resemble monocytic-like (CD11b(+)Ly6C(high)) and granulocytic-like (CD11b(+)Gr1(high)) MDSCs. Both populations suppress T cell proliferation in vitro and traffic to the cardiac allografts in vivo to mediate their protection via inhibition of local CD8 T cell accumulation and potentially also via induction and homing of regulatory T cells. Importantly, repeated treatments with ECDI-SPs induce the CD11b(+)Gr1(high) cells to produce a high level of IFN-gamma and to exhibit an enhanced responsiveness to IFN-gamma by expressing higher levels of downstream effector molecules ido and nos2. Consequently, neutralization of IFN-gamma completely abolishes the suppressive capacity of this population. We conclude that donor ECDI-SPs induce the expansion of two populations of MDSCs important for allograft protection mediated in part by intrinsic IFN-gamma-dependent mechanisms. This form of preemptive donor apoptotic cell infusions has significant potential for the therapeutic manipulation of MDSCs for transplant tolerance induction.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
Norris, B. A., et al. (2013). "Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity" Immunity 38(2): 309-321. PubMed
Resolution of acute and chronic viral infections requires activation of innate cells to initiate and maintain adaptive immune responses. Here we report that infection with acute Armstrong (ARM) or chronic Clone 13 (C13) strains of lymphocytic choriomeningitis virus (LCMV) led to two distinct phases of innate immune response. During the first 72 hr of infection, dendritic cells upregulated activation markers and stimulated antiviral CD8(+) T cells, independent of viral strain. Seven days after infection, there was an increase in Ly6C(hi) monocytic and Gr-1(hi) neutrophilic cells in lymphoid organs and blood. This expansion in cell numbers was enhanced and sustained in C13 infection, whereas it occurred only transiently with ARM infection. These cells resembled myeloid-derived suppressor cells and potently suppressed T cell proliferation. The reduction of monocytic cells in Ccr2(-/-) mice or after Gr-1 antibody depletion enhanced antiviral T cell function. Thus, innate cells have an important immunomodulatory role throughout chronic infection.
in vivo depletion of Gr-1+ myeloid cells, Flow Cytometry
van der Merwe, M., et al. (2013). "Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplantation" J Immunol 191(11): 5764-5776. PubMed
We showed previously that nonmyeloablative total lymphoid irradiation/rabbit anti-thymocyte serum (TLI/ATS) conditioning facilitates potent donor-recipient immune tolerance following bone marrow transplantation (BMT) across MHC barriers via recipient invariant NKT (iNKT) cell-derived IL-4-dependent expansion of donor Foxp3(+) naturally occurring regulatory T cells (nTregs). In this study, we report a more specific mechanism. Wild-type (WT) BALB/c (H-2(d)) hosts were administered TLI/ATS and BMT from WT or STAT6(-/-) C57BL/6 (H-2(b)) donors. Following STAT6(-/-) BMT, donor nTregs demonstrated no loss of proliferation in vivo, indicating that an IL-4-responsive population in the recipient, rather than the donor, drives donor nTreg proliferation. In graft-versus-host disease (GVHD) target organs, three recipient CD11b(+) cell subsets (Gr-1(high)CD11c(-), Gr-1(int)CD11c(-), and Gr-1(low)CD11c(+)) were enriched early after TLI/ATS + BMT versus total body irradiation/ATS + BMT. Gr-1(low)CD11c(+) cells induced potent H-2K(b+)CD4(+)Foxp3(+) nTreg proliferation in vitro in 72-h MLRs. Gr-1(low)CD11c(+) cells were reduced significantly in STAT6(-/-) and iNKT cell-deficient Jalpha18(-/-) BALB/c recipients after TLI/ATS + BMT. Depletion of CD11b(+) cells resulted in severe acute GVHD, and adoptive transfer of WT Gr-1(low)CD11c(+) cells to Jalpha18(-/-) BALB/c recipients of TLI/ATS + BMT restored day-6 donor Foxp3(+) nTreg proliferation and protection from CD8 effector T cell-mediated GVHD. Blockade of programmed death ligand 1 and 2, but not CD40, TGF-beta signaling, arginase 1, or iNOS, inhibited nTreg proliferation in cocultures of recipient-derived Gr-1(low)CD11c(+) cells with donor nTregs. Through iNKT-dependent Th2 polarization, myeloid-derived immunomodulatory dendritic cells are expanded after nonmyeloablative TLI/ATS conditioning and allogeneic BMT, induce PD-1 ligand-dependent donor nTreg proliferation, and maintain potent graft-versus-host immune tolerance.
in vivo depletion of Gr-1+ myeloid cells
Ordonez-Rueda, D., et al. (2012). "A hypomorphic mutation in the Gfi1 transcriptional repressor results in a novel form of neutropenia" Eur J Immunol 42(9): 2395-2408. PubMed
Using N-ethyl-N-nitrosourea-induced mutagenesis, we established a mouse model with a novel form of neutropenia resulting from a point mutation in the transcriptional repressor Growth Factor Independence 1 (Gfi1). These mice, called Genista, had normal viability and no weight loss, in contrast to mice expressing null alleles of the Gfi1 gene. Furthermore, the Genista mutation had a very limited impact on lymphopoiesis or on T- and B-cell function. Within the bone marrow (BM), the Genista mutation resulted in a slight increase of monopoiesis and in a block of terminal granulopoiesis. This block occurred just after the metamyelocytic stage and resulted in the generation of small numbers of atypical CD11b(+) Ly-6G(int) neutrophils, the nuclear morphology of which resembled that of mature WT neutrophils. Unexpectedly, once released from the BM, these atypical neutrophils contributed to induce mild forms of autoantibody-induced arthritis and of immune complex-mediated lung alveolitis. They additionally failed to provide resistance to acute bacterial infection. Our study demonstrates that a hypomorphic mutation in the Gfi1 transcriptional repressor results in a novel form of neutropenia characterized by a split pattern of functional responses, reflecting the distinct thresholds required for eliciting neutrophil-mediated inflammatory and anti-infectious responses.
in vivo depletion of Gr-1+ myeloid cells
Carr, K. D., et al. (2011). "Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection" Eur J Immunol 41(9): 2666-2676. PubMed
Previous studies have suggested that neutrophils are required for resistance during infection with multiple pathogenic microorganisms. However, the depleting antibody used in those studies binds to both Ly6G and Ly6C (anti-Gr-1; clone RB6-8C5). This antibody has been shown to deplete not only neutrophils but also monocytes and a subset of CD8(+) T cells. Recently, an antibody against Ly6G, which specifically depletes neutrophils, was characterized. In the present study, neutrophils are depleted using the antibody against Ly6G during infection with the intracellular bacterium Listeria monocytogenes (LM). Our data show that neutrophil-depleted mice are much less susceptible to infection than mice depleted with anti-Gr-1. Although neutrophils are required for clearance of LM, their importance is more pronounced in the liver and during a high-dose bacterial challenge. Furthermore, we demonstrate that the protection mediated by neutrophils is due to the production of TNF-alpha, but not IFN-gamma. Additionally, neutrophils are not required for the recruitment of monocytes or the generation of adaptive T-cell responses during LM infection. This study highlights the importance of neutrophils during LM infection, and indicate that depletion of neutrophils is less detrimental to the host than depletion of all Gr-1-expressing cell populations.
in vivo depletion of Gr-1+ myeloid cells
Waight, J. D., et al. (2011). "Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism" PLoS One 6(11): e27690. PubMed
Myeloid-derived suppressor cells (MDSC) are induced under diverse pathologic conditions, including neoplasia, and suppress innate and adaptive immunity. While the mechanisms by which MDSC mediate immunosuppression are well-characterized, details on how they develop remain less understood. This is complicated further by the fact that MDSC comprise multiple myeloid cell types, namely monocytes and granulocytes, reflecting diverse stages of differentiation and the proportion of these subpopulations vary among different neoplastic models. Thus, it is thought that the type and quantities of inflammatory mediators generated during neoplasia dictate the composition of the resultant MDSC response. Although much interest has been devoted to monocytic MDSC biology, a fundamental gap remains in our understanding of the derivation of granulocytic MDSC. In settings of heightened granulocytic MDSC responses, we hypothesized that inappropriate production of G-CSF is a key initiator of granulocytic MDSC accumulation. We observed abundant amounts of G-CSF in vivo, which correlated with robust granulocytic MDSC responses in multiple tumor models. Using G-CSF loss- and gain-of-function approaches, we demonstrated for the first time that: 1) abrogating G-CSF production significantly diminished granulocytic MDSC accumulation and tumor growth; 2) ectopically over-expressing G-CSF in G-CSF-negative tumors significantly augmented granulocytic MDSC accumulation and tumor growth; and 3) treatment of naive healthy mice with recombinant G-CSF protein elicited granulocytic-like MDSC remarkably similar to those induced under tumor-bearing conditions. Collectively, we demonstrated that tumor-derived G-CSF enhances tumor growth through granulocytic MDSC-dependent mechanisms. These findings provide us with novel insights into MDSC subset development and potentially new biomarkers or targets for cancer therapy.
Immunohistochemistry (paraffin)
Li, M., et al. (2006). "Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis" Proc Natl Acad Sci U S A 103(31): 11736-11741. PubMed
We have demonstrated that cytokine thymic stromal lymphopoietin (TSLP), whose expression is rapidly induced upon keratinocyte-selective ablation of retinoid X receptors (RXRs) -alpha and -beta in the mouse (RXRalphabeta(ep-/-) mice), plays a key role in initiating a skin and systemic atopic dermatitis-like phenotype. We show here that topical application of the physiologically active ligand [1alpha,25-(OH)(2)D(3); calcitriol] of the vitamin D receptor, or of its low-calcemic analog MC903 (calcipotriol; Dovonex), induces TSLP expression in epidermal keratinocytes, which results in an atopic dermatitis-like syndrome mimicking that seen in RXRalphabeta(ep-/-) mutants and transgenic mice overexpressing TSLP in keratinocytes. Furthermore, topical application of retinoic acid receptor RARgamma-selective agonist BMS961 also induces TSLP expression either on its own or synergistically with 1alpha,25-(OH)(2)D(3). Our data demonstrate that RXR/vitamin D receptor and RXR/retinoic acid receptor-gamma heterodimers and their ligands cell-autonomously control the expression of TSLP in epidermal keratinocytes of the mouse. We propose molecular mechanisms through which vitamin D3 and retinoic acid signalings could be involved in the pathogenesis of atopic diseases.
Immunohistochemistry (frozen)
Brown, C. R., et al. (2004). "Treatment of mice with the neutrophil-depleting antibody RB6-8C5 results in early development of experimental lyme arthritis via the recruitment of Gr-1- polymorphonuclear leukocyte-like cells" Infect Immun 72(9): 4956-4965. PubMed
Recently, we demonstrated that blocking the entry of neutrophils into Borrelia burgdorferi-infected joints in mice deficient in the chemokine receptor CXCR2 prevented the development of experimental Lyme arthritis. Neutrophils were marginalized in blood vessels at the site of infection but could not enter the joint tissue. In the present study, we treated both genetically arthritis-resistant DBA/2J (DBA) and arthritis-susceptible C3H/HeJ (C3H) mice with the neutrophil-depleting monoclonal antibody RB6-8C5 (RB6) to determine the effect on arthritis development. Surprisingly, both DBA and C3H mice treated with RB6 developed arthritis at 1 week postinfection, approximately 1 week earlier than the control-treated C3H mice. The early development of arthritis in the RB6-treated mice was accompanied by an influx into the joints of cells with ring-shaped polymorphonuclear leukocyte (PMN) cell morphology that were negative for the Gr-1 neutrophil maturation marker. RB6 treatment of mice also resulted in increased numbers of B. burgdorferi cells in the joints at 7 days postinfection and earlier expression of the chemokines KC and monocyte chemoattractant protein 1 in the joints compared to control-treated animals. Together, these results suggest that recruitment of neutrophils or PMN-like cells into an infected joint is a key requirement for Lyme arthritis development and that altered recruitment of these cells into the joints of arthritis-resistant mice can exacerbate the development of pathology.
- Neuroscience,
- Mus musculus (House mouse)
VEGF-A in serum protects against memory impairment in APP/PS1 transgenic mice by blocking neutrophil infiltration.
In Molecular Psychiatry on 1 October 2023 by Qi, F., Zuo, Z., et al.
PubMed
Activation of innate immunity in the brain is a prominent feature of Alzheimer's disease (AD). The present study investigated the regulation of innate immunity by wild-type serum injection in a transgenic AD mouse model. We found that treatment with wild-type mouse serum significantly reduced the number of neutrophils and microglial reactivity in the brains of APP/PS1 mice. Mimicking this effect, neutrophil depletion via Ly6G neutralizing antibodies resulted in improvements in AD brain functions. Serum proteomic analysis identified vascular endothelial growth factor-A (VEGF-A) and chemokine (C-X-C motif) ligand 1 (CXCL1) as factors enriched in serum samples, which are crucial for neutrophil migration and chemotaxis, leukocyte migration, and cell chemotaxis. Exogenous VEGF-A reversed amyloid β (Aβ)-induced decreases in cyclin-dependent kinase 5 (Cdk5) and increases in CXCL1 in vitro and blocked neutrophil infiltration into the AD brain. Endothelial Cdk5 overexpression conferred an inhibitory effect on CXCL1 and neutrophil infiltration, thereby restoring memory abilities in APP/PS1 mice. Our findings uncover a previously unknown link between blood-derived VEGF signaling and neutrophil infiltration and support targeting endothelial Cdk5 signaling as a potential therapeutic strategy for AD. © 2023. The Author(s).
- Immunology and Microbiology,
- Cancer Research,
- Mus musculus (House mouse)
Abnormal activation of NF-κB and MAPK signaling pathways affect osimertinib resistance and influence the recruitment of myeloid-derived suppressor cells to shape the immunosuppressive tumor immune microenvironment.
In Thoracic Cancer on 1 July 2023 by Wang, C., Fei, K., et al.
PubMed
Osimertinib is the first-line treatment for patients with epidermal growth factor receptor (EGFR) mutations, but the treatment options after drug resistance are limited. Previous studies have suggested that EGFR is in an immunosuppressive tumor immune microenvironment (TIME). However, the evolution of TIME after osimertinib resistance and whether this resistance can be overcome by targeting TIME needs to be further investigated. The remodeling process and mechanism of TIME during the treatment with osimertinib were studied. The proportion of EGFRL858R+T790M mutant tumor immune infiltrating cells was extremely low. Osimertinib treatment transiently triggered inflammatory cells, but several immunosuppressive cells infiltrated after drug resistance and formed a myeloid-derived suppressor cell (MDSC)-enriched TIME. The programmed cell death protein-1 monoclonal antibody was not able to reverse the MDSC-enriched TIME. Further analysis revealed that the activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways recruited a large number of MDSCs via cytokines. Finally, MDSC secreted high levels of interleukin-10 and arginase-1 and created an immunosuppressive TIME. Thus, our findings lay the foundation for the evolution of TIME in osimertinib treatment, establish the mechanism of immunosuppressive TIME after osimertinib resistance, and propose potential solutions. © 2023 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
A neutrophil response linked to tumor control in immunotherapy.
In Cell on 30 March 2023 by Gungabeesoon, J., Gort-Freitas, N. A., et al.
PubMed
Neutrophils accumulate in solid tumors, and their abundance correlates with poor prognosis. Neutrophils are not homogeneous, however, and could play different roles in cancer therapy. Here, we investigate the role of neutrophils in immunotherapy, leading to tumor control. We show that successful therapies acutely expanded tumor neutrophil numbers. This expansion could be attributed to a Sellhi state rather than to other neutrophils that accelerate tumor progression. Therapy-elicited neutrophils acquired an interferon gene signature, also seen in human patients, and appeared essential for successful therapy, as loss of the interferon-responsive transcription factor IRF1 in neutrophils led to failure of immunotherapy. The neutrophil response depended on key components of anti-tumor immunity, including BATF3-dependent DCs, IL-12, and IFNγ. In addition, we found that a therapy-elicited systemic neutrophil response positively correlated with disease outcome in lung cancer patients. Thus, we establish a crucial role of a neutrophil state in mediating effective cancer therapy. Copyright © 2023 Elsevier Inc. All rights reserved.
- Cancer Research
Conditional Knockout of Hypoxia-Inducible Factor 1-Alpha in Tumor-Infiltrating Neutrophils Protects against Pancreatic Ductal Adenocarcinoma.
In International Journal of Molecular Sciences on 1 January 2023 by Sieow, J. L., Penny, H. L., et al.
PubMed
Large numbers of neutrophils infiltrate tumors and comprise a notable component of the inflammatory tumor microenvironment. While it is established that tumor cells exhibit the Warburg effect for energy production, the contribution of the neutrophil metabolic state to tumorigenesis is unknown. Here, we investigated whether neutrophil infiltration and metabolic status promotes tumor progression in an orthotopic mouse model of pancreatic ductal adenocarcinoma (PDAC). We observed a large increase in the proportion of neutrophils in the blood and tumor upon orthotopic transplantation. Intriguingly, these tumor-infiltrating neutrophils up-regulated glycolytic factors and hypoxia-inducible factor 1-alpha (HIF-1α) expression compared to neutrophils from the bone marrow and blood of the same mouse. This enhanced glycolytic signature was also observed in human PDAC tissue samples. Strikingly, neutrophil-specific deletion of HIF-1α (HIF-1αΔNφ) significantly reduced tumor burden and improved overall survival in orthotopic transplanted mice, by converting the pro-tumorigenic neutrophil phenotype to an anti-tumorigenic phenotype. This outcome was associated with elevated reactive oxygen species production and activated natural killer cells and CD8+ cytotoxic T cells compared to littermate control mice. These data suggest a role for HIF-1α in neutrophil metabolism, which could be exploited as a target for metabolic modulation in cancer.
- Cardiovascular biology
Mfsd2b and Spns2 are essential for maintenance of blood vessels during development and in anaphylactic shock.
In Cell Reports on 16 August 2022 by Le, T. N. U., Nguyen, T. Q., et al.
PubMed
Sphingosine-1-phosphate (S1P) is a potent lipid mediator that is secreted by several cell types. We recently showed that Mfsd2b is an S1P transporter from hematopoietic cells that contributes approximately 50% plasma S1P. Here we report the characterization of compound deletion of Mfsd2b and Spns2, another S1P transporter active primarily in endothelial cells. Global deletion of Mfsd2b and Spns2 (global double knockout [gDKO]) results in embryonic lethality beyond embryonic day 14.5 (E14.5), with severe hemorrhage accompanied by defects of tight junction proteins, indicating that Mfsd2b and Spns2 provide S1P for signaling, which is essential for blood vessel integrity. Compound postnatal deletion of Mfsd2b and Spns2 using Mx1Cre (ctDKO-Mx1Cre) results in maximal 80% reduction of plasma S1P. ctDKO-Mx1Cre mice exhibit severe susceptibility to anaphylaxis, indicating that S1P from Mfsd2b and Spns2 is indispensable for vascular homeostasis. Our results show that S1P export from Mfsd2b and Spns2 is essential for developing and mature vasculature. Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Neuroscience
Young serum protects against memory impairment in APP/PS1 transgenic mice by blocking neutrophil infiltration
Preprint on Research Square on 9 August 2022 by Guo, K., Qi, F., et al.
PubMed
Activation of innate immunity in the brain is a prominent feature of Alzheimer's disease (AD). The present study investigated the regulation of innate immunity by young serum in a transgenic AD mouse model. We found that young serum significantly reduced the number of neutrophils and microglial reactivity in the brains of APP/PS1 mice. Neutrophil depletion via Ly6G neutralizing antibodies mimicked the benefits of young serum on AD brain functions. Serum proteomic analysis of young serum revealed significant enrichment of the factors VEGF-A and CXCL1, which are crucial for neutrophil migration and chemotaxis, leukocyte migration, and cell chemotaxis. Intravenously injected VEGF-A reversed Aβ-induced decreases in Cdk5 and increases in CXCL1 in vitro and blocked neutrophil infiltration into the AD brain. Endothelial Cdk5 overexpression conferred an inhibitory effect on CXCL1 and neutrophil infiltration and thereby restored memory in APP/PS1 mice. Our data uncover a previously unknown link between blood-derived VEGF signaling and neutrophil infiltration and provide a rationale for targeting endothelial Cdk5 signaling as a potential therapeutic strategy for AD.
- Cardiovascular biology,
- Mus musculus (House mouse)
Unique Angiogenesis From Cardiac Arterioles During Pericardial Adhesion Formation.
In Frontiers in Cardiovascular Medicine on 22 February 2022 by Namiguchi, K., Sakaue, T., et al.
PubMed
The molecular mechanisms underlying post-operative pericardial adhesions remain poorly understood. We aimed to unveil the temporal molecular and cellular mechanisms underlying tissue dynamics during adhesion formation, including inflammation, angiogenesis, and fibrosis. We visualized cell-based tissue dynamics during pericardial adhesion using histological evaluations. To determine the molecular mechanism, RNA-seq was performed. Chemical inhibitors were administered to confirm the molecular mechanism underlying adhesion formation. A high degree of adhesion formation was observed during the stages in which collagen production was promoted. Histological analyses showed that arterioles excessively sprouted from pericardial tissues after the accumulation of neutrophils on the heart surface in mice as well as humans. The combination of RNA-seq and histological analyses revealed that hyperproliferative endothelial and smooth muscle cells with dedifferentiation appeared in cytokine-exposed sprouting vessels and adhesion tissue but not in quiescent vessels in the heart. SMAD2/3 and ERK activation was observed in sprouting vessels. The simultaneous abrogation of PI3K/ERK or TGF-β/MMP9 signaling significantly decreased angiogenic sprouting, followed by inhibition of adhesion formation. Depleting MMP9-positive neutrophils shortened mice survival and decreased angiogenic sprouting and fibrosis in the adhesion. Our data suggest that TGF-β/matrix metalloproteinase-dependent tissue remodeling and PI3K/ERK signaling activation might contribute to unique angiogenesis with dedifferentiation of vascular smooth muscle cells from the contractile to the synthetic phenotype for fibrosis in the pericardial cavity. Our findings provide new insights in developing prevention strategies for pericardial adhesions by targeting the recruitment of vascular cells from heart tissues. Copyright © 2022 Namiguchi, Sakaue, Okazaki, Kanno, Komoda, Shikata, Kurata, Ota, Kubota, Kurobe, Nishimura, Masumoto, Higashiyama and Izutani.
- Immunology and Microbiology
Neutrophil-Derived IL-17 Promotes Ventilator-Induced Lung Injury via p38 MAPK/MCP-1 Pathway Activation.
In Frontiers in Immunology on 4 January 2022 by Liao, X., Zhang, W., et al.
PubMed
Ventilator-induced lung injury (VILI) is one of the most common complications of mechanical ventilation and can severely affect health. VILI appears to involve excessive inflammatory responses, but its pathogenesis has not yet been clarified. Since interleukin-17 (IL-17) plays a critical role in the immune system and the development of infectious and inflammatory diseases, we investigated here whether it plays a role in VILI. In a mouse model of VILI, mechanical ventilation with high tidal volume promoted the accumulation of lung neutrophils, leading to increased IL-17 levels in the lung, which in turn upregulated macrophage chemoattractant protein-1 via p38 mitogen-activated protein kinase. Depletion of neutrophils decreases the production IL-17 in mice and inhibition of IL-17 significantly reduced HTV-induced lung injury and inflammatory response. These results were confirmed in vitro using RAW264.7 macrophage cultures. Our results suggest that IL-17 plays a pro-inflammatory role in VILI and could serve as a new target for its treatment. Copyright © 2021 Liao, Zhang, Dai, Jing, Ye, Ge, Pei and Pan.
- Cardiovascular biology
Mfsd2b and Spns2 are essential for maintenance of blood vessels during development and protection of anaphylaxis
Preprint on BioRxiv : the Preprint Server for Biology on 16 November 2021 by Le, T. N. U., Nguyen, T. Q., et al.
PubMed
h4>Summary/h4> Sphingosine-1-phosphate (S1P) is a potent lipid mediator that is secreted by several cell types to induce signaling. We recently showed that Mfsd2b is an S1P transporter from hematopoietic cells, which contributes approximately 50% plasma S1P. To further determine the sources of plasma S1P, here, we report the characterizations of compound deletions of Mfsd2b and Spns2, another S1P transporter from endothelial cells. Global deletion of Mfsd2b and Spns2 (gDKO) resulted in embryonic lethality between E13.5 and E14.5 with severe hemorrhage that largely recapitulated the phenotypes from global S1P1 knockout mice, indicating that together with Mfsd2b, Spns2 also provides embryonic source of S1P for S1P1 stimulation. The hemorrhagic phenotypes in gDKO embryos were accompanied by increased angiogenesis and defects of tight junction proteins, indicating that S1P from Mfsd2b and Spns2 is essential for blood vessel integrity and maturation. The various sources of S1P in postnatal stages are yet to be fully understood. Postnatal ablation of S1P synthesis enzymes using Mx1Cre shows that Mx1Cre-sensitive cells provide most of plasma S1P. Interestingly, we showed that compound postnatal deletion of Mfsd2b and Spns2 using Mx1Cre (ctDKO-Mx1Cre) resulted in maximal reduction of 80% plasma S1P. Thus, a small amount of plasma S1P is supplied from other sources independent of Mfsd2b and Spns2. Nevertheless, the vasculature in the lung of ctDKO-Mx1Cre mice was compromised. Furthermore, ctDKO-Mx1Cre mice also exhibited severe susceptibility to anaphylaxis, indicating that S1P from Mfsd2b and Spns2 is indispensable during vascular stress. Together, our results show that Mfsd2b and Spns2 provide a critical source of S1P for embryonic development and they also provide a majority of plasma S1P for vascular homeostasis.
Driving regeneration, instead of healing, in adult mammals: the decisive role of resident macrophages through efferocytosis.
In Npj Regenerative Medicine on 3 August 2021 by Rabiller, L., Robert, V., et al.
PubMed
Tissue repair after lesion usually leads to scar healing and thus loss of function in adult mammals. In contrast, other adult vertebrates such as amphibians have the ability to regenerate and restore tissue homeostasis after lesion. Understanding the control of the repair outcome is thus a concerning challenge for regenerative medicine. We recently developed a model of induced tissue regeneration in adult mice allowing the comparison of the early steps of regenerative and scar healing processes. By using studies of gain and loss of function, specific cell depletion approaches, and hematopoietic chimeras we demonstrate here that tissue regeneration in adult mammals depends on an early and transient peak of granulocyte producing reactive oxygen species and an efficient efferocytosis specifically by tissue-resident macrophages. These findings highlight key and early cellular pathways able to drive tissue repair towards regeneration in adult mammals. © 2021. The Author(s).
- Cancer Research,
- Immunology and Microbiology
Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy.
In Science Immunology on 2 July 2021 by Siwicki, M., Gort-Freitas, N. A., et al.
PubMed
Immunotherapy is revolutionizing cancer treatment but is often restricted by toxicities. What distinguishes adverse events from concomitant antitumor reactions is poorly understood. Here, using anti-CD40 treatment in mice as a model of TH1-promoting immunotherapy, we showed that liver macrophages promoted local immune-related adverse events. Mechanistically, tissue-resident Kupffer cells mediated liver toxicity by sensing lymphocyte-derived IFN-γ and subsequently producing IL-12. Conversely, dendritic cells were dispensable for toxicity but drove tumor control. IL-12 and IFN-γ were not toxic themselves but prompted a neutrophil response that determined the severity of tissue damage. We observed activation of similar inflammatory pathways after anti-PD-1 and anti-CTLA-4 immunotherapies in mice and humans. These findings implicated macrophages and neutrophils as mediators and effectors of aberrant inflammation in TH1-promoting immunotherapy, suggesting distinct mechanisms of toxicity and antitumor immunity. Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
- Endocrinology and Physiology,
- Immunology and Microbiology,
- Mus musculus (House mouse)
Neutrophil-Macrophage Imbalance Drives the Development of Renal Scarring during Experimental Pyelonephritis.
In Journal of the American Society of Nephrology : JASN on 1 January 2021 by Ruíz-Rosado, J. D., Robledo-Avila, F., et al.
PubMed
In children, the acute pyelonephritis that can result from urinary tract infections (UTIs), which commonly ascend from the bladder to the kidney, is a growing concern because it poses a risk of renal scarring and irreversible loss of kidney function. To date, the cellular mechanisms underlying acute pyelonephritis-driven renal scarring remain unknown. We used a preclinical model of uropathogenic Escherichia coli-induced acute pyelonephritis to determine the contribution of neutrophils and monocytes to resolution of the condition and the subsequent development of kidney fibrosis. We used cell-specific monoclonal antibodies to eliminate neutrophils, monocytes, or both. Bacterial ascent and the cell dynamics of phagocytic cells were assessed by biophotonic imaging and flow cytometry, respectively. We used quantitative RT-PCR and histopathologic analyses to evaluate inflammation and renal scarring. We found that neutrophils are critical to control bacterial ascent, which is in line with previous studies suggesting a protective role for neutrophils during a UTI, whereas monocyte-derived macrophages orchestrate a strong, but ineffective, inflammatory response against uropathogenic, E. coli-induced, acute pyelonephritis. Experimental neutropenia during acute pyelonephritis resulted in a compensatory increase in the number of monocytes and heightened macrophage-dependent inflammation in the kidney. Exacerbated macrophage-mediated inflammatory responses promoted renal scarring and compromised renal function, as indicated by elevated serum creatinine, BUN, and potassium. These findings reveal a previously unappreciated outcome for neutrophil-macrophage imbalance in promoting host susceptibility to acute pyelonephritis and the development of permanent renal damage. This suggests targeting dysregulated macrophage responses might be a therapeutic tool to prevent renal scarring during acute pyelonephritis. Copyright © 2021 by the American Society of Nephrology.
Neutrophil extracellular traps impair regeneration
Preprint on BioRxiv : the Preprint Server for Biology on 6 July 2020 by Wier, E., Asada, M., et al.
PubMed
Fibrosis is a major health burden across diseases and organs. To remedy this, we study wound induced hair follicle regeneration (WIHN) as a model of non-fibrotic healing that recapitulates embryogenesis for de novo hair follicle morphogenesis after wounding. We have previously demonstrated that TLR3 promotes WIHN through binding dsRNA, but the source of which is still unclear. Here, we demonstrate that multiple distinct contexts of high WIHN all show a strong neutrophil signature, and given the likelihood of nuclear dsRNA release during the production of neutrophil extracellular trap (NETs), we hypothesized that mature neutrophils and NETs might promote WIHN. Consistent with this, in addition to the presence of mature neutrophils shortly after wounding, neutrophils remain within the wound after the barrier is reestablished, where they produce extracellular traps (NETs) that likely release spliceosomal U1 dsRNA. Contrary to our hypothesis, genetic models of neutrophil depletion show enhanced WIHN. Pad4 null mice that are defective in NET production also augment WIHN. Finally, using single-cell RNA sequencing, we identified a dramatic increase in mature neutrophils in the wound beds of low regenerating Tlr3-/- mice. Taken together, these results demonstrate that although mature neutrophils are stimulated by a common pro-regenerative cue, their presence and NETs hinder WIHN.
- Mus musculus (House mouse),
- Genetics,
- Immunology and Microbiology
TLR9 Sensing of Self-DNA Controls Cell-Mediated Immunity to Listeria Infection via Rapid Conversion of Conventional CD4+ T Cells to Treg.
In Cell Reports on 7 April 2020 by Dolina, J. S., Lee, J., et al.
PubMed
CD4+ T lymphocytes are crucial for controlling a range of innate and adaptive immune effectors. For CD8+ cytotoxic T lymphocyte (CTL) responses, CD4+ T cells can function as helpers (TH) to amplify magnitude and functionality or as regulatory cells (Treg) capable of profound inhibition. It is unclear what determines differentiation to these phenotypes and whether pathogens provoke alternate programs. We find that, depending on the size of initial dose, Listeria infection drives CD4+ T cells to act as TH or induces rapid polyclonal conversion to immunosuppressive Treg. Conversion to Treg depends on the TLR9 and IL-12 pathways elicited by CD8α+ dendritic cell (DC) sensing of danger-associated neutrophil self-DNA. These findings resolve long-standing questions regarding the conditional requirement for TH amongst pathogens and reveal a remarkable degree of plasticity in the function of CD4+ T cells, which can be quickly converted to Tregin vivo by infection-mediated immune modulation. Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
- IHC,
- Cancer Research,
- Endocrinology and Physiology,
- Mus musculus (House mouse)
Loss of testosterone impairs anti-tumor neutrophil function.
In Nature Communications on 31 March 2020 by Markman, J. L., Porritt, R. A., et al.
PubMed
In men, the incidence of melanoma rises rapidly after age 50, and nearly two thirds of melanoma deaths are male. The immune system is known to play a key role in controlling the growth and spread of malignancies, but whether age- and sex-dependent changes in immune cell function account for this effect remains unknown. Here, we show that in castrated male mice, neutrophil maturation and function are impaired, leading to elevated metastatic burden in two models of melanoma. Replacement of testosterone effectively normalized the tumor burden in castrated male mice. Further, the aberrant neutrophil phenotype was also observed in prostate cancer patients receiving androgen deprivation therapy, highlighting the evolutionary conservation and clinical relevance of the phenotype. Taken together, these results provide a better understanding of the role of androgen signaling in neutrophil function and the impact of this biology on immune control of malignancies.
- In Vivo,
- Mus musculus (House mouse)
Clec10a regulates mite-induced dermatitis.
In Science Immunology on 6 December 2019 by Kanemaru, K., Noguchi, E., et al.
PubMed
House dust mite (HDM) is a major allergen that causes allergic diseases such as atopic dermatitis. However, the regulatory mechanisms of HDM-induced immune responses are incompletely understood. NC/Nga mice are an inbred strain that is more susceptible to HDM and develops more severe dermatitis than other strains. Using whole-exome sequencing, we found that NC/Nga mice carry a stop-gain mutation in Clec10a, which encodes a C-type lectin receptor, Clec10a (MGL1/CD301a). The repair of this gene mutation using the CRISPR-Cas9 system ameliorated HDM-induced dermatitis, indicating that the Clec10a mutation is responsible for hypersensitivity to HDM in NC/Nga mice. Similarly, Clec10a -/- mice on the C57BL/6J background showed exacerbated HDM-induced dermatitis. Clec10a expressed on skin macrophages inhibits HDM-induced Toll-like receptor 4 (TLR4)-mediated inflammatory cytokine production through the inhibitory immunoreceptor tyrosine activating motif in its cytoplasmic portion. We identified asialoglycoprotein receptor 1 (Asgr1) as a functional homolog of mouse Clec10a in humans. Moreover, we found that a mucin-like molecule in HDM is a ligand for mouse Clec10a and human Asgr1. Skin application of the ligand ameliorated a TLR4 ligand-induced dermatitis in mice. Our findings suggest that Clec10a in mice and Asgr1 in humans play an important role in skin homeostasis against inflammation associated with HDM-induced dermatitis.Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Selective Cannabinoid 2 Receptor Agonists as Potential Therapeutic Drugs for the Treatment of Endotoxin-Induced Uveitis.
In Molecules (Basel, Switzerland) on 13 September 2019 by Porter, R. F., Szczesniak, A. M., et al.
PubMed
(1) Background: The cannabinoid 2 receptor (CB2R) is a promising anti-inflammatory drug target and development of selective CB2R ligands may be useful for treating sight-threatening ocular inflammation. (2) Methods: This study examined the pharmacology of three novel chemically-diverse selective CB2R ligands: CB2R agonists, RO6871304, and RO6871085, as well as a CB2R inverse agonist, RO6851228. In silico molecular modelling and in vitro cell-based receptor assays were used to verify CB2R interactions, binding, cell signaling (ß-arrestin and cAMP) and early absorption, distribution, metabolism, excretion, and toxicology (ADMET) profiling of these receptor ligands. All ligands were evaluated for their efficacy to modulate leukocyte-neutrophil activity, in comparison to the reported CB2R ligand, HU910, using an in vivo mouse model of endotoxin-induced uveitis (EIU) in wild-type (WT) and CB2R-/- mice. The actions of RO6871304 on neutrophil migration and adhesion were examined in vitro using isolated neutrophils from WT and CB2R-/- mice, and in vivo in WT mice with EIU using adoptive transfer of WT and CB2R-/- neutrophils, respectively. (3) Results: Molecular docking studies indicated that RO6871304 and RO6871085 bind to the orthosteric site of CB2R. Binding studies and cell signaling assays for RO6871304 and RO6871085 confirmed high-affinity binding to CB2R and selectivity for CB2R > CB1R, with both ligands acting as full agonists in cAMP and ß-arrestin assays (EC50s in low nM range). When tested in EIU, topical application of RO6871304 and RO6871085 decreased leukocyte-endothelial adhesion and this effect was antagonized by the inverse agonist, RO6851228. The CB2R agonist, RO6871304, decreased in vitro neutrophil migration of WT neutrophils but not neutrophils from CB2R-/-, and attenuated adhesion of adoptively-transferred leukocytes in EIU. (4) Conclusions: These unique ligands are potent and selective for CB2R and have good immunomodulating actions in the eye. RO6871304 and RO6871085, as well as HU910, decreased leukocyte adhesion in EIU through inhibition of resident ocular immune cells. The data generated with these three structurally-diverse and highly-selective CB2R agonists support selective targeting of CB2R for treating ocular inflammatory diseases.
- Mus musculus (House mouse)
Locally renewing resident synovial macrophages provide a protective barrier for the joint.
In Nature on 1 August 2019 by Culemann, S., Grüneboom, A., et al.
PubMed
Macrophages are considered to contribute to chronic inflammatory diseases such as rheumatoid arthritis1. However, both the exact origin and the role of macrophages in inflammatory joint disease remain unclear. Here we use fate-mapping approaches in conjunction with three-dimensional light-sheet fluorescence microscopy and single-cell RNA sequencing to perform a comprehensive spatiotemporal analysis of the composition, origin and differentiation of subsets of macrophages within healthy and inflamed joints, and study the roles of these macrophages during arthritis. We find that dynamic membrane-like structures, consisting of a distinct population of CX3CR1+ tissue-resident macrophages, form an internal immunological barrier at the synovial lining and physically seclude the joint. These barrier-forming macrophages display features that are otherwise typical of epithelial cells, and maintain their numbers through a pool of locally proliferating CX3CR1- mononuclear cells that are embedded into the synovial tissue. Unlike recruited monocyte-derived macrophages, which actively contribute to joint inflammation, these epithelial-like CX3CR1+ lining macrophages restrict the inflammatory reaction by providing a tight-junction-mediated shield for intra-articular structures. Our data reveal an unexpected functional diversification among synovial macrophages and have important implications for the general role of macrophages in health and disease.
- Cancer Research
Regulation of Tumor-Associated Myeloid Cell Activity by CBP/EP300 Bromodomain Modulation of H3K27 Acetylation.
In Cell Reports on 2 April 2019 by De Almeida Nagata, D. E., Chiang, E. Y., et al.
PubMed
Myeloid-derived suppressor cells (MDSCs) are found in most cancer malignancies and support tumorigenesis by suppressing immunity and promoting tumor growth. Here we identify the bromodomain (BRD) of CBP/EP300 as a critical regulator of H3K27 acetylation (H3K27ac) in MDSCs across promoters and enhancers of pro-tumorigenic target genes. In preclinical tumor models, in vivo administration of a CBP/EP300-BRD inhibitor (CBP/EP300-BRDi) alters intratumoral MDSCs and attenuates established tumor growth in immunocompetent tumor-bearing mice, as well as in MDSC-dependent xenograft models. Inhibition of CBP/EP300-BRD redirects tumor-associated MDSCs from a suppressive to an inflammatory phenotype through downregulation of STAT pathway-related genes and inhibition of Arg1 and iNOS. Similarly, CBP/EP300-BRDi decreases differentiation and suppressive function of human MDSCs in vitro. Our findings uncover a role of CBP/EP300-BRD in intratumoral MDSCs that may be targeted therapeutically to boost anti-tumor immunity.Published by Elsevier Inc.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma.
In Cancer Research on 15 January 2019 by Elahi-Gedwillo, K. Y., Carlson, M., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) remains one of the deadliest forms of cancer, in part, because it is largely refractory to current therapies. The failure of most standard therapies in PDA, as well as promising immune therapies, may be largely ascribed to highly unique and protective stromal microenvironments that present significant biophysical barriers to effective drug delivery, that are immunosuppressive, and that can limit the distribution and function of antitumor immune cells. Here, we utilized stromal reengineering to disrupt these barriers and move the stroma toward normalization using a potent antifibrotic agent, halofuginone. In an autochthonous genetically engineered mouse model of PDA, halofuginone disrupted physical barriers to effective drug distribution by decreasing fibroblast activation and reducing key extracellular matrix elements that drive stromal resistance. Concomitantly, halofuginone treatment altered the immune landscape in PDA, with greater immune infiltrate into regions of low hylauronan, which resulted in increased number and distribution of both classically activated inflammatory macrophages and cytotoxic T cells. In concert with a direct effect on carcinoma cells, this led to widespread intratumoral necrosis and reduced tumor volume. These data point to the multifunctional and critical role of the stroma in tumor protection and survival and demonstrate how compromising tumor integrity to move toward a more normal physiologic state through stroma-targeting therapy will likely be an instrumental component in treating PDA. SIGNIFICANCE: This work demonstrates how focused stromal re-engineering approaches to move toward normalization of the stroma disrupt physical barriers to effective drug delivery and promote antitumor immunity.See related commentary by Huang and Brekken, p. 328. ©2018 American Association for Cancer Research.