InVivoMAb anti-human CD71 (TfR1)
Product Description
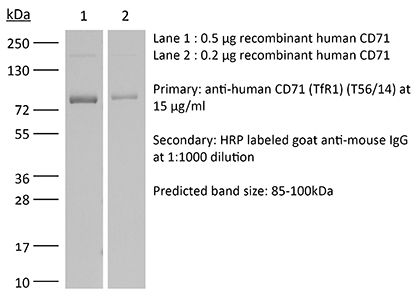
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human hematopoietic cell line K562 |
| Reported Applications |
Flow cytometry Immunoprecipitation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Purification | Protein G |
| RRID | AB_2927507 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
Olivier, E. N., et al (2006). "Large-scale production of embryonic red blood cells from human embryonic stem cells" Exp Hematol 34(12): 1635-1642.
PubMed
OBJECTIVE: To develop a method to produce in culture large number of erythroid cells from human embryonic stem cells. MATERIALS AND METHODS: Human H1 embryonic stem cells were differentiated into hematopoietic cells by coculture with a human fetal liver cell line, and the resulting CD34-positive cells were expanded in vitro in liquid culture using a three-step method. The erythroid cells produced were then analyzed by light microscopy and flow cytometry. Globin expression was characterized by quantitative reverse-transcriptase polymerase chain reaction and by high-performance liquid chromatography. RESULTS: CD34-positive cells produced from human embryonic stem cells could be efficiently differentiated into erythroid cells in liquid culture leading to a more than 5000-fold increase in cell number. The erythroid cells produced are similar to primitive erythroid cells present in the yolk sac of early human embryos and did not enucleate. They are fully hemoglobinized and express a mixture of embryonic and fetal globins but no beta-globin. CONCLUSIONS: We have developed an experimental protocol to produce large numbers of primitive erythroid cells starting from undifferentiated human embryonic stem cells. As the earliest human erythroid cells, the nucleated primitive erythroblasts, are not very well characterized because experimental material at this stage of development is very difficult to obtain, this system should prove useful to answer a number of experimental questions regarding the biology of these cells. In addition, production of mature red blood cells from human embryonic stem cells is of great potential practical importance because it could eventually become an alternate source of cell for transfusion.
Flow Cytometry
Abendroth, A., et al (2001). "Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells" J Virol 75(10): 4878-4888.
PubMed
We sought to examine the effects of varicella-zoster virus (VZV) infection on the expression of major histocompatibility complex class I (MHC I) molecules by human fibroblasts and T lymphocytes. By flow cytometry, VZV infection reduced the cell surface expression of MHC I molecules on fibroblasts significantly, yet the expression of transferrin receptor was not affected. Importantly, when human fetal thymus/liver implants in SCID-hu mice were inoculated with VZV, cell surface MHC I expression was downregulated specifically on VZV-infected human CD3+ T lymphocytes, a prominent target that sustains VZV viremia. The stage in the MHC I assembly process that was disrupted by VZV in fibroblasts was examined in pulse-chase and immunoprecipitation experiments in the presence of endoglycosidase H. MHC I complexes continued to be assembled in VZV-infected cells and were not retained in the endoplasmic reticulum. In contrast, immunofluorescence and confocal microscopy showed that VZV infection resulted in an accumulation of MHC I molecules which colocalized to the Golgi compartment. Inhibition of late viral gene expression by treatment of infected fibroblasts with phosphonoacetic acid did not influence the modulation of MHC I expression, nor did transfection of cells with plasmids expressing immediate early viral proteins. However, cells transfected with a plasmid carrying the early gene ORF66 did result in a significant downregulation of MHC I expression, suggesting that this gene encodes a protein with an immunomodulatory function. Thus, VZV downregulates MHC I expression by impairing the transport of MHC I molecules from the Golgi compartment to the cell surface; this effect may enable the virus to evade CD8+ T-cell immune recognition during VZV pathogenesis, including the critical phase of T-lymphocyte-associated viremia.
Franco, A., et al (1992). "Transferrin receptor mediates uptake and presentation of hepatitis B envelope antigen by T lymphocytes" J Exp Med 175(5): 1195-1205.
PubMed
Human activated T lymphocytes expressing class II molecules are able to present only complex antigens that bind to their own surface receptors, and thus can be captured, internalized, and processed through the class II major histocompatibility complex processing pathway. We have used the antigen-presenting T cell system to identify the viral receptor used by hepatitis B virus (HBV) to enter cells, as well as the sequence of HB envelope antigen (HBenvAg) involved in this interaction. Results show that both CD4+ and CD8+ T clones can process and present HBenvAg to class II-restricted cytotoxic T lymphocytes and that the CD71 transferrin receptor (TfR) is involved in efficient HBenvAg uptake by T cells. Moreover, we provide evidence that the HBenvAg sequence interacting with the T cell surface is contained within the pre-S2 region. Since TfR is also expressed on hepatocytes, it might represent a portal of cellular entry for HBV infection. This system of antigen presentation by T cells may serve as a model to study both lymphocyte receptors used by lymphocytotropic viruses and viral proteins critical to bind them.
Immunoprecipitation
Trowbridge, I. S. and M. B. Omary (1981). "Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin" Proc Natl Acad Sci U S A 78(5): 3039-3043.
PubMed
A cell surface glycoprotein antigen with an apparent molecular weight of about 100,000 that is selectively expressed on proliferating cells was purified from deoxycholate-solubilized membranes of a cultured human leukemic thymus-derived (T) cell line by affinity chromatography on a monoclonal antibody-Sepharose column. A conventional xenoantiserum prepared by immunization with the affinity-purified glycoprotein was found to contain antibodies against a serum component that bound tightly to cultured cells. This molecule was shown to be specifically associated with the cell surface glycoprotein purified by immunoprecipitation from lysates of cells. We have identified the serum component as transferrin and conclude that the membrane glycoprotein is the cell surface transferrin receptor.