InVivoPlus anti-mouse IL-4
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
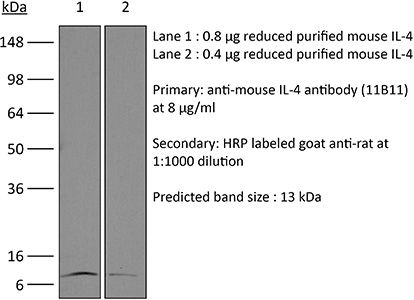
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Partially purified native mouse IL-4 |
| Reported Applications |
in vivo IL-4 neutralization in vitro IL-4 neutralization in vivo IL-4 receptor stimulation (as a complex with IL-4) Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107707 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-4 neutralization
in vivo IL-4 receptor stimulation (as a complex with IL-4)
Gaya, M., et al (2018). "Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells" Cell 172(3): 517-533 e520.
PubMed
B cells constitute an essential line of defense from pathogenic infections through the generation of class-switched antibody-secreting cells (ASCs) in germinal centers. Although this process is known to be regulated by follicular helper T (TfH) cells, the mechanism by which B cells initially seed germinal center reactions remains elusive. We found that NKT cells, a population of innate-like T lymphocytes, are critical for the induction of B cell immunity upon viral infection. The positioning of NKT cells at the interfollicular areas of lymph nodes facilitates both their direct priming by resident macrophages and the localized delivery of innate signals to antigen-experienced B cells. Indeed, NKT cells secrete an early wave of IL-4 and constitute up to 70% of the total IL-4-producing cells during the initial stages of infection. Importantly, the requirement of this innate immunity arm appears to be evolutionarily conserved because early NKT and IL-4 gene signatures also positively correlate with the levels of neutralizing antibodies in Zika-virus-infected macaques. In conclusion, our data support a model wherein a pre-TfH wave of IL-4 secreted by interfollicular NKT cells triggers the seeding of germinal center cells and serves as an innate link between viral infection and B cell immunity.
in vitro IL-4 neutralization
Clever, D., et al (2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche" Cell 166(5): 1117-1131 e1114.
PubMed
Cancer cells must evade immune responses at distant sites to establish metastases. The lung is a frequent site for metastasis. We hypothesized that lung-specific immunoregulatory mechanisms create an immunologically permissive environment for tumor colonization. We found that T-cell-intrinsic expression of the oxygen-sensing prolyl-hydroxylase (PHD) proteins is required to maintain local tolerance against innocuous antigens in the lung but powerfully licenses colonization by circulating tumor cells. PHD proteins limit pulmonary type helper (Th)-1 responses, promote CD4(+)-regulatory T (Treg) cell induction, and restrain CD8(+) T cell effector function. Tumor colonization is accompanied by PHD-protein-dependent induction of pulmonary Treg cells and suppression of IFN-gamma-dependent tumor clearance. T-cell-intrinsic deletion or pharmacological inhibition of PHD proteins limits tumor colonization of the lung and improves the efficacy of adoptive cell transfer immunotherapy. Collectively, PHD proteins function in T cells to coordinate distinct immunoregulatory programs within the lung that are permissive to cancer metastasis.
in vitro IL-4 neutralization
Flow Cytometry
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vitro IL-4 neutralization
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro IL-4 neutralization
Hou, L., et al (2015). "The protease cathepsin L regulates Th17 cell differentiation" J Autoimmun. S 0896-8411(15): 30024-X.
PubMed
Previously we reported that IL-17+ T cells, primarily IL-17+ gammadelta cells, are increased in mice lacking the protease inhibitor serpinB1 (serpinb1-/- mice). Here we show that serpinB1-deficient CD4 cells exhibit a cell-autonomous and selective deficiency in suppressing T helper 17 (Th17) cell differentiation. This suggested an opposing role for one or more protease in promoting Th17 differentiation. We found that several SerpinB1-inhibitable cysteine cathepsins are induced in Th17 cells, most prominently cathepsin L (catL); this was verified by peptidase assays, active site labeling and Western blots. Moreover, Th17 differentiation was suppressed by both broad cathepsin inhibitors and catL selective inhibitors. CatL is present in Th17 cells as single chain (SC)- and two-chain (TC)-forms. Inhibiting asparagine endopeptidase (AEP) blocked conversion of SC-catL to TC-catL and increased generation of serpinb1-/- Th17 cells, but not wild-type Th17 cells. These findings suggest that SC-catL is biologically active in promoting Th17 generation and is counter-regulated by serpinB1 and secondarily by AEP. Thus, in addition to regulation by cytokines and transcription factors, differentiation of CD4 cells to Th17 cells is actively regulated by a catL-serpinB1-AEP module. Targeting this protease regulatory module could be an approach to treating Th17 cell-driven autoimmune disorders.
in vitro IL-4 neutralization
Kim, Y. U., et al (2015). "Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells" PLoS One 10(3): e0120294.
PubMed
BXD2 mice spontaneously develop autoantibodies and subsequent glomerulonephritis, offering a useful animal model to study autoimmune lupus. Although initial studies showed a critical contribution of IL-17 and Th17 cells in mediating autoimmune B cell responses in BXD2 mice, the role of follicular helper T (Tfh) cells remains incompletely understood. We found that both the frequency of Th17 cells and the levels of IL-17 in circulation in BXD2 mice were comparable to those of wild-type. By contrast, the frequency of PD-1+ CXCR5+ Tfh cells was significantly increased in BXD2 mice compared with wild-type mice, while the frequency of PD-1+ CXCR5+ Foxp3+ follicular regulatory T (Tfr) cells was reduced in the former group. The frequency of Tfh cells rather than that of Th17 cells was positively correlated with the frequency of germinal center B cells as well as the levels of autoantibodies to dsDNA. More importantly, CXCR5+ CD4+ T cells isolated from BXD2 mice induced the production of IgG from naive B cells in an IL-21-dependent manner, while CCR6+ CD4+ T cells failed to do so. These results together demonstrate that Tfh cells rather than Th17 cells contribute to the autoimmune germinal center reactions in BXD2 mice.
in vitro IL-4 neutralization
Hill, E. V., et al (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115.
PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro IL-4 neutralization
Burton, B. R., et al (2014). "Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy" Nat Commun 5: 4741.
PubMed
Antigen-specific immunotherapy combats autoimmunity or allergy by reinstating immunological tolerance to target antigens without compromising immune function. Optimization of dosing strategy is critical for effective modulation of pathogenic CD4(+) T-cell activity. Here we report that dose escalation is imperative for safe, subcutaneous delivery of the high self-antigen doses required for effective tolerance induction and elicits anergic, interleukin (IL)-10-secreting regulatory CD4(+) T cells. Analysis of the CD4(+) T-cell transcriptome, at consecutive stages of escalating dose immunotherapy, reveals progressive suppression of transcripts positively regulating inflammatory effector function and repression of cell cycle pathways. We identify transcription factors, c-Maf and NFIL3, and negative co-stimulatory molecules, LAG-3, TIGIT, PD-1 and TIM-3, which characterize this regulatory CD4(+) T-cell population and whose expression correlates with the immunoregulatory cytokine IL-10. These results provide a rationale for dose escalation in T-cell-directed immunotherapy and reveal novel immunological and transcriptional signatures as surrogate markers of successful immunotherapy.
in vitro IL-4 neutralization
Tang, W., et al (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566.
PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vitro IL-4 neutralization
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vitro IL-4 neutralization
McKinstry, K. K., et al (2014). "Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2" Nat Commun 5: 5377.
PubMed
It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation.
in vivo IL-4 neutralization
Mishra, P. K., et al (2013). "Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response" Mucosal Immunol 6(2): 297-308.
PubMed
Helminth infection can prevent type 1 diabetes (T1D); however, the regulatory mechanisms inhibiting disease remain largely undefined. In these studies, nonobese diabetic (NOD) IL-4(-/-) mice were infected with the strictly enteric nematode parasite, Heligmosomoides polygyrus. Short-term infection, 5-7 weeks of age, inhibited T1D onset, as late as 40 weeks of age. CD4(+) T-cell STAT6 phosphorylation was inhibited, while suppressed signal transducer and activator of transcription 1 phosphorylation was sustained, as were increases in FOXP3(-), CD4(+) T-cell interleukin (IL)-10 production. Blockade of IL-10 signaling in NOD-IL-4(-/-), but not in NOD, mice during this short interval abrogated protective effects resulting in pancreatic beta-cell destruction and ultimately T1D. Transfer of CD4(+) T cells from H. polygyrus (Hp)-inoculated NOD IL-4(-/-) mice to NOD mice blocked the onset of T1D. These studies indicate that Hp infection induces non-T-regulatory cells to produce IL-10 independently of STAT6 signaling and that in this Th2-deficient environment IL-10 is essential for T1D inhibition.
in vitro IL-4 neutralization
Li, X., et al (2012). "Divergent requirement for Galphas and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets" J Clin Invest 122(3): 963-973.
PubMed
cAMP, the intracellular signaling molecule produced in response to GPCR signaling, has long been recognized as an immunosuppressive agent that inhibits T cell receptor activation and T cell function. However, recent studies show that cAMP also promotes T cell-mediated immunity. Central to cAMP production downstream of GPCR activation is the trimeric G protein Gs. In order to reconcile the reports of divergent effects of cAMP in T cells and to define the direct effect of cAMP in T cells, we engineered mice in which the stimulatory Galpha subunit of Gs (Galphas) could be deleted in T cells using CD4-Cre (Gnas(DeltaCD4)). Gnas(DeltaCD4) CD4(+) T cells had reduced cAMP accumulation and Ca2(+) influx. In vitro and in vivo, Gnas(DeltaCD4) CD4(+) T cells displayed impaired differentiation to specific Th subsets: Th17 and Th1 cells were reduced or absent, but Th2 and regulatory T cells were unaffected. Furthermore, Gnas(DeltaCD4) CD4(+) T cells failed to provoke colitis in an adoptive transfer model, indicating reduced inflammatory function. Restoration of cAMP levels rescued the impaired phenotype of Gnas(DeltaCD4) CD4(+) T cells, reinstated the PKA-dependent influx of Ca2(+), and enhanced the ability of these cells to induce colitis. Our findings thus define an important role for cAMP in the differentiation of Th subsets and their subsequent inflammatory responses, and provide evidence that altering cAMP levels in CD4(+) T cells could provide an immunomodulatory approach targeting specific Th subsets.
in vitro IL-4 neutralization
Ueda, A., et al (2012). "Fyn promotes Th17 differentiation by regulating the kinetics of RORgammat and Foxp3 expression" J Immunol 188(11): 5247-5256.
PubMed
Th17 cells constitute a proinflammatory CD4(+) T cell subset that is important for microbial clearance, but also are implicated as propagators of various autoimmune pathologies. Evidence suggests that Th17 cells share common progenitors with immunosuppressive CD4(+) inducible regulatory T cells (T(REG)) and that the developmental pathways of these two subsets are reciprocally regulated. In this study, we show evidence that the Src family tyrosine kinase Fyn helps regulate this Th17/T(REG) balance. When placed under Th17-skewing conditions, CD4(+) T cells from fyn(-/-) mice had decreased levels of IL-17, but increased expression of the T(REG) transcription factor Foxp3. The defect in IL-17 expression occurred independently of the ectopic Foxp3 expression and correlated with a delay in retinoic acid-related orphan receptor gammat upregulation and an inability to maintain normal STAT3 activation. Fyn-deficient Th17 cells also exhibited delayed upregulation of Il23r, Il21, Rora, and Irf4, as well as aberrant expression of Socs3, suggesting that Fyn may function upstream of a variety of molecular pathways that contribute to Th17 polarization. The fyn(-/-) mice had fewer IL-17(+)CD4(+) T cells in the large intestinal lamina propria compared with littermate controls. Furthermore, after transfer of either wild-type or fyn(-/-) naive CD4(+) T cells into Rag1(-/-) hosts, recipients receiving fyn(-/-) cells had fewer IL-17-producing T cells, indicating that Fyn may also regulate Th17 differentiation in vivo. These results identify Fyn as a possible novel regulator of the developmental balance between the Th17 cell and T(REG) subsets.
Product Citations
-
-
Immunology and Microbiology
-
COVID-19
IL-4 and TGF-β regulate inflammatory cytokines and cellular infiltration in the lung and systemic IL-6 in mouse-adapted SARS-CoV-2 infection.
In Immunohorizons on 25 August 2025 by Taye Sima, S., Puebla-Clark, L., et al.
PubMed
The pathology of severe COVID-19 is due to a hyperinflammatory immune response persisting after viral clearance. To understand how the immune response to SARS-CoV-2 is regulated to avoid severe COVID-19, we tested relevant immunoregulatory cytokines. Transforming growth factor β (TGF-β), interleukin (IL)-10, and IL-4 were neutralized upon infection with mouse-adapted SARS-CoV-2 (CMA3p20), a model of mild disease; lung inflammation was quantified by histology and flow cytometry at early and late time points. Mild weight loss and lung inflammation including consolidation and alveolar thickening were evident 3 d postinfection (dpi), and inflammation persisted to 7 dpi. Coinciding with early monocytic infiltrates, CCL2 and granulocyte colony-stimulating factor were transiently produced 3 dpi, while IL-12 and CCL5 persisted to 7 dpi, modeling viral and inflammatory phases of disease. Neutralization of TGF-β, but not IL-10 or IL-4, significantly increased lung inflammatory monocytes and elevated serum but not lung IL-6. Neutralization of IL-4 prolonged weight loss and increased early perivascular infiltration without changing viral titer. Anti-IL-4 reduced expression of Arg1, a gene associated with alternative activation of macrophages. Neutralizing TGF-β and IL-4 had differential effects on pathology after virus control. Lung perivascular infiltration was reduced 7 dpi by neutralization of IL-4 or TGF-β, and periairway inflammation was affected by anti-TGF-β, while alveolar infiltrates were not affected by either. Anti-IL-4 prolonged IL-12 to 7 dpi along with reduced IL-10 in lungs. Overall, the immunoregulatory cytokines TGF-β and IL-4 dampen initial inflammation in this mouse-adapted SARS-CoV-2 infection, suggesting that promotion of immunoregulation could help patients in early stages of disease.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
LincR-PPP2R5C deficiency enhancing the fungicidal activity of neutrophils in pulmonary cryptococcosis is linked to the upregulation of IL-4.
In MBio on 16 October 2024 by Yang, C., Gong, Y., et al.
PubMed
Pulmonary cryptococcosis is a common complication in immunocompromised patients. In a mouse model of pulmonary cryptococcosis, Cryptococcus neoformans induces a type 2 immune response that is detrimental to host protection. Long non-coding RNAs (lncRNAs) have emerged as key players in the pathogenesis of infectious diseases. However, the roles and mechanisms of lncRNAs in fungal infection are largely elusive. In the present study, we aimed to explore the roles of LincR-PPP2R5C in pulmonary cryptococcosis. We observed an increase in the level of LincR-PPP2R5C in the lung tissues of C57BL/6J mice after tracheal infection with C. neoformans. Subsequently, we intratracheally infected LincR-PPP2R5C knockout (KO) mice and wild-type mice with C. neoformans. LincR-PPP2R5C deficiency mitigates C. neoformans infection, which can be demonstrated by extending survival time and decreasing fungal burden in the lung. In the lung tissues of infected LincR-PPP2R5C KO mice, there was a notable increase in the levels of type 2 cytokines [interleukin (IL)-4 and IL-5] and an increase in the number of neutrophils in both the lung tissue and bronchoalveolar lavage fluid. Mechanistically, the lack of LincR-PPP2R5C results in increased protein phosphatase 2A phosphorylation, thereby enhancing the fungicidal activity of neutrophils against Cryptococcus neoformans, with IL-4 playing a synergistic role in this process. Overall, LincR-PPP2R5C deficiency mitigated pulmonary cryptococcosis by increasing the fungicidal activity of neutrophils, which was associated with increased IL-4 levels. Our study presented specific evidence of the role of host-derived lncRNAs in the regulation of C. neoformans infection.
-
-
-
Mus musculus (Mouse)
Stage-specific GATA3 induction promotes ILC2 development after lineage commitment.
In Nat Commun on 5 July 2024 by Furuya, H., Toda, Y., et al.
PubMed
Group 2 innate lymphoid cells (ILC2s) are a subset of innate lymphocytes that produce type 2 cytokines, including IL-4, IL-5, and IL-13. GATA3 is a critical transcription factor for ILC2 development at multiple stages. However, when and how GATA3 is induced to the levels required for ILC2 development remains unclear. Herein, we identify ILC2-specific GATA3-related tandem super-enhancers (G3SE) that induce high GATA3 in ILC2-committed precursors. G3SE-deficient mice exhibit ILC2 deficiency in the bone marrow, lung, liver, and small intestine with minimal impact on other ILC lineages or Th2 cells. Single-cell RNA-sequencing and subsequent flow cytometry analysis show that GATA3 induction mechanism, which is required for entering the ILC2 stage, is lost in IL-17RB+PD-1- late ILC2-committed precursor stage in G3SE-deficient mice. Cnot6l, part of the CCR4-NOT deadenylase complex, is a possible GATA3 target during ILC2 development. Our findings implicate a stage-specific regulatory mechanism for GATA3 expression during ILC2 development.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
Sphingolipid biosynthesis is essential for metabolic rewiring during TH17 cell differentiation.
In Sci Adv on 26 April 2024 by Abimannan, T., Parthibane, V., et al.
PubMed
T helper 17 (TH17) cells are implicated in autoimmune diseases, and several metabolic processes are shown to be important for their development and function. In this study, we report an essential role for sphingolipids synthesized through the de novo pathway in TH17 cell development. Deficiency of SPTLC1, a major subunit of serine palmitoyl transferase enzyme complex that catalyzes the first and rate-limiting step of de novo sphingolipid synthesis, impaired glycolysis in differentiating TH17 cells by increasing intracellular reactive oxygen species (ROS) through enhancement of nicotinamide adenine dinucleotide phosphate oxidase 2 activity. Increased ROS leads to impaired activation of mammalian target of rapamycin C1 and reduced expression of hypoxia-inducible factor 1-alpha and c-Myc-induced glycolytic genes. SPTLCI deficiency protected mice from developing experimental autoimmune encephalomyelitis and experimental T cell transfer colitis. Our results thus show a critical role for de novo sphingolipid biosynthetic pathway in shaping adaptive immune responses with implications in autoimmune diseases.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Interleukin-9 promotes mast cell progenitor proliferation and CCR2-dependent mast cell migration in allergic airway inflammation.
In Mucosal Immunol on 1 August 2023 by Pajulas, A., Fu, Y., et al.
PubMed
Allergic asthma is a chronic lung disease characterized by airway hyperresponsiveness and cellular infiltration that is exacerbated by immunoglobulin E-dependent mast cell (MC) activation. Interleukin-9 (IL-9) promotes MC expansion during allergic inflammation but precisely how IL-9 expands tissue MCs and promotes MC function is unclear. In this report, using multiple models of allergic airway inflammation, we show that both mature MCs (mMCs) and MC progenitors (MCp) express IL-9R and respond to IL-9 during allergic inflammation. IL-9 acts on MCp in the bone marrow and lungs to enhance proliferative capacity. Furthermore, IL-9 in the lung stimulates the mobilization of CCR2+ mMC from the bone marrow and recruitment to the allergic lung. Mixed bone marrow chimeras demonstrate that these are intrinsic effects in the MCp and mMC populations. IL-9-producing T cells are both necessary and sufficient to increase MC numbers in the lung in the context of allergic inflammation. Importantly, T cell IL-9-mediated MC expansion is required for the development of antigen-induced and MC-dependent airway hyperreactivity. Collectively, these data demonstrate that T cell IL-9 induces lung MC expansion and migration by direct effects on the proliferation of MCp and the migration of mMC to mediate airway hyperreactivity.
-
-
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Transcription factor RORα enforces stability of the Th17 cell effector program by binding to a Rorc cis-regulatory element.
In Immunity on 8 November 2022 by Hall, J. A., Pokrovskii, M., et al.
PubMed
T helper 17 (Th17) cells regulate mucosal barrier defenses but also promote multiple autoinflammatory diseases. Although many molecular determinants of Th17 cell differentiation have been elucidated, the transcriptional programs that sustain Th17 cells in vivo remain obscure. The transcription factor RORγt is critical for Th17 cell differentiation; however, it is not clear whether the closely related RORα, which is co-expressed in Th17 cells, has a distinct role. Here, we demonstrated that although dispensable for Th17 cell differentiation, RORα was necessary for optimal Th17 responses in peripheral tissues. The absence of RORα in T cells led to reductions in both RORγt expression and effector function among Th17 cells. Cooperative binding of RORα and RORγt to a previously unidentified Rorc cis-regulatory element was essential for Th17 lineage maintenance in vivo. These data point to a non-redundant role of RORα in Th17 lineage maintenance via reinforcement of the RORγt transcriptional program.
-
-
-
Cancer Research
-
Immunology and Microbiology
Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression.
In Nat Commun on 16 February 2022 by Qi, M., Xia, Y., et al.
PubMed
The formation of pre-metastatic niche is a key step in the metastatic burden. The pluripotent factor Lin28B is frequently expressed in breast tumors and is particularly upregulated in the triple negative breast cancer subtype. Here, we demonstrate that Lin28B promotes lung metastasis of breast cancer by building an immune-suppressive pre-metastatic niche. Lin28B enables neutrophil recruitment and N2 conversion. The N2 neutrophils are then essential for immune suppression in pre-metastatic lung by PD-L2 up-regulation and a dysregulated cytokine milieu. We also identify that breast cancer-released exosomes with low let-7s are a prerequisite for Lin28B-induced immune suppression. Moreover, Lin28B-induced breast cancer stem cells are the main sources of low-let-7s exosomes. Clinical data further verify that high Lin28B and low let-7s in tumors are both indicators for poor prognosis and lung metastasis in breast cancer patients. Together, these data reveal a mechanism by which Lin28B directs the formation of an immune-suppressive pre-metastatic niche.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
-
Flow cytometry/Cell sorting
BFAR coordinates TGFβ signaling to modulate Th9-mediated cancer immunotherapy.
In J Exp Med on 5 July 2021 by Pei, S., Huang, M., et al.
PubMed
TGFβ is essential for the generation of anti-tumor Th9 cells; on the other hand, it causes resistance against anti-tumor immunity. Despite recent progress, the underlying mechanism reconciling the double-edged effect of TGFβ signaling in Th9-mediated cancer immunotherapy remains elusive. Here, we find that TGFβ-induced down-regulation of bifunctional apoptosis regulator (BFAR) represents the key mechanism preventing the sustained activation of TGFβ signaling and thus impairing Th9 inducibility. Mechanistically, BFAR mediates K63-linked ubiquitination of TGFβR1 at K268, which is critical to activate TGFβ signaling. Thus, BFAR deficiency or K268R knock-in mutation suppresses TGFβR1 ubiquitination and Th9 differentiation, thereby inhibiting Th9-mediated cancer immunotherapy. More interestingly, BFAR-overexpressed Th9 cells exhibit promising therapeutic efficacy to curtail tumor growth and metastasis and promote the sensitivity of anti-PD-1-mediated checkpoint immunotherapy. Thus, our findings establish BFAR as a key TGFβ-regulated gene to fine-tune TGFβ signaling that causes Th9 induction insensitivity, and they highlight the translational potential of BFAR in promoting Th9-mediated cancer immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Oncogenic KRAS-Driven Metabolic Reprogramming in Pancreatic Cancer Cells Utilizes Cytokines from the Tumor Microenvironment.
In Cancer Discov on 1 April 2020 by Dey, P., Li, J., et al.
PubMed
A hallmark of pancreatic ductal adenocarcinoma (PDAC) is an exuberant stroma comprised of diverse cell types that enable or suppress tumor progression. Here, we explored the role of oncogenic KRAS in protumorigenic signaling interactions between cancer cells and host cells. We show that KRAS mutation (KRAS*) drives cell-autonomous expression of type I cytokine receptor complexes (IL2rγ-IL4rα and IL2rγ-IL13rα1) in cancer cells that in turn are capable of receiving cytokine growth signals (IL4 or IL13) provided by invading Th2 cells in the microenvironment. Early neoplastic lesions show close proximity of cancer cells harboring KRAS* and Th2 cells producing IL4 and IL13. Activated IL2rγ-IL4rα and IL2rγ-IL13rα1 receptors signal primarily via JAK1-STAT6. Integrated transcriptomic, chromatin occupancy, and metabolomic studies identified MYC as a direct target of activated STAT6 and that MYC drives glycolysis. Thus, paracrine signaling in the tumor microenvironment plays a key role in the KRAS*-driven metabolic reprogramming of PDAC. SIGNIFICANCE: Type II cytokines, secreted by Th2 cells in the tumor microenvironment, can stimulate cancer cell-intrinsic MYC transcriptional upregulation to drive glycolysis. This KRAS*-driven heterotypic signaling circuit in the early and advanced tumor microenvironment enables cooperative protumorigenic interactions, providing candidate therapeutic targets in the KRAS* pathway for this intractable disease.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease.
In Cell on 9 January 2020 by Lee, J. Y., Hall, J. A., et al.
PubMed
Lymphoid cells that produce interleukin (IL)-17 cytokines protect barrier tissues from pathogenic microbes but are also prominent effectors of inflammation and autoimmune disease. T helper 17 (Th17) cells, defined by RORγt-dependent production of IL-17A and IL-17F, exert homeostatic functions in the gut upon microbiota-directed differentiation from naive CD4+ T cells. In the non-pathogenic setting, their cytokine production is regulated by serum amyloid A proteins (SAA1 and SAA2) secreted by adjacent intestinal epithelial cells. However, Th17 cell behaviors vary markedly according to their environment. Here, we show that SAAs additionally direct a pathogenic pro-inflammatory Th17 cell differentiation program, acting directly on T cells in collaboration with STAT3-activating cytokines. Using loss- and gain-of-function mouse models, we show that SAA1, SAA2, and SAA3 have distinct systemic and local functions in promoting Th17-mediated inflammatory diseases. These studies suggest that T cell signaling pathways modulated by the SAAs may be attractive targets for anti-inflammatory therapies.
-
-
Fate mapping via Ms4a3 expression history traces monocyte-derived cells
In bioRxiv on 27 May 2019 by Liu, Z., Gu, Y., et al.
-
-
Mus musculus (Mouse)
-
Cell Biology
A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling.
In Cell on 12 July 2018 by Schneider, C., O'Leary, C. E., et al.
PubMed
The small intestinal tuft cell-ILC2 circuit mediates epithelial responses to intestinal helminths and protists by tuft cell chemosensory-like sensing and IL-25-mediated activation of lamina propria ILC2s. Small intestine ILC2s constitutively express the IL-25 receptor, which is negatively regulated by A20 (Tnfaip3). A20 deficiency in ILC2s spontaneously triggers the circuit and, unexpectedly, promotes adaptive small-intestinal lengthening and remodeling. Circuit activation occurs upon weaning and is enabled by dietary polysaccharides that render mice permissive for Tritrichomonas colonization, resulting in luminal accumulation of acetate and succinate, metabolites of the protist hydrogenosome. Tuft cells express GPR91, the succinate receptor, and dietary succinate, but not acetate, activates ILC2s via a tuft-, TRPM5-, and IL-25-dependent pathway. Also induced by parasitic helminths, circuit activation and small intestinal remodeling impairs infestation by new helminths, consistent with the phenomenon of concomitant immunity. We describe a metabolic sensing circuit that may have evolved to facilitate mutualistic responses to luminal pathosymbionts.
-