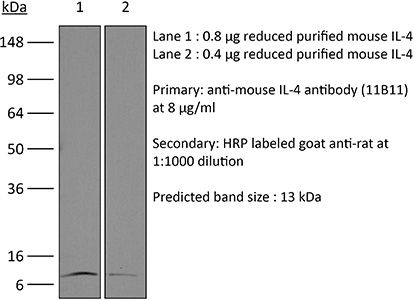
InVivoMAb anti-mouse IL-4
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Partially purified native mouse IL-4 |
| Reported Applications |
in vivo IL-4 neutralization in vitro IL-4 neutralization in vivo IL-4 receptor stimulation (as a complex with IL-4) Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107707 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-4 neutralization
in vivo IL-4 receptor stimulation (as a complex with IL-4)
Gaya, M., et al (2018). "Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells" Cell 172(3): 517-533 e520.
PubMed
B cells constitute an essential line of defense from pathogenic infections through the generation of class-switched antibody-secreting cells (ASCs) in germinal centers. Although this process is known to be regulated by follicular helper T (TfH) cells, the mechanism by which B cells initially seed germinal center reactions remains elusive. We found that NKT cells, a population of innate-like T lymphocytes, are critical for the induction of B cell immunity upon viral infection. The positioning of NKT cells at the interfollicular areas of lymph nodes facilitates both their direct priming by resident macrophages and the localized delivery of innate signals to antigen-experienced B cells. Indeed, NKT cells secrete an early wave of IL-4 and constitute up to 70% of the total IL-4-producing cells during the initial stages of infection. Importantly, the requirement of this innate immunity arm appears to be evolutionarily conserved because early NKT and IL-4 gene signatures also positively correlate with the levels of neutralizing antibodies in Zika-virus-infected macaques. In conclusion, our data support a model wherein a pre-TfH wave of IL-4 secreted by interfollicular NKT cells triggers the seeding of germinal center cells and serves as an innate link between viral infection and B cell immunity.
in vitro IL-4 neutralization
Clever, D., et al (2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche" Cell 166(5): 1117-1131 e1114.
PubMed
Cancer cells must evade immune responses at distant sites to establish metastases. The lung is a frequent site for metastasis. We hypothesized that lung-specific immunoregulatory mechanisms create an immunologically permissive environment for tumor colonization. We found that T-cell-intrinsic expression of the oxygen-sensing prolyl-hydroxylase (PHD) proteins is required to maintain local tolerance against innocuous antigens in the lung but powerfully licenses colonization by circulating tumor cells. PHD proteins limit pulmonary type helper (Th)-1 responses, promote CD4(+)-regulatory T (Treg) cell induction, and restrain CD8(+) T cell effector function. Tumor colonization is accompanied by PHD-protein-dependent induction of pulmonary Treg cells and suppression of IFN-gamma-dependent tumor clearance. T-cell-intrinsic deletion or pharmacological inhibition of PHD proteins limits tumor colonization of the lung and improves the efficacy of adoptive cell transfer immunotherapy. Collectively, PHD proteins function in T cells to coordinate distinct immunoregulatory programs within the lung that are permissive to cancer metastasis.
in vitro IL-4 neutralization
Flow Cytometry
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vitro IL-4 neutralization
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro IL-4 neutralization
Hou, L., et al (2015). "The protease cathepsin L regulates Th17 cell differentiation" J Autoimmun. S 0896-8411(15): 30024-X.
PubMed
Previously we reported that IL-17+ T cells, primarily IL-17+ gammadelta cells, are increased in mice lacking the protease inhibitor serpinB1 (serpinb1-/- mice). Here we show that serpinB1-deficient CD4 cells exhibit a cell-autonomous and selective deficiency in suppressing T helper 17 (Th17) cell differentiation. This suggested an opposing role for one or more protease in promoting Th17 differentiation. We found that several SerpinB1-inhibitable cysteine cathepsins are induced in Th17 cells, most prominently cathepsin L (catL); this was verified by peptidase assays, active site labeling and Western blots. Moreover, Th17 differentiation was suppressed by both broad cathepsin inhibitors and catL selective inhibitors. CatL is present in Th17 cells as single chain (SC)- and two-chain (TC)-forms. Inhibiting asparagine endopeptidase (AEP) blocked conversion of SC-catL to TC-catL and increased generation of serpinb1-/- Th17 cells, but not wild-type Th17 cells. These findings suggest that SC-catL is biologically active in promoting Th17 generation and is counter-regulated by serpinB1 and secondarily by AEP. Thus, in addition to regulation by cytokines and transcription factors, differentiation of CD4 cells to Th17 cells is actively regulated by a catL-serpinB1-AEP module. Targeting this protease regulatory module could be an approach to treating Th17 cell-driven autoimmune disorders.
in vitro IL-4 neutralization
Kim, Y. U., et al (2015). "Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells" PLoS One 10(3): e0120294.
PubMed
BXD2 mice spontaneously develop autoantibodies and subsequent glomerulonephritis, offering a useful animal model to study autoimmune lupus. Although initial studies showed a critical contribution of IL-17 and Th17 cells in mediating autoimmune B cell responses in BXD2 mice, the role of follicular helper T (Tfh) cells remains incompletely understood. We found that both the frequency of Th17 cells and the levels of IL-17 in circulation in BXD2 mice were comparable to those of wild-type. By contrast, the frequency of PD-1+ CXCR5+ Tfh cells was significantly increased in BXD2 mice compared with wild-type mice, while the frequency of PD-1+ CXCR5+ Foxp3+ follicular regulatory T (Tfr) cells was reduced in the former group. The frequency of Tfh cells rather than that of Th17 cells was positively correlated with the frequency of germinal center B cells as well as the levels of autoantibodies to dsDNA. More importantly, CXCR5+ CD4+ T cells isolated from BXD2 mice induced the production of IgG from naive B cells in an IL-21-dependent manner, while CCR6+ CD4+ T cells failed to do so. These results together demonstrate that Tfh cells rather than Th17 cells contribute to the autoimmune germinal center reactions in BXD2 mice.
in vitro IL-4 neutralization
Hill, E. V., et al (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115.
PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro IL-4 neutralization
Burton, B. R., et al (2014). "Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy" Nat Commun 5: 4741.
PubMed
Antigen-specific immunotherapy combats autoimmunity or allergy by reinstating immunological tolerance to target antigens without compromising immune function. Optimization of dosing strategy is critical for effective modulation of pathogenic CD4(+) T-cell activity. Here we report that dose escalation is imperative for safe, subcutaneous delivery of the high self-antigen doses required for effective tolerance induction and elicits anergic, interleukin (IL)-10-secreting regulatory CD4(+) T cells. Analysis of the CD4(+) T-cell transcriptome, at consecutive stages of escalating dose immunotherapy, reveals progressive suppression of transcripts positively regulating inflammatory effector function and repression of cell cycle pathways. We identify transcription factors, c-Maf and NFIL3, and negative co-stimulatory molecules, LAG-3, TIGIT, PD-1 and TIM-3, which characterize this regulatory CD4(+) T-cell population and whose expression correlates with the immunoregulatory cytokine IL-10. These results provide a rationale for dose escalation in T-cell-directed immunotherapy and reveal novel immunological and transcriptional signatures as surrogate markers of successful immunotherapy.
in vitro IL-4 neutralization
Tang, W., et al (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566.
PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vitro IL-4 neutralization
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vitro IL-4 neutralization
McKinstry, K. K., et al (2014). "Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2" Nat Commun 5: 5377.
PubMed
It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation.
in vivo IL-4 neutralization
Mishra, P. K., et al (2013). "Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response" Mucosal Immunol 6(2): 297-308.
PubMed
Helminth infection can prevent type 1 diabetes (T1D); however, the regulatory mechanisms inhibiting disease remain largely undefined. In these studies, nonobese diabetic (NOD) IL-4(-/-) mice were infected with the strictly enteric nematode parasite, Heligmosomoides polygyrus. Short-term infection, 5-7 weeks of age, inhibited T1D onset, as late as 40 weeks of age. CD4(+) T-cell STAT6 phosphorylation was inhibited, while suppressed signal transducer and activator of transcription 1 phosphorylation was sustained, as were increases in FOXP3(-), CD4(+) T-cell interleukin (IL)-10 production. Blockade of IL-10 signaling in NOD-IL-4(-/-), but not in NOD, mice during this short interval abrogated protective effects resulting in pancreatic beta-cell destruction and ultimately T1D. Transfer of CD4(+) T cells from H. polygyrus (Hp)-inoculated NOD IL-4(-/-) mice to NOD mice blocked the onset of T1D. These studies indicate that Hp infection induces non-T-regulatory cells to produce IL-10 independently of STAT6 signaling and that in this Th2-deficient environment IL-10 is essential for T1D inhibition.
in vitro IL-4 neutralization
Li, X., et al (2012). "Divergent requirement for Galphas and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets" J Clin Invest 122(3): 963-973.
PubMed
cAMP, the intracellular signaling molecule produced in response to GPCR signaling, has long been recognized as an immunosuppressive agent that inhibits T cell receptor activation and T cell function. However, recent studies show that cAMP also promotes T cell-mediated immunity. Central to cAMP production downstream of GPCR activation is the trimeric G protein Gs. In order to reconcile the reports of divergent effects of cAMP in T cells and to define the direct effect of cAMP in T cells, we engineered mice in which the stimulatory Galpha subunit of Gs (Galphas) could be deleted in T cells using CD4-Cre (Gnas(DeltaCD4)). Gnas(DeltaCD4) CD4(+) T cells had reduced cAMP accumulation and Ca2(+) influx. In vitro and in vivo, Gnas(DeltaCD4) CD4(+) T cells displayed impaired differentiation to specific Th subsets: Th17 and Th1 cells were reduced or absent, but Th2 and regulatory T cells were unaffected. Furthermore, Gnas(DeltaCD4) CD4(+) T cells failed to provoke colitis in an adoptive transfer model, indicating reduced inflammatory function. Restoration of cAMP levels rescued the impaired phenotype of Gnas(DeltaCD4) CD4(+) T cells, reinstated the PKA-dependent influx of Ca2(+), and enhanced the ability of these cells to induce colitis. Our findings thus define an important role for cAMP in the differentiation of Th subsets and their subsequent inflammatory responses, and provide evidence that altering cAMP levels in CD4(+) T cells could provide an immunomodulatory approach targeting specific Th subsets.
in vitro IL-4 neutralization
Ueda, A., et al (2012). "Fyn promotes Th17 differentiation by regulating the kinetics of RORgammat and Foxp3 expression" J Immunol 188(11): 5247-5256.
PubMed
Th17 cells constitute a proinflammatory CD4(+) T cell subset that is important for microbial clearance, but also are implicated as propagators of various autoimmune pathologies. Evidence suggests that Th17 cells share common progenitors with immunosuppressive CD4(+) inducible regulatory T cells (T(REG)) and that the developmental pathways of these two subsets are reciprocally regulated. In this study, we show evidence that the Src family tyrosine kinase Fyn helps regulate this Th17/T(REG) balance. When placed under Th17-skewing conditions, CD4(+) T cells from fyn(-/-) mice had decreased levels of IL-17, but increased expression of the T(REG) transcription factor Foxp3. The defect in IL-17 expression occurred independently of the ectopic Foxp3 expression and correlated with a delay in retinoic acid-related orphan receptor gammat upregulation and an inability to maintain normal STAT3 activation. Fyn-deficient Th17 cells also exhibited delayed upregulation of Il23r, Il21, Rora, and Irf4, as well as aberrant expression of Socs3, suggesting that Fyn may function upstream of a variety of molecular pathways that contribute to Th17 polarization. The fyn(-/-) mice had fewer IL-17(+)CD4(+) T cells in the large intestinal lamina propria compared with littermate controls. Furthermore, after transfer of either wild-type or fyn(-/-) naive CD4(+) T cells into Rag1(-/-) hosts, recipients receiving fyn(-/-) cells had fewer IL-17-producing T cells, indicating that Fyn may also regulate Th17 differentiation in vivo. These results identify Fyn as a possible novel regulator of the developmental balance between the Th17 cell and T(REG) subsets.
Product Citations
-
-
Immunology and Microbiology
Harnessing the dual immunomodulatory function of myeloid-derived suppressor cells to reshape the inflammatory microenvironment for osteoarthritis therapy.
In Mater Today Bio on 1 December 2025 by Guo, Z., Chen, T., et al.
PubMed
Osteoarthritis (OA) pathogenesis is profoundly influenced by dysregulated immune dynamics, where persistent interleukin-17 (IL-17)/T helper 17 (Th17) cell mediated inflammation coordinates with failed regenerative processes to perpetuate joint destruction. Here, we unveil the role of myeloid-derived suppressor cells (MDSCs) as dual-phase regulators that paradoxically orchestrate both inflammatory escalation and tissue repair in OA progression. Intra-articular administration of MDSCs in OA mice amplified IL-17 dependent inflammatory cascades and chemokine-driven leukocyte recruitment, revealing a context-dependent pro-inflammatory phenotype. Unexpectedly, MDSC depletion failed to attenuate joint damage, implying their indispensable yet multifaceted role in OA pathogenesis. Mechanistically, MDSCs exhibited functional plasticity by upregulating arginase-1 to polarize M2 macrophages, fostering a regenerative niche alongside their inflammatory activity. To resolve this duality, we developed a bio-responsive hydrogel-microsphere system integrating transforming growth factor β1 (TGF-β1) and interleukin-1 β1 antibody (anti-IL-1β) loaded mesoporous silica nanoparticles (MSNs). This spatiotemporally controlled platform selectively suppressed MDSC-mediated Th17 cell expansion while harnessing their intrinsic capacity to drive M2 macrophage polarization and chondrogenesis. The resultant shift from a pro-inflammatory to pro-regenerative microenvironment significantly attenuated cartilage erosion and restored joint integrity in OA models. Our findings redefine MDSCs as bifunctional immune orchestrators in OA and establish precision biomaterial guided immune decoding as a paradigm-shifting therapeutic strategy. By engineering MDSCs plasticity through antagonistic cytokine delivery, this work provides a blueprint for microenvironment remodeling in degenerative joint diseases.
-
-
-
Cell Biology
-
Genetics
-
Immunology and Microbiology
Cytoplasmic Histone Is a Marker of Pro-Inflammatory CD4 + T Cells.
In Immunology on 1 September 2025 by Xu, B., Liu, G., et al.
PubMed
Although extranuclear histones have been identified for a long time, their role in lymphocytes, particularly cytoplasmic histones, remains elusive. In this study, we conducted a visual and quantitative analysis of cytoplasmic histones in CD4+ T cells and discovered that effector CD4+ T cells contain higher levels of cytoplasmic histones compared to naïve T cells. We observed a significant increase in cytoplasmic histones following T cell receptor (TCR) activation, with further elevation during the differentiation of Th1 and Th17 cells. Interestingly, double-stranded RNA (dsRNA) specifically enhanced cytoplasmic histones in Th17, but not in Th1 cells. Additionally, we confirmed that cytoplasmic H2B-positive Th17 cells exhibited increased expression of inflammatory molecules, including pro-inflammatory cytokines. Data are available via ProteomeXchange with identifier PXD063335. Importantly, CD4+ T cells from peripheral blood mononuclear cells (PBMCs) of patients with systemic lupus erythematosus (SLE) presented elevated levels of cytoplasmic histones. These findings define cytoplasmic histones as markers of proinflammatory CD4+ T cells and suggest their potential as biomarkers for autoimmune disease.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Neuroscience
Tumor-infiltrating nociceptor neurons promote immunosuppression.
In Sci Signal on 5 August 2025 by Restaino, A. C., Ahmadi, M., et al.
PubMed
Small extracellular vesicles (sEVs) released from tumors recruit nociceptor neurons to the tumor bed. Here, we found that ablating these neurons in mouse models of head and neck carcinoma and melanoma reduced the infiltration of myeloid-derived suppressor cells (MDSCs). Moreover, sEV-deficient tumors failed to develop in mice lacking nociceptor neurons. We investigated the interplay between tumor-infiltrating nociceptors and immune cells in head and neck squamous cell carcinoma (HNSCC) and melanoma. Upon exposure to cancer-derived sEVs, mouse dorsal root ganglion (DRG) neurons secreted increased amounts of substance P, IL-6, and injury-associated neuronal markers. Patient-derived sEVs sensitized DRG neurons to capsaicin, implying enhanced nociceptor responsiveness. Furthermore, nociceptors cultured with sEVs induced an immunosuppressed state in CD8+ T cells. Incubation with conditioned medium from cocultures of neurons and cancer cells resulted in increased expression of markers of MDSCs and suppressive function in primary bone marrow cells, and the combination of neuron-conditioned medium and cancer sEVs promoted checkpoint receptor expression on T cells. Together, these findings reveal that nociceptor neurons facilitate CD8+ T cell exhaustion and bolster MDSC infiltration into HNSCC and melanoma. Consequently, targeting nociceptors may provide a strategy to disrupt detrimental neuroimmune cross-talk in cancer and potentiate antitumor immunity.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
Phosphoglycerate mutase regulates Treg differentiation through control of serine synthesis and one-carbon metabolism.
In Elife on 28 July 2025 by Godfrey, W. H., Lee, J. J., et al.
PubMed
The differentiation and suppressive functions of regulatory CD4 T cells (Tregs) are supported by a broad array of metabolic changes, providing potential therapeutic targets for immune modulation. In this study, we focused on the regulatory role of glycolytic enzymes in Tregs and identified phosphoglycerate mutase (PGAM) as being differentially overexpressed in Tregs and associated with a highly suppressive phenotype. Pharmacologic or genetic inhibition of PGAM reduced Treg differentiation and suppressive function while reciprocally inducing markers of a pro-inflammatory, T helper 17 (Th17)-like state. The regulatory role of PGAM was dependent on the contribution of 3-phosphoglycerate (3 PG), the PGAM substrate, to de novo serine synthesis. Blocking de novo serine synthesis from 3 PG reversed the effect of PGAM inhibition on Treg polarization, while exogenous serine directly inhibited Treg polarization. Additionally, altering serine levels in vivo with a serine/glycine-free diet increased peripheral Tregs and attenuated autoimmunity in a murine model of multiple sclerosis. Mechanistically, we found that serine limits Treg polarization by contributing to one-carbon metabolism and methylation of Treg-associated genes. Inhibiting one-carbon metabolism increased Treg polarization and suppressive function both in vitro and in vivo in a murine model of autoimmune colitis. Our study identifies a novel physiologic role for PGAM and highlights the metabolic interconnectivity between glycolysis, serine synthesis, one-carbon metabolism, and epigenetic regulation of Treg differentiation and suppressive function.
-
-
-
Immunology and Microbiology
Intermittent fasting exacerbates colon inflammation by promoting Th17 cell differentiation through inhibition of gut microbiota-derived indoleacrylic acid.
In World J Gastroenterol on 14 June 2025 by Fu, R., Zhang, P., et al.
PubMed
Intermittent fasting (IF), particularly time-restricted feeding (TRF), is increasingly popular has gained popularity for weight loss, yet management, but its effects impact on gut health remain unclear. Remains inadequately understood. This study explores how investigated the effects of TRF effects on intestinal health and explored the underlying mechanisms.
-
-
-
Mus musculus (Mouse)
High-salt-driven gut microbiota dysfunction aggravates prostatitis by promoting AHR/SGK1/FOXO1 axis-mediated Th17 cell differentiation.
In Mil Med Res on 19 May 2025 by Chen, J., Feng, R., et al.
PubMed
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a frequently encountered disorder characterized by voiding symptoms and pelvic or perineal pain. Proinflammatory T helper 17 (Th17) cells are essential for triggering the development of CP/CPPS. High-salt diet (HSD) consumption has been found to cause an accumulation of sodium chloride in peripheral organs, inducing autoimmune responses via the Th17 cell axis. It is currently unknown whether HSD affects the etiology and course of CP/CPPS.
-
-
-
Cancer Research
Hepatic iNKT cells facilitate colorectal cancer metastasis by inducing a fibrotic niche in the liver.
In iScience on 16 May 2025 by Nater, M., Brügger, M., et al.
PubMed
The liver is an important metastatic organ that contains many innate immune cells, yet little is known about their role in anti-metastatic defense. We investigated how invariant natural killer T (iNKT) cells influence colorectal cancer-derived liver metastasis using different models in immunocompetent mice. We found that hepatic iNKT cells promote metastasis by creating a supportive niche for disseminated cancer cells. Mechanistically, iNKT cells respond to disseminating cancer cells by producing the fibrogenic cytokines interleukin-4 (IL-4) and IL-13 in a T cell receptor-independent manner. Selective abrogation of IL-4 and IL-13 sensing in hepatic stellate cells prevented their transdifferentiation into extracellular matrix-producing myofibroblasts, which hindered metastatic outgrowth of disseminated cancer cells. This study highlights a novel tumor-promoting axis driven by iNKT cells in the initial stages of metastasis.
-
-
-
Immunology and Microbiology
IGF1R Promotes Th17/Treg Cell Development in Experimental Autoimmune Prostatitis.
In J Inflamm Res on 5 May 2025 by Guan, Y., Yue, S., et al.
PubMed
Chronic prostatitis is a common urological disorder in young and middle-aged men, characterized by frequent relapses and an unknown etiology. We investigated the potential function of insulin-like growth factor 1 (IGF1) -related ligands in chronic prostatitis in the current study.
-
-
-
Immunology and Microbiology
Synergizing adaptive immunity and regenerative signals to enhance osteochondral defects repair.
In Bioact Mater on 1 April 2025 by Lin, C., Ying, C., et al.
PubMed
In clinical practice, repairing osteochondral defects (OCDs) is challenging because of the complex cartilage/subchondral bone structure and intricate immunological microenvironment. Here, we identify the crucial role of adaptive immunity dysfunction by revealing that an increase of T helper 17 (Th17) cells exacerbated osteochondral tissue degradation via its pro-inflammatory cytokine interleukin-17 (IL-17) in the early-stage OCDs. Next, we leveraged this adaptive immunity mechanism and combined it with regenerative signals to develop a multifunctional hydrogel system capable of simultaneously tackling immune dysfunction and regenerative deficiency. Rapid IL-4 release from the methacrylated hyaluronic acid (HAMA) hydrogel exerts a potent immunomodulatory effect by inhibiting the differentiation and function of Th17 cells. Moreover, transforming growth factor-beta1 anchored on methacrylated hyaluronic acid and heparin (HAMA@HepMA) microparticles provides sustained regenerative signals, which synergistically transform the pro-inflammatory microenvironment into a pro-regenerative niche for enhanced OCDs healing. Our study suggests that targeting specific immune pathways can significantly enhance the efficacy of regenerative strategies, paving the way for innovative treatments in orthopedic medicine.
-
-
Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling.
In Molecules on 11 February 2025 by Jo, H., Kim, M., et al.
PubMed
Rocaglamide (Roc-A), a natural phytochemical isolated from Aglaia species, is known to exert anticancer effects. Allergic inflammation can enhance the tumorigenic potential of cancer cells. We hypothesized that Roc-A could regulate allergic inflammation. Roc-A prevented an antigen from increasing the hallmarks of allergic reactions in vitro. Roc-A suppressed passive cutaneous anaphylaxis (PCA) and passive systemic anaphylaxis (PSA). RNA sequencing analysis showed that Roc-A prevented the antigen from increasing the expression of IL-4 in RBL2H3 cells. Roc-A also prevented the antigen from increasing the expression of interleukin-4 receptor (IL-4R). Roc-A was found to form a hydrogen-bonding network with residues N92 and L64 of IL-4R in a molecular docking simulation. Roc-A prevented the antigen from inducing the binding of IL-4R to JAK1. Chromatin immunoprecipitation (ChIP) assays showed that C-Jun could bind to promoter sequences of IL-4 and IL-4R. Mouse recombinant IL-4 protein increased β-hexosaminidase activity, IL-4R expression, and the hallmarks of allergic inflammation in the antigen-independent manner. Mouse recombinant IL-4 protein increased the expressions of CD163 and arghinase-1 and markers of M2 macrophages, but decreased the expression of iNOS, a marker of M1 macrophages in lung macrophages. Roc-A regulated the effects of a culture medium of antigen-stimulated RBL2H3 cells on the expressions of iNOS and arginase-1 in RAW264.7 macrophages. The blocking of IL-4 or downregulation of IL-4R exerted negative effects on the hallmarks of allergic reactions in vitro. The blocking of IL-4 or downregulation of IL-4R also exerted negative effects on PCA, and the downregulation of IL-4R exerted negative effects on PSA. An miR-34a mimic exerted negative effects on allergic reactions in vitro. The downregulation of IL-4R prevented the antigen from decreasing the expression of miR-34a in RBL2H3 cells. We identified chemicals that could bind to IL-4R via molecular docking analysis. The IL-4R docking chemical 1536801 prevented the antigen from increasing β-hexosaminidase activity and the hallmarks of allergic reactions. The IL-4R docking chemical 1536801 also exerted a negative effect on PCA. TargetScan analysis predicted miR-34a as a negative regulator of IL-4R. We found that the anti-allergic effect of Roc-A and its mechanisms were associated with miR-34a. Taken together, our results show that understanding IL-4R-mediated allergic reactions can provide clues for the development of anti-allergy therapeutics.
-
-
Mus musculus (Mouse)
A20’s Linear Ubiquitin Binding Motif Restrains Pathogenic Activation of TH17/22 cells and IL-22 Driven Enteritis
In bioRxiv on 2 January 2025 by Bowman, C. J., Stibor, D., et al.
-
-
Natural lung-tropic TH9 cells: a sharp weapon for established lung metastases.
In J Immunother Cancer on 4 December 2024 by Chen, T., Qiao, C., et al.
PubMed
Lung metastasis remains the primary cause of tumor-related mortality, with limited treatment options and unsatisfactory efficacy. In preclinical studies, T helper 9 (TH9) cells have shown promise in treating solid tumors. However, it is unclear whether TH9 cells can tackle more challenging situations, such as established lung metastases. Moreover, comprehensive exploration into the nuanced biological attributes of TH9 cells is imperative to further unravel their therapeutic potential.
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Neurotrophic factor Neuritin modulates T cell electrical and metabolic state for the balance of tolerance and immunity.
In Elife on 20 November 2024 by Yu, H., Nishio, H., et al.
PubMed
The adaptive T cell response is accompanied by continuous rewiring of the T cell's electric and metabolic state. Ion channels and nutrient transporters integrate bioelectric and biochemical signals from the environment, setting cellular electric and metabolic states. Divergent electric and metabolic states contribute to T cell immunity or tolerance. Here, we report in mice that neuritin (Nrn1) contributes to tolerance development by modulating regulatory and effector T cell function. Nrn1 expression in regulatory T cells promotes its expansion and suppression function, while expression in the T effector cell dampens its inflammatory response. Nrn1 deficiency in mice causes dysregulation of ion channel and nutrient transporter expression in Treg and effector T cells, resulting in divergent metabolic outcomes and impacting autoimmune disease progression and recovery. These findings identify a novel immune function of the neurotrophic factor Nrn1 in regulating the T cell metabolic state in a cell context-dependent manner and modulating the outcome of an immune response.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Sorafenib-induced macrophage extracellular traps via ARHGDIG/IL4/PADI4 axis confer drug resistance through inhibiting ferroptosis in hepatocellular carcinoma.
In Biol Direct on 11 November 2024 by Huang, X., Yi, N., et al.
PubMed
Hepatocellular carcinoma (HCC) is one of the most common as well as leading causes of mortality worldwide, and sorafenib is the first-line treatment in HCC patients. Unfortunately, drug resistance to sorafenib often develops. However, the underlying mechanism remains unclear. Here, we reveal the important role of macrophage extracellular traps (METs)-mediated crosstalk between macrophages and tumor cells in sorafenib resistance.
-
-
-
Biochemistry and Molecular biology
TRAF3 regulates STAT6 activation and T-helper cell differentiation by modulating the phosphatase PTP1B.
In J Biol Chem on 1 October 2024 by Arkee, T., Hornick, E. L., et al.
PubMed
The adaptor protein tumor necrosis factor receptor-associated factor 3 (TRAF3) is a multifaceted regulator of lymphocyte biology that plays key roles in modulation of the molecular signals required for T-cell activation and function. TRAF3 regulates signals mediated by the T-cell receptor (TCR), costimulatory molecules, and cytokine receptors, which each drive activation of the serine/threonine kinase Akt. The impact of TRAF3 upon TCR-CD28-mediated activation of Akt, and thus on the diverse cellular processes regulated by Akt, including CD4 T-cell fate decisions, remains poorly understood. We show here that TRAF3 deficiency led to impaired Akt activation and thus to impaired in vitro skewing of CD4 T cells into the TH1 and TH2 fates. We investigated the role of TRAF3 in regulation of signaling pathways that drive TH1 and TH2 differentiation and found that TRAF3 enhanced activation of signal transducer and activator of transcription 6 (STAT6), thus promoting skewing toward the TH2 fate. TRAF3 promoted STAT6 activation by regulating recruitment of the inhibitory molecule protein tyrosine phosphatase 1B to the IL-4R signaling complex, in a manner that required integration of TCR-CD28- and IL-4R-mediated signals. This work reveals a new mechanism for TRAF3-mediated regulation of STAT6 activation in CD4 T cells and adds to our understanding of the diverse roles played by TRAF3 as an important regulator of T-cell biology.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
The type 2 cytokine Fc-IL-4 revitalizes exhausted CD8+ T cells against cancer.
In Nature on 1 October 2024 by Feng, B., Bai, Z., et al.
PubMed
Current cancer immunotherapy predominately focuses on eliciting type 1 immune responses fighting cancer; however, long-term complete remission remains uncommon1,2. A pivotal question arises as to whether type 2 immunity can be orchestrated alongside type 1-centric immunotherapy to achieve enduring response against cancer3,4. Here we show that an interleukin-4 fusion protein (Fc-IL-4), a typical type 2 cytokine, directly acts on CD8+ T cells and enriches functional terminally exhausted CD8+ T (CD8+ TTE) cells in the tumour. Consequently, Fc-IL-4 enhances antitumour efficacy of type 1 immunity-centric adoptive T cell transfer or immune checkpoint blockade therapies and induces durable remission across several syngeneic and xenograft tumour models. Mechanistically, we discovered that Fc-IL-4 signals through both signal transducer and activator of transcription 6 (STAT6) and mammalian target of rapamycin (mTOR) pathways, augmenting the glycolytic metabolism and the nicotinamide adenine dinucleotide (NAD) concentration of CD8+ TTE cells in a lactate dehydrogenase A-dependent manner. The metabolic modulation mediated by Fc-IL-4 is indispensable for reinvigorating intratumoural CD8+ TTE cells. These findings underscore Fc-IL-4 as a potent type 2 cytokine-based immunotherapy that synergizes effectively with type 1 immunity to elicit long-lasting responses against cancer. Our study not only sheds light on the synergy between these two types of immune responses, but also unveils an innovative strategy for advancing next-generation cancer immunotherapy by integrating type 2 immune factors.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
HIF-2α-dependent induction of miR-29a restrains TH1 activity during T cell dependent colitis.
In Nat Commun on 14 September 2024 by Czopik, A. K., McNamee, E. N., et al.
PubMed
Metabolic imbalance leading to inflammatory hypoxia and stabilization of hypoxia-inducible transcription factors (HIFs) is a hallmark of inflammatory bowel diseases. We hypothesize that HIF could be stabilized in CD4+ T cells during intestinal inflammation and alter the functional responses of T cells via regulation of microRNAs. Our assays reveal markedly increased T cell-intrinsic hypoxia and stabilization of HIF protein during experimental colitis. microRNA screen in primary CD4+ T cells points us towards miR-29a and our subsequent studies identify a selective role for HIF-2α in CD4-cell-intrinsic induction of miR-29a during hypoxia. Mice with T cell-intrinsic HIF-2α deletion display elevated T-bet (target of miR-29a) levels and exacerbated intestinal inflammation. Mice with miR-29a deficiency in T cells show enhanced intestinal inflammation. T cell-intrinsic overexpression of HIF-2α or delivery of miR-29a mimetic dampen TH1-driven colitis. In this work, we show a previously unrecognized function for hypoxia-dependent induction of miR-29a in attenuating TH1-mediated inflammation.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
An intestinal TH17 cell-derived subset can initiate cancer.
In Nat Immunol on 1 September 2024 by Fesneau, O., Thevin, V., et al.
PubMed
Approximately 25% of cancers are preceded by chronic inflammation that occurs at the site of tumor development. However, whether this multifactorial oncogenic process, which commonly occurs in the intestines, can be initiated by a specific immune cell population is unclear. Here, we show that an intestinal T cell subset, derived from interleukin-17 (IL-17)-producing helper T (TH17) cells, induces the spontaneous transformation of the intestinal epithelium. This subset produces inflammatory cytokines, and its tumorigenic potential is not dependent on IL-17 production but on the transcription factors KLF6 and T-BET and interferon-γ. The development of this cell type is inhibited by transforming growth factor-β1 (TGFβ1) produced by intestinal epithelial cells. TGFβ signaling acts on the pretumorigenic TH17 cell subset, preventing its progression to the tumorigenic stage by inhibiting KLF6-dependent T-BET expression. This study therefore identifies an intestinal T cell subset initiating cancer.
-
-
-
Immunology and Microbiology
The CXCL10/CXCR3 axis regulates Th1 cell differentiation and migration in experimental autoimmune prostatitis through the PI3K/AKT pathway.
In Andrology on 1 September 2024 by Yue, S. Y., Niu, D., et al.
PubMed
To investigate the mechanism of the CXCL10/CXCR3 axis regulating Th1 cell differentiation and migration through the PI3K/AKT pathway in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
-