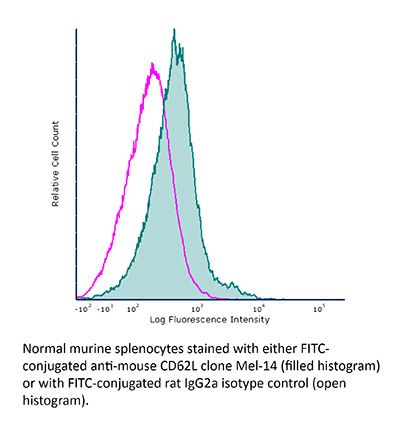
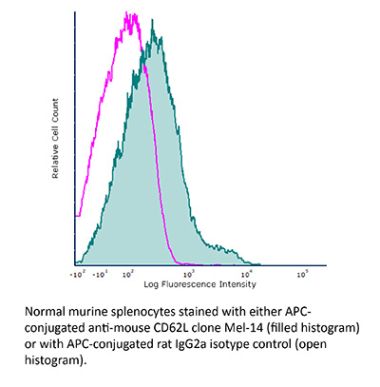
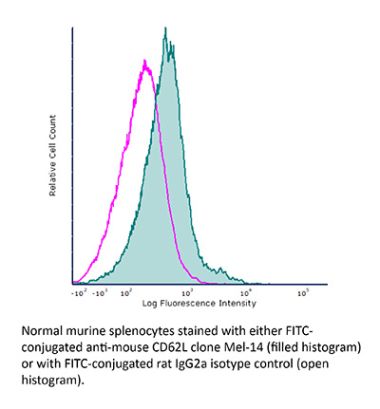
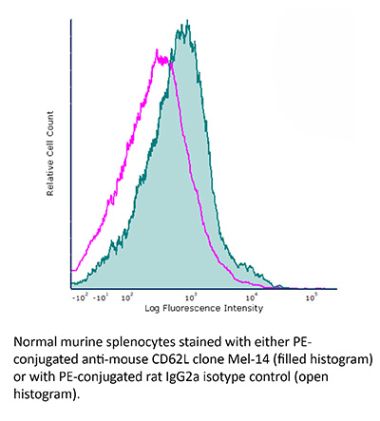
InVivoMAb anti-mouse L-Selectin (CD62L)
Product Details
The Mel-14 monoclonal antibody reacts with mouse CD62L also known as L-selectin and MEL-14. CD62L is a 76 kDa glycoprotein and a member of the selectin family that is expressed by neutrophils, monocytes, the majority of naïve T and B cells, a subset of memory T cells, NK cells, and most thymocytes. CD62L is a cell adhesion molecule that binds to many glycoprotein ligands including CD34, GlyCAM-1, MAdCAM-1, and PSGL-1 and acts as a “homing receptor” for lymphocytes to enter secondary lymphoid tissues via high endothelial venules.The Mel-14 monoclonal antibody reacts with mouse CD62L also known as L-selectin and MEL-14. CD62L is a 76 kDa glycoprotein and a member of the selectin family that is expressed by neutrophils, monocytes, the majority of naïve T and B cells, a subset of memory T cells, NK cells, and most thymocytes. CD62L is a cell adhesion molecule that binds to many glycoprotein ligands including CD34, GlyCAM-1, MAdCAM-1, and PSGL-1 and acts as a “homing receptor” for lymphocytes to enter secondary lymphoid tissues via high endothelial venules.Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C3H/eb mouse B lymphoma 38C-13 |
| Reported Applications | in vivo CD62L neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107665 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
In vivo CD62L neutralization
Brinkman, C. C., et al. (2016). "Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration" Nat Commun 7: 12021. PubMed
Regulatory T cells (Tregs) are essential to suppress unwanted immunity or inflammation. After islet allo-transplant Tregs must migrate from blood to allograft, then via afferent lymphatics to draining LN to protect allografts. Here we show that Tregs but not non-Treg T cells use lymphotoxin (LT) during migration from allograft to draining LN, and that LT deficiency or blockade prevents normal migration and allograft protection. Treg LTalphabeta rapidly modulates cytoskeletal and membrane structure of lymphatic endothelial cells; dependent on VCAM-1 and non-canonical NFkappaB signalling via LTbetaR. These results demonstrate a form of T-cell migration used only by Treg in tissues that serves an important role in their suppressive function and is a unique therapeutic focus for modulating suppression.
In vivo CD62L neutralization
Guidotti, L. G., et al. (2015). "Immunosurveillance of the liver by intravascular effector CD8(+) T cells" Cell 161(3): 486-500. PubMed
Effector CD8(+) T cells (CD8 TE) play a key role during hepatotropic viral infections. Here, we used advanced imaging in mouse models of hepatitis B virus (HBV) pathogenesis to understand the mechanisms whereby these cells home to the liver, recognize antigens, and deploy effector functions. We show that circulating CD8 TE arrest within liver sinusoids by docking onto platelets previously adhered to sinusoidal hyaluronan via CD44. After the initial arrest, CD8 TE actively crawl along liver sinusoids and probe sub-sinusoidal hepatocytes for the presence of antigens by extending cytoplasmic protrusions through endothelial fenestrae. Hepatocellular antigen recognition triggers effector functions in a diapedesis-independent manner and is inhibited by the processes of sinusoidal defenestration and capillarization that characterize liver fibrosis. These findings reveal the dynamic behavior whereby CD8 TE control hepatotropic pathogens and suggest how liver fibrosis might reduce CD8 TE immune surveillance toward infected or transformed hepatocytes.
In vivo CD62L neutralization
Cremasco, V., et al. (2014). "B cell homeostasis and follicle confines are governed by fibroblastic reticular cells" Nat Immunol 15(10): 973-981. PubMed
Fibroblastic reticular cells (FRCs) are known to inhabit T cell-rich areas of lymphoid organs, where they function to facilitate interactions between T cells and dendritic cells. However, in vivo manipulation of FRCs has been limited by a dearth of genetic tools that target this lineage. Here, using a mouse model to conditionally ablate FRCs, we demonstrated their indispensable role in antiviral T cell responses. Unexpectedly, loss of FRCs also attenuated humoral immunity due to impaired B cell viability and follicular organization. Follicle-resident FRCs established a favorable niche for B lymphocytes via production of the cytokine BAFF. Thus, our study indicates that adaptive immunity requires an intact FRC network and identifies a subset of FRCs that control B cell homeostasis and follicle identity.
In vivo CD62L neutralization
Harp, J. R., et al. (2010). "Memory T cells are enriched in lymph nodes of selectin-ligand-deficient mice" J Immunol 185(10): 5751-5761. PubMed
Fucosyltransferase-IV and -VII double knockout (FtDKO) mice reveal profound impairment in T cell trafficking to lymph nodes (LNs) due to an inability to synthesize selectin ligands. We observed an increase in the proportion of memory/effector (CD44(high)) T cells in LNs of FtDKO mice. We infected FtDKO mice with lymphocytic choriomeningitis virus to generate and track Ag-specific CD44(high)CD8 T cells in secondary lymphoid organs. Although frequencies were similar, total Ag-specific effector CD44(high)CD8 T cells were significantly reduced in LNs, but not blood, of FtDKO mice at day 8. In contrast, frequencies of Ag-specific memory CD44(high)CD8 T cells were up to 8-fold higher in LNs of FtDKO mice at day 60. Because wild-type mice treated with anti-CD62L treatment also showed increased frequencies of CD44(high) T cells in LNs, we hypothesized that memory T cells were preferentially retained in, or preferentially migrated to, FtDKO LNs. We analyzed T cell entry and egress in LNs using adoptive transfer of bone fide naive or memory T cells. Memory T cells were not retained longer in LNs compared with naive T cells; however, T cell exit slowed significantly as T cell numbers declined. Memory T cells were profoundly impaired in entering LNs of FtDKO mice; however, memory T cells exhibited greater homeostatic proliferation in FtDKO mice. These results suggest that memory T cells are enriched in LNs with T cell deficits by several mechanisms, including longer T cell retention and increased homeostatic proliferation.
In vivo CD62L neutralization
Harp, J. R. and T. M. Onami. (2010). "Naive T cells re-distribute to the lungs of selectin ligand deficient mice" PLoS One 5(6): e10973. PubMed
BACKGROUND: Selectin mediated tethering represents one of the earliest steps in T cell extravasation into lymph nodes via high endothelial venules and is dependent on the biosynthesis of sialyl Lewis X (sLe(x)) ligands by several glycosyltransferases, including two fucosyltransferases, fucosyltransferase-IV and -VII. Selectin mediated binding also plays a key role in T cell entry to inflamed organs. METHODOLOGY/PRINCIPAL FINDINGS: To understand how loss of selectin ligands (sLe(x)) influences T cell migration to the lung, we examined fucosyltransferase-IV and -VII double knockout (FtDKO) mice. We discovered that FtDKO mice showed significant increases (approximately 5-fold) in numbers of naive T cells in non-inflamed lung parenchyma with no evidence of induced bronchus-associated lymphoid tissue. In contrast, activated T cells were reduced in inflamed lungs of FtDKO mice following viral infection, consistent with the established role of selectin mediated T cell extravasation into inflamed lung. Adoptive transfer of T cells into FtDKO mice revealed impaired T cell entry to lymph nodes, but selective accumulation in non-lymphoid organs. Moreover, inhibition of T cell entry to the lymph nodes by blockade of L-selectin, or treatment of T cells with pertussis toxin to inhibit chemokine dependent G-coupled receptor signaling, also resulted in increased T cells in non-lymphoid organs. Conversely, inhibition of T cell egress from lymph nodes using FTY720 agonism of S1P1 impaired T cell migration into non-lymphoid organs. CONCLUSIONS/SIGNIFICANCE: Taken together, our results suggest that impaired T cell entry into lymph nodes via high endothelial venules due to genetic deficiency of selectin ligands results in the selective re-distribution and accumulation of T cells in non-lymphoid organs, and correlates with their increased frequency in the blood. Re-distribution of T cells into organs could potentially play a role in the initiation of T cell mediated organ diseases.
- Immunology and Microbiology,
CD301b+ dendritic cell-derived IL-2 dictates CD4+ T helper cell differentiation.
In Nat Commun on 26 February 2025 by Tatsumi, N., El-Fenej, J., et al.
PubMed
T helper (Th) cell differentiation is fundamental to functional adaptive immunity. Different subsets of dendritic cells (DC) preferentially induce different types of Th cells, but the DC-derived mechanism for Th type 2 (Th2) differentiation is not fully understood. Here, we show that in mice, CD301b+ DCs, a major Th2-inducing DC subset, drive Th2 differentiation through cognate interaction by rapidly inducing IL-2 receptor signalling in CD4+ T cells. Mechanistically, CD40 engagement prompts IL-2 production selectively from CD301b+ DCs to maximize CD25 expression in CD4+ T cells, which instructs the Th2 fate decision, while simultaneously skewing CD4+ T cells away from the T follicular helper fate. Moreover, CD301b+ DCs utilize their own CD25 to facilitate directed action of IL-2 toward cognate CD4+ T cells, as genetic deletion of CD25 in CD301b+ DCs results in reduced IL-2-mediated signalling in antigen-specific CD4+ T cells and hence their Th2 differentiation. These results highlight the critical role of DC-intrinsic CD40-IL-2 axis in Th cell fate decision.
- Mus musculus (Mouse),
- Immunology and Microbiology
A lymphoid tissue chemokine checkpoint prevents loss of CD8+T cell functionality
In bioRxiv on 22 September 2024 by Altenburger, L. M., Claudino Carvoeiro, D., et al.
- In vivo experiments,
- Mus musculus (Mouse)
Therapeutic potential of co-signaling receptor modulation in hepatitis B.
In Cell on 25 July 2024 by Andreata, F., Laura, C., et al.
PubMed
Reversing CD8+ T cell dysfunction is crucial in treating chronic hepatitis B virus (HBV) infection, yet specific molecular targets remain unclear. Our study analyzed co-signaling receptors during hepatocellular priming and traced the trajectory and fate of dysfunctional HBV-specific CD8+ T cells. Early on, these cells upregulate PD-1, CTLA-4, LAG-3, OX40, 4-1BB, and ICOS. While blocking co-inhibitory receptors had minimal effect, activating 4-1BB and OX40 converted them into antiviral effectors. Prolonged stimulation led to a self-renewing, long-lived, heterogeneous population with a unique transcriptional profile. This includes dysfunctional progenitor/stem-like (TSL) cells and two distinct dysfunctional tissue-resident memory (TRM) populations. While 4-1BB expression is ubiquitously maintained, OX40 expression is limited to TSL. In chronic settings, only 4-1BB stimulation conferred antiviral activity. In HBeAg+ chronic patients, 4-1BB activation showed the highest potential to rejuvenate dysfunctional CD8+ T cells. Targeting all dysfunctional T cells, rather than only stem-like precursors, holds promise for treating chronic HBV infection.
- Mus musculus (Mouse),
- Immunology and Microbiology
Rapid activation of IL-2 receptor signaling by CD301b+DC-derived IL-2 dictates the outcome of helper T cell differentiation
In bioRxiv on 31 October 2023 by Tatsumi, N., El-Fenej, J., et al.
- Flow cytometry/Cell sorting,
- Mus musculus (Mouse),
- Neuroscience,
- Immunology and Microbiology
Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks.
In Immunity on 8 August 2023 by Ugur, M., Labios, R. J., et al.
PubMed
Unlike macrophage networks composed of long-lived tissue-resident cells within specific niches, conventional dendritic cells (cDCs) that generate a 3D network in lymph nodes (LNs) are short lived and continuously replaced by DC precursors (preDCs) from the bone marrow (BM). Here, we examined whether specific anatomical niches exist within which preDCs differentiate toward immature cDCs. In situ photoconversion and Prtn3-based fate-tracking revealed that the LN medullary cords are preferential entry sites for preDCs, serving as specific differentiation niches. Repopulation and fate-tracking approaches demonstrated that the cDC1 network unfolded from the medulla along the vascular tree toward the paracortex. During inflammation, collective maturation and migration of resident cDC1s to the paracortex created discontinuity in the medullary cDC1 network and temporarily impaired responsiveness. The decrease in local cDC1 density resulted in higher Flt3L availability in the medullary niche, which accelerated cDC1 development to restore the network. Thus, the spatiotemporal development of the cDC1 network is locally regulated in dedicated LN niches via sensing of cDC1 densities.
- Immunology and Microbiology
Multitier mechanics control stromal adaptations in the swelling lymph node.
In Nat Immunol on 1 August 2022 by Assen, F. P., Abe, J., et al.
PubMed
Lymph nodes (LNs) comprise two main structural elements: fibroblastic reticular cells that form dedicated niches for immune cell interaction and capsular fibroblasts that build a shell around the organ. Immunological challenge causes LNs to increase more than tenfold in size within a few days. Here, we characterized the biomechanics of LN swelling on the cellular and organ scale. We identified lymphocyte trapping by influx and proliferation as drivers of an outward pressure force, causing fibroblastic reticular cells of the T-zone (TRCs) and their associated conduits to stretch. After an initial phase of relaxation, TRCs sensed the resulting strain through cell matrix adhesions, which coordinated local growth and remodeling of the stromal network. While the expanded TRC network readopted its typical configuration, a massive fibrotic reaction of the organ capsule set in and countered further organ expansion. Thus, different fibroblast populations mechanically control LN swelling in a multitier fashion.
- Genetics,
- Immunology and Microbiology,
- Mus musculus (Mouse),
- In vivo experiments
Treg tissue stability depends on lymphotoxin beta-receptor- and adenosine-receptor-driven lymphatic endothelial cell responses.
In Cell Rep on 19 April 2022 by Saxena, V., Piao, W., et al.
PubMed
Regulatory T cell (Treg) lymphatic migration is required for resolving inflammation and prolonging allograft survival. Focusing on Treg interactions with lymphatic endothelial cells (LECs), we dissect mechanisms and functional consequences of Treg transendothelial migration (TEM). Using three genetic mouse models of pancreatic islet transplantation, we show that Treg lymphotoxin (LT) αβ and LEC LTβ receptor (LTβR) signaling are required for efficient Treg migration and suppressive function to prolong allograft survival. Inhibition of LT signaling increases Treg conversion to Foxp3loCD25lo exTregs. In a transwell-based model of TEM across polarized LECs, non-migrated Tregs become exTregs. Such conversion is regulated by LTβR nuclear factor κB (NF-κB) signaling in LECs, which increases interleukin-6 (IL-6) production and drives exTreg conversion. Migrating Tregs are ectonucleotidase CD39hi and resist exTreg conversion in an adenosine-receptor-2A-dependent fashion. Human Tregs migrating across human LECs behave similarly. These molecular interactions can be targeted for therapeutic manipulation of immunity and suppression.
- Immunology and Microbiology
Extracellular ATP Limits Homeostatic T Cell Migration Within Lymph Nodes.
In Front Immunol on 11 January 2022 by Kobayashi, D., Sugiura, Y., et al.
PubMed
Whereas adenosine 5'-triphosphate (ATP) is the major energy source in cells, extracellular ATP (eATP) released from activated/damaged cells is widely thought to represent a potent damage-associated molecular pattern that promotes inflammatory responses. Here, we provide suggestive evidence that eATP is constitutively produced in the uninflamed lymph node (LN) paracortex by naïve T cells responding to C-C chemokine receptor type 7 (CCR7) ligand chemokines. Consistently, eATP was markedly reduced in naïve T cell-depleted LNs, including those of nude mice, CCR7-deficient mice, and mice subjected to the interruption of the afferent lymphatics in local LNs. Stimulation with a CCR7 ligand chemokine, CCL19, induced ATP release from LN cells, which inhibited CCR7-dependent lymphocyte migration in vitro by a mechanism dependent on the purinoreceptor P2X7 (P2X7R), and P2X7R inhibition enhanced T cell retention in LNs in vivo. These results collectively indicate that paracortical eATP is produced by naïve T cells in response to constitutively expressed chemokines, and that eATP negatively regulates CCR7-mediated lymphocyte migration within LNs via a specific subtype of ATP receptor, demonstrating its fine-tuning role in homeostatic cell migration within LNs.
- Mus musculus (Mouse),
- Immunology and Microbiology
Effective CD4 T cell priming requires repertoire scanning by CD301b+ migratory cDC2 cells upon lymph node entry.
In Sci Immunol on 10 December 2021 by Tatsumi, N., Codrington, A. L., et al.
PubMed
During the initiation of adaptive immune responses, millions of lymphocytes must be scanned to find the few cognate clones. The activation mechanisms of CD4 T cells have been extensively studied, but the cellular mechanisms that drive selection of cognate clones are not completely understood. Here, we show that recently homed naïve polyclonal CD4 T cells are temporarily retained before leaving the lymph node. This stop-and-go traffic of CD4 T cells provides an adequate time window for efficient scanning and timely priming of antigen-specific cognate clones. CD301b+ DCs, a major subset of migratory cDC2 cells, localize to the areas around high endothelial venules, where they retain incoming polyclonal CD4 T cells through MHCII-dependent but antigen-independent mechanisms, while concurrently providing cognate stimuli for priming. These results indicate that CD301b+ DCs function as an immunological “display window” for CD4 T cells to efficiently scan their antigen specificity.
- Immunology and Microbiology
Inflammation rapidly recruits mammalian GMP and MDP from bone marrow into regional lymphatics.
In Elife on 8 April 2021 by Serrano-López, J., Hegde, S., et al.
PubMed
Innate immune cellular effectors are actively consumed during systemic inflammation, but the systemic traffic and the mechanisms that support their replenishment remain unknown. Here, we demonstrate that acute systemic inflammation induces the emergent activation of a previously unrecognized system of rapid migration of granulocyte-macrophage progenitors and committed macrophage-dendritic progenitors, but not other progenitors or stem cells, from bone marrow (BM) to regional lymphatic capillaries. The progenitor traffic to the systemic lymphatic circulation is mediated by Ccl19/Ccr7 and is NF-κB independent, Traf6/IκB-kinase/SNAP23 activation dependent, and is responsible for the secretion of pre-stored Ccl19 by a subpopulation of CD205+/CD172a+ conventional dendritic cells type 2 and upregulation of BM myeloid progenitor Ccr7 signaling. Mature myeloid Traf6 signaling is anti-inflammatory and necessary for lymph node myeloid cell development. This report unveils the existence and the mechanistic basis of a very early direct traffic of myeloid progenitors from BM to lymphatics during inflammation.
- Immunology and Microbiology
TSLP drives acute TH2-cell differentiation in lungs.
In J Allergy Clin Immunol on 1 December 2020 by Lai, J. F., Thompson, L. J., et al.
PubMed
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine that is important for the development of type 2 inflammatory responses at mucosal surfaces.
Optimal CD4T Cell Priming in Lymph Nodes Requires Repertoire Scanning by CD301bsup>+/sup> Migratory cDC2 Cells
In bioRxiv on 31 August 2020 by Tatsumi, N., Codrington, A. L., et al.
- Immunology and Microbiology
Neutrophils Recirculate through Lymph Nodes to Survey Tissues for Pathogens.
In J Immunol on 1 May 2020 by Bogoslowski, A., Wijeyesinghe, S., et al.
PubMed
The adaptive immune function of lymph nodes is dependent on constant recirculation of lymphocytes. In this article, we identify neutrophils present in the lymph node at steady state, exhibiting the same capacity for recirculation. In germ-free mice, neutrophils still recirculate through lymph nodes, and in mice cohoused with wild microbiome mice, the level of neutrophils in lymph nodes increases significantly. We found that at steady state, neutrophils enter the lymph node entirely via L-selectin and actively exit via efferent lymphatics via an S1P dependent mechanism. The small population of neutrophils in the lymph node can act as reconnaissance cells to recruit additional neutrophils in the event of bacterial dissemination to the lymph node. Without these reconnaissance cells, there is a delay in neutrophil recruitment to the lymph node and a reduction in swarm formation following Staphylococcus aureus infection. This ability to recruit additional neutrophils by lymph node neutrophils is initiated by LTB4. This study establishes the capacity of neutrophils to recirculate, much like lymphocytes via L-selectin and high endothelial venules in lymph nodes and demonstrates how the presence of neutrophils at steady state fortifies the lymph node in case of an infection disseminating through lymphatics.
- Immunology and Microbiology
The physical form of microbial ligands bypasses the need for dendritic cell migration to stimulate adaptive immunity
In bioRxiv on 5 March 2020 by Borriello, F., Spreafico, R., et al.
- Immunology and Microbiology
Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming.
In Nature on 1 October 2019 by Benechet, A. P., De Simone, G., et al.
PubMed
The responses of CD8+ T cells to hepatotropic viruses such as hepatitis B range from dysfunction to differentiation into effector cells, but the mechanisms that underlie these distinct outcomes remain poorly understood. Here we show that priming by Kupffer cells, which are not natural targets of hepatitis B, leads to differentiation of CD8+ T cells into effector cells that form dense, extravascular clusters of immotile cells scattered throughout the liver. By contrast, priming by hepatocytes, which are natural targets of hepatitis B, leads to local activation and proliferation of CD8+ T cells but not to differentiation into effector cells; these cells form loose, intravascular clusters of motile cells that coalesce around portal tracts. Transcriptomic and chromatin accessibility analyses reveal unique features of these dysfunctional CD8+ T cells, with limited overlap with those of exhausted or tolerant T cells; accordingly, CD8+ T cells primed by hepatocytes cannot be rescued by treatment with anti-PD-L1, but instead respond to IL-2. These findings suggest immunotherapeutic strategies against chronic hepatitis B infection.
- Immunology and Microbiology,
- Neuroscience
The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction.
In Cell on 22 August 2019 by Collins, N., Han, S. J., et al.
PubMed
Mammals evolved in the face of fluctuating food availability. How the immune system adapts to transient nutritional stress remains poorly understood. Here, we show that memory T cells collapsed in secondary lymphoid organs in the context of dietary restriction (DR) but dramatically accumulated within the bone marrow (BM), where they adopted a state associated with energy conservation. This response was coordinated by glucocorticoids and associated with a profound remodeling of the BM compartment, which included an increase in T cell homing factors, erythropoiesis, and adipogenesis. Adipocytes, as well as CXCR4-CXCL12 and S1P-S1P1R interactions, contributed to enhanced T cell accumulation in BM during DR. Memory T cell homing to BM during DR was associated with enhanced protection against infections and tumors. Together, this work uncovers a fundamental host strategy to sustain and optimize immunological memory during nutritional challenges that involved a temporal and spatial reorganization of the memory pool within "safe haven" compartments.
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8+ T Cells.
In Cell Metab on 6 August 2019 by Hu, Z., Qu, G., et al.
PubMed
CD8+ T cell expansions and functions rely on glycolysis, but the mechanisms underlying CD8+ T cell glycolytic metabolism remain elusive. Here, we show that acylglycerol kinase (AGK) is required for the establishment and maintenance of CD8+ T cell metabolic and functional fitness. AGK deficiency dampens CD8+ T cell antitumor functions in vivo and perturbs CD8+ T cell proliferation in vitro. Activation of phosphatidylinositol-3-OH kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling, which mediates elevated CD8+ T cell glycolysis, is tightly dependent on AGK kinase activity. Mechanistically, T cell antigen receptor (TCR)- and CD28-stimulated recruitment of PTEN to the plasma membrane facilitates AGK-PTEN interaction and AGK-triggered PTEN phosphorylation, thereby restricting PTEN phosphatase activity in CD8+ T cells. Collectively, these results demonstrate that AGK maintains CD8+ T cell metabolic and functional state by restraining PTEN activity and highlight a critical role for AGK in CD8+ T cell metabolic programming and effector function.
- In vivo experiments,
- In vivo experiments,
- Mus musculus (Mouse),
- Immunology and Microbiology
Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues.
In Immunity on 18 December 2018 by He, W., Holtkamp, S., et al.
PubMed
The number of leukocytes present in circulation varies throughout the day, reflecting bone marrow output and emigration from blood into tissues. Using an organism-wide circadian screening approach, we detected oscillations in pro-migratory factors that were distinct for specific vascular beds and individual leukocyte subsets. This rhythmic molecular signature governed time-of-day-dependent homing behavior of leukocyte subsets to specific organs. Ablation of BMAL1, a transcription factor central to circadian clock function, in endothelial cells or leukocyte subsets demonstrated that rhythmic recruitment is dependent on both microenvironmental and cell-autonomous oscillations. These oscillatory patterns defined leukocyte trafficking in both homeostasis and inflammation and determined detectable tumor burden in blood cancer models. Rhythms in the expression of pro-migratory factors and migration capacities were preserved in human primary leukocytes. The definition of spatial and temporal expression profiles of pro-migratory factors guiding leukocyte migration patterns to organs provides a resource for the further study of the impact of circadian rhythms in immunity.
- Immunology and Microbiology
Mononuclear cell dynamics in M. tuberculosis infection provide opportunities for therapeutic intervention.
In PLoS Pathog on 1 October 2018 by Norris, B. A., Ernst, J. D., et al.
PubMed
Mycobacterium tuberculosis causes chronic infection of mononuclear phagocytes, especially resident (alveolar) macrophages, recruited macrophages, and dendritic cells. Despite the importance of these cells in tuberculosis (TB) pathogenesis and immunity, little is known about the population dynamics of these cells at the sites of infection. We used a combination of congenic monocyte adoptive transfer, and pulse-chase labeling of DNA, to determine the kinetics and characteristics of trafficking, differentiation, and infection of mononuclear phagocytes during the chronic, adaptive immune phase of M. tuberculosis infection in mice. We found that Ly6Chi monocytes traffic rapidly to the lungs, where a subpopulation become Ly6Clo and remain in the lung vascular space, while the remainder migrate into the lung parenchyma and differentiate into Ly6Chi dendritic cells, CD11b+ dendritic cells, and recruited macrophages. As in humans with TB, M. tuberculosis-infected mice have increased numbers of blood monocytes; this is due to increased egress from the bone marrow, and not delayed egress from the blood. Pulse-chase labeling of dividing cells and flow cytometry analysis revealed a T1/2 of ~15 hrs for Ly6Chi monocytes, indicating that they differentiate rapidly upon entry to the parenchyma of infected lungs; in contrast, cells that differentiate from Ly6Chi monocytes turn over more slowly, but diminish in frequency in less than one week. New cells (identified by pulse-chase labeling) acquire bacteria within 1-3 days of appearance in the lungs, indicating that bacteria regularly encounter new cellular niches, even during the chronic stage of infection. Our findings that mononuclear phagocyte populations at the site of M. tuberculosis infection are highly dynamic provide support for specific approaches for host-directed therapies directed at monocytes, including trained immunity, as potential interventions in TB, by replacing cells with limited antimycobacterial capabilities with newly-recruited cells better able to restrict and kill M. tuberculosis.
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
Metabolic control of regulatory T cell stability and function by TRAF3IP3 at the lysosome.
In J Exp Med on 3 September 2018 by Yu, X., Teng, X. L., et al.
PubMed
Metabolic programs are crucial for regulatory T (T reg) cell stability and function, but the underlying mechanisms that regulate T reg cell metabolism are elusive. Here, we report that lysosomal TRAF3IP3 acts as a pivotal regulator in the maintenance of T reg cell metabolic fitness. T reg-specific deletion of Traf3ip3 impairs T reg cell function, causing the development of inflammatory disorders and stronger antitumor T cell responses in mice. Excessive mechanistic target of rapamycin complex 1 (mTORC1)-mediated hyper-glycolytic metabolism is responsible for the instability of TRAF3IP3-deficient T reg cells. Mechanistically, TRAF3IP3 restricts mTORC1 signaling by recruiting the serine-threonine phosphatase catalytic subunit (PP2Ac) to the lysosome, thereby facilitating the interaction of PP2Ac with the mTORC1 component Raptor. Our results define TRAF3IP3 as a metabolic regulator in T reg cell stability and function and suggest a lysosome-specific mTORC1 signaling mechanism that regulates T reg cell metabolism.