InVivoPlus anti-mouse CD71 (TfR1)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse erythroleukemia cell line 745.6 |
| Reported Applications | in vivo depletion of CD71+ cells |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
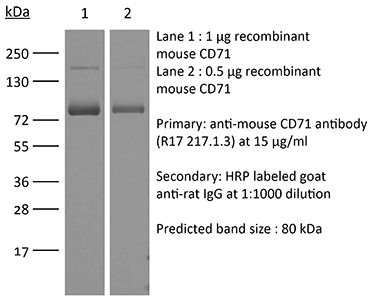
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950526 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Antibody-oligonucleotide conjugate (AOC) synthesis
Weeden T, Picariello T, Quinn B, Spring S, Shen PY, Qiu Q, Vieira BF, Schlaefke L, Russo RJ, Chang YA, Cui J, Yao M, Wen A, Hsia N, Evron T, Ovington K, Tsai PN, Yoder N, Lan B, Venkatesan R, Hall J, Desjardins CA, Qatanani M, Hilderbrand S, Najim J,
PubMed
Background: We developed the FORCETM platform to overcome limitations of oligonucleotide delivery to muscle and enable their applicability to neuromuscular disorders. The platform consists of an antigen-binding fragment, highly specific for the human transferrin receptor 1 (TfR1), conjugated to an oligonucleotide via a cleavable valine-citrulline linker. Myotonic dystrophy type 1 (DM1) is a neuromuscular disorder caused by expanded CUG triplets in the DMPK RNA, which sequester splicing proteins in the nucleus, lead to spliceopathy, and drive disease progression. Methods: Multiple surrogate conjugates were generated to characterize the FORCE platform. DYNE-101 is the conjugate designed to target DMPK and correct spliceopathy for the treatment of DM1. HSALR and TfR1hu/mu;DMSXLTg/Tg mice were used as models of myotonic dystrophy, the latter expresses human TfR1 and a human DMPK RNA with >1,000 CUG repeats. Cynomolgus monkeys were used to determine translatability of DYNE-101 pharmacology to higher species. Results: In HSALR mice, a surrogate FORCE conjugate achieves durable correction of spliceopathy and improves myotonia to a greater extent than unconjugated ASO. In patient-derived myoblasts, DYNE-101 reduces DMPK RNA and nuclear foci, consequently improving spliceopathy. In TfR1hu/mu;DMSXLTg/Tg mice, DYNE-101 reduces mutant DMPK RNA in muscle, thereby correcting splicing. Reduction of DMPK foci in cardiomyocyte nuclei accompanies these effects. Low monthly dosing of DYNE-101 in TfR1hu/mu;DMSXLWT/Tg mice or cynomolgus monkeys leads to a profound reduction of DMPK expression in muscle. Conclusions: These data validate FORCE as a drug delivery platform and support the notion that DM1 may be treatable with low and infrequent dosing of DYNE-101.
Antibody-oligonucleotide conjugate (AOC) synthesis
Malecova B, Burke RS, Cochran M, Hood MD, Johns R, Kovach PR, Doppalapudi VR, Erdogan G, Arias JD, Darimont B, Miller CD, Huang H, Geall A, Younis HS, Levin AA (2023). "Targeted tissue delivery of RNA therapeutics using antibody-oligonucleotide conju
PubMed
Although targeting TfR1 to deliver oligonucleotides to skeletal muscle has been demonstrated in rodents, effectiveness and pharmacokinetic/pharmacodynamic (PKPD) properties remained unknown in higher species. We developed antibody-oligonucleotide conjugates (AOCs) towards mice or monkeys utilizing anti-TfR1 monoclonal antibodies (αTfR1) conjugated to various classes of oligonucleotides (siRNA, ASOs and PMOs). αTfR1 AOCs delivered oligonucleotides to muscle tissue in both species. In mice, αTfR1 AOCs achieved a > 15-fold higher concentration to muscle tissue than unconjugated siRNA. A single dose of an αTfR1 conjugated to an siRNA against Ssb mRNA produced > 75% Ssb mRNA reduction in mice and monkeys, and mRNA silencing was greatest in skeletal and cardiac (striated) muscle with minimal to no activity in other major organs. In mice the EC50 for Ssb mRNA reduction in skeletal muscle was >75-fold less than in systemic tissues. Oligonucleotides conjugated to control antibodies or cholesterol produced no mRNA reduction or were 10-fold less potent, respectively. Tissue PKPD of AOCs demonstrated mRNA silencing activity primarily driven by receptor-mediated delivery in striated muscle for siRNA oligonucleotides. In mice, we show that AOC-mediated delivery is operable across various oligonucleotide modalities. AOC PKPD properties translated to higher species, providing promise for a new class of oligonucleotide therapeutics.
in vivo depletion of CD71+ cells
Wynn, J. L., et al (2015). "Neonatal CD71+ Erythroid Cells Do Not Modify Murine Sepsis Mortality" J Immunol 195(3): 1064-1070.
PubMed
Sepsis is a major cause of neonatal mortality and morbidity worldwide. A recent report suggested that murine neonatal host defense against infection could be compromised by immunosuppressive CD71(+) erythroid splenocytes. We examined the impact of CD71(+) erythroid splenocytes on murine neonatal mortality to endotoxin challenge or polymicrobial sepsis and characterized circulating CD71(+) erythroid (CD235a(+)) cells in human neonates. Adoptive transfer or an Ab-mediated reduction in neonatal CD71(+) erythroid splenocytes did not alter murine neonatal survival to endotoxin challenge or polymicrobial sepsis challenge. Ex vivo immunosuppression of stimulated adult CD11b(+) cells was not limited to neonatal splenocytes; it also occurred with adult and neonatal bone marrow. Animals treated with anti-CD71 Ab showed reduced splenic bacterial load following bacterial challenge compared with isotype-treated mice. However, adoptive transfer of enriched CD71(+) erythroid splenocytes to CD71(+)-reduced animals did not reduce bacterial clearance. Human CD71(+)CD235a(+) cells were common among cord blood mononuclear cells and were shown to be reticulocytes. In summary, a lack of effect on murine survival to polymicrobial sepsis following adoptive transfer or diminution of CD71(+) erythroid splenocytes under these experimental conditions suggests that the impact of these cells on neonatal infection risk and progression may be limited. An unanticipated immune priming effect of anti-CD71 Ab treatment, rather than a reduction in immunosuppressive CD71(+) erythroid splenocytes, was likely responsible for the reported enhanced bacterial clearance. In humans, the well-described rapid decrease in circulating reticulocytes after birth suggests that they may have a limited role in reducing inflammation secondary to microbial colonization.
in vivo depletion of CD71+ cells
Torow, N., et al (2015). "Active suppression of intestinal CD4(+)TCRalphabeta(+) T-lymphocyte maturation during the postnatal period" Nat Commun 6: 7725.
PubMed
Priming of the mucosal immune system during the postnatal period substantially influences host-microbial interaction and susceptibility to immune-mediated diseases in adult life. The underlying mechanisms are ill defined. Here we show that shortly after birth, CD4 T cells populate preformed lymphoid structures in the small intestine and quickly acquire a distinct transcriptional profile. T-cell recruitment is independent of microbial colonization and innate or adaptive immune stimulation but requires beta7 integrin expression. Surprisingly, neonatal CD4 T cells remain immature throughout the postnatal period under homeostatic conditions but undergo maturation and gain effector function on barrier disruption. Maternal SIgA and regulatory T cells act in concert to prevent immune stimulation and maintain the immature phenotype of CD4 T cells in the postnatal intestine during homeostasis. Active suppression of CD4 T-cell maturation during the postnatal period might contribute to prevent auto-reactivity, sustain a broad TCR repertoire and establish life-long immune homeostasis.