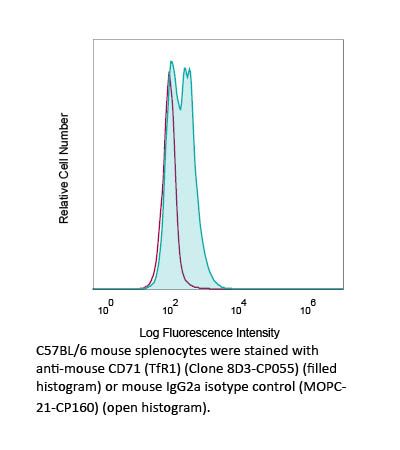
RecombiMAb anti-mouse CD71 (TfR1)
(switched from rat IgG2a, kappa)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse transformed endothelioma cell line t.end1 |
| Reported Applications |
Targeted drug delivery to the brain Immunohistochemistry Flow Cytometry Western Blot In vivo depletion of CD71+ cells In vivo anti-tumor activity *Reported for the original 8D3 antibody. For information on in vivo applications, please contact technicalservice@bioxcell.com |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from HEK293 cell supernatant in an animal-free facility |
| Purification | Protein G |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo anti-tumor activity
Candelaria PV, Leoh LS, Penichet ML, Daniels-Wells TR (2021). "Antibodies Targeting the Transferrin Receptor 1 (TfR1) as Direct Anti-cancer Agents" Front Immunol .
PubMed
The transferrin receptor 1 (TfR1), also known as cluster of differentiation 71 (CD71), is a type II transmembrane glycoprotein that binds transferrin (Tf) and performs a critical role in cellular iron uptake through the interaction with iron-bound Tf. Iron is required for multiple cellular processes and is essential for DNA synthesis and, thus, cellular proliferation. Due to its central role in cancer cell pathology, malignant cells often overexpress TfR1 and this increased expression can be associated with poor prognosis in different types of cancer. The elevated levels of TfR1 expression on malignant cells, together with its extracellular accessibility, ability to internalize, and central role in cancer cell pathology make this receptor an attractive target for antibody-mediated therapy. The TfR1 can be targeted by antibodies for cancer therapy in two distinct ways: (1) indirectly through the use of antibodies conjugated to anti-cancer agents that are internalized by receptor-mediated endocytosis or (2) directly through the use of antibodies that disrupt the function of the receptor and/or induce Fc effector functions, such as antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC). Although TfR1 has been used extensively as a target for antibody-mediated cancer therapy over the years, interest continues to increase for both targeting the receptor for delivery purposes and for its use as direct anti-cancer agents. This review focuses on the developments in the use of antibodies targeting TfR1 as direct anti-tumor agents.
transport across the BBB
Boado RJ, Zhang Y, Wang Y, Pardridge WM (2009). "Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse" Biotechnol Bioeng 102(4):1251-8.
PubMed
Protein therapeutics may be delivered across the blood-brain barrier (BBB) by genetic fusion to a BBB molecular Trojan horse. The latter is an endogenous peptide or a peptidomimetic monoclonal antibody (MAb) against a BBB receptor, such as the insulin receptor or the transferrin receptor (TfR). Fusion proteins have been engineered with the MAb against the human insulin receptor (HIR). However, the HIRMAb is not active against the rodent insulin receptor, and cannot be used for drug delivery across the mouse BBB. The rat 8D3 MAb against the mouse TfR is active as a drug delivery system in the mouse, and the present studies describe the cloning and sequencing of the variable region of the heavy chain (VH) and light chain (VL) of the rat 8D3 TfRMAb. The VH and VL were fused to the constant region of mouse IgG1 heavy chain and mouse kappa light chain, respectively, to produce a new chimeric TfRMAb. The chimeric TfRMAb was expressed in COS cells following dual transfection with the heavy and light chain expression plasmids, and was purified by protein G affinity chromatography. The affinity of the chimeric TfRMAb for the murine TfR was equal to the 8D3 MAb using a radio-receptor assay and mouse fibroblasts. The chimeric TfRMAb was radio-labeled and injected into mice for a pharmacokinetics study of the clearance of the chimeric TfRMAb. The chimeric TfRMAb was rapidly taken up by mouse brain in vivo at a level comparable to the rat 8D3 MAb. In summary, these studies describe the genetic engineering, expression, and validation of a chimeric TfRMAb with high activity for the mouse TfR, which can be used in future engineering of therapeutic fusion proteins for BBB drug delivery in the mouse.