InVivoMAb recombinant CTLA-4-Ig (hum/hum)
Specifications
| Recommended Isotype Control(s) | InVivoMAb recombinant human IgG1 Fc |
|---|---|
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949064 |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Rochman, Y., et al (2015). "Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig" PLoS One 10(4): e0122198.
PubMed
During activation, T cells integrate multiple signals from APCs and cytokine milieu. The blockade of these signals can have clinical benefits as exemplified by CTLA4-Ig, which blocks interaction of B7 co-stimulatory molecules on APCs with CD28 on T cells. Variants of CTLA4-Ig, abatacept and belatacept are FDA approved as immunosuppressive agents in arthritis and transplantation, yet murine studies suggested that CTLA4-Ig could be beneficial in a number of other diseases. However, detailed analysis of human CD4 cell hyporesponsivness induced by CTLA4-Ig has not been performed. Herein, we established a model to study the effect of CTLA4-Ig on the activation of human naive T cells in a human mixed lymphocytes system. Comparison of human CD4 cells activated in the presence or absence of CTLA4-Ig showed that co-stimulation blockade during TCR activation does not affect NFAT signaling but results in decreased activation of NF-kappaB and AP-1 transcription factors followed by a profound decrease in proliferation and cytokine production. The resulting T cells become hyporesponsive to secondary activation and, although capable of receiving TCR signals, fail to proliferate or produce cytokines, demonstrating properties of anergic cells. However, unlike some models of T cell anergy, these cells did not possess increased levels of the TCR signaling inhibitor CBLB. Rather, the CTLA4-Ig-induced hyporesponsiveness was associated with an elevated level of p27kip1 cyclin-dependent kinase inhibitor.
Schroder, P. M., et al (2013). "Transient combination therapy targeting the immune synapse abrogates T cell responses and prolongs allograft survival in mice" PLoS One 8(7): e69397.
PubMed
T cells play a major role in allograft rejection, which occurs after T cell activation by the engagement of several functional molecules to form an immune synapse with alloantigen presenting cells. In this study, the immune synapse was targeted using mAbs directed to the TCR beta-chain (TCRbeta) and lymphocyte function-associated antigen-1 (LFA1) to induce long-term allograft survival. Evaluation of antigen-specific T cell responses was performed by adoptively transferring CFSE labeled transgenic OT-II cells into wild-type mice and providing OVA peptide by intravenous injection. Graft survival studies were performed in mice by transplanting BALB/c ear skins onto the flanks of C57BL/6 recipients. The anti-TCRbeta plus anti-LFA1 mAb combination (but not either mAb alone) abrogated antigen-specific T cell responses invitro and invivo. Transient combination therapy with these agents resulted in significantly prolonged skin allograft survival in mice (51+/-10 days; p<0.01) when compared to treatment with either anti-TCRbeta mAb (24+/-5 days) or anti-LFA1 mAb (19+/-3 days) alone or no treatment (10+/-1 days). When lymphoid tissues from these mice were analyzed at different times post-transplant, only those receiving the combination of anti-TCRbeta and anti-LFA1 mAbs demonstrated long-lasting reductions in total T cell numbers, cellular and humoral anti-donor responses, and expression of CD3 on the surface of T cells. These results demonstrate that transient anti-TCRbeta and anti-LFA1 mAb combination therapy abrogates antigen-reactive T cell responses with long-lasting effects that significantly prolong allograft survival.
Chen, Y., et al (2012). "Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression" J Immunol 188(4): 1698-1707.
PubMed
Regulatory T cells (Treg) are crucial for self-tolerance. It has been an enigma that Treg exhibit an anergic phenotype reflected by hypoproliferation in vitro after TCR stimulation but undergo vigorous proliferation in vivo. We report in this study that murine Treg are prone to death but hyperproliferative in vitro and in vivo, which is different from conventional CD4(+)Foxp3(-) T cells (Tcon). During in vitro culture, most Treg die with or without TCR stimulation, correlated with constitutive activation of the intrinsic death pathway. However, a small portion of the Treg population is more sensitive to TCR stimulation, particularly weak stimulation, proliferates more vigorously than CD4(+) Tcon, and is resistant to activation-induced cell death. Treg proliferation is enhanced by IL-2 but is less dependent on CD28-mediated costimulation than that of Tcon. We demonstrate further that the surviving and proliferative Treg are ICOS(+) whereas the death-prone Treg are ICOS(-). Moreover, ICOS(+) Treg contain much stronger suppressive activity than that of ICOS(-) Treg. Our data indicate that massive death contributes to the anergic phenotype of Treg in vitro and suggest modulation of Treg survival as a therapeutic strategy for treatment of autoimmune diseases and cancer.
Raedler, H., et al (2011). "Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice" Am J Transplant 11(7): 1397-1406.
PubMed
While activation of serum complement mediates antibody-initiated vascular allograft injury, increasing evidence indicates that complement also functions as a modulator of alloreactive T cells. We tested whether blockade of complement activation at the C5 convertase step affects T cell-mediated cardiac allograft rejection in mice. The anti-C5 mAb BB5.1, which prevents the formation of C5a and C5b, synergized with subtherapeutic doses of CTLA4Ig to significantly prolong the survival of C57BL/6 heart grafts that were transplanted into naive BALB/c recipients. Anti-C5 mAb treatment limited the induction of donor-specific IFNgamma-producing T cell alloimmunity without inducing Th2 or Th17 immunity in vivo and inhibited primed T cells from responding to donor antigens in secondary mixed lymphocyte responses. Additional administration of anti-C5 mAb to the donor prior to graft recovery further prolonged graft survival and concomitantly reduced both the in vivo trafficking of primed T cells into the transplanted allograft and decreased expression of T cell chemoattractant chemokines within the graft. Together these results support the novel concept that C5 blockade can inhibit T cell-mediated allograft rejection through multiple mechanisms, and suggest that C5 blockade may constitute a viable strategy to prevent and/or treat T cell-mediated allograft rejection in humans.
Whitlock, E. L., et al (2010). "Dynamic quantification of host Schwann cell migration into peripheral nerve allografts" Exp Neurol 225(2): 310-319.
PubMed
Host Schwann cell (SC) migration into nerve allografts is the limiting factor in the duration of immunosuppression following peripheral nerve allotransplantation, and may be affected by different immunosuppressive regimens. Our objective was to compare SC migration patterns between clinical and experimental immunosuppression regimens both over time and at the harvest endpoint. Eighty mice that express GFP under the control of the Schwann cell specific S100 promoter were engrafted with allogeneic, nonfluorescent sciatic nerve grafts. Mice received immunosuppression with either tacrolimus (FK506), or experimental T-cell triple costimulation blockade (CSB), consisting of CTLA4-immunoglobulin fusion protein, anti-CD40 monoclonal antibody, and anti-inducible costimulator monoclonal antibody. Migration of GFP-expressing host SCs into wild-type allografts was assessed in vivo every 3 weeks until 15 weeks postoperatively, and explanted allografts were evaluated for immunohistochemical staining patterns to differentiate graft from host SCs. Immunosuppression with tacrolimus exhibited a plateau of SC migration, characterized by significant early migration (< 3 weeks) followed by a constant level of host SCs in the graft (15 weeks). At the endpoint, graft fluorescence was decreased relative to surrounding host nerve, and donor SCs persisted within the graft. CSB-treated mice displayed gradually increasing migration of host SCs into the graft, without the plateau noted in tacrolimus-treated mice, and also maintained a population of donor SCs at the 15-week endpoint. SC migration patterns are affected by immunosuppressant choice, particularly in the immediate postoperative period, and the use of a single treatment of CSB may allow for gradual population of nerve allografts with host SCs.
Shen, H. and D. R. Goldstein (2009). "IL-6 and TNF-alpha synergistically inhibit allograft acceptance" J Am Soc Nephrol 20(5): 1032-1040.
PubMed
Previous studies suggested that activation of the innate immune system impairs the induction of transplantation tolerance, but the responsible inflammatory mediators have not been identified. In this study, we examined whether IL-6 and TNF-alpha promote resistance to transplantation tolerance. Using a highly immunogenic murine skin allograft model, we found that the absence of both IL-6 and TNF-alpha in the graft recipient synergized with co-stimulatory blockade to induce tolerance. Furthermore, IL-6 and TNF-alpha acted together to promote T cell alloimmune responses both in vitro and in vivo and to impair the ability of regulatory T cells to suppress effector T cell alloimmunity. In addition, deficiency of recipient IRAK-M, a negative regulator of certain innate immune pathways, augmented cellular IL-6 and TNF-alpha responses and impaired the ability of co-stimulatory blockade to extend allograft survival. In summary, IL-6 and TNF-alpha synergistically impair the efficacy of therapies that promote allograft acceptance.
Product Citations
-
Immunomodulatory Nanoparticles Enable Combination Therapies To Enhance Disease Prevention and Flare Control in Rheumatoid Arthritis.
In ACS Cent Sci on 24 September 2025 by Johnson, W. T., Wilkinson, E. L., et al.
PubMed
Disease-modifying antirheumatic drugs (DMARDs) have greatly improved the treatment of rheumatoid arthritis (RA), but strategies to prevent disease onset and recurring flares remain limited. While abatacept (CTLA-4 IgG) can delay RA onset and corticosteroids are used for flare control, the benefit is temporary. We report that combining standard-of-care treatments with a locally administered immunomodulatory agent, termed Agg-CLNP, enhances both disease prevention and flare mitigation. Agg-CLNP consists of polymer nanoparticles conjugated with an immunodominant aggrecan peptide and encapsulate calcitriol. These nanoparticles are optimized for uptake by dendritic cells (DC) in lymph nodes proximal to arthritic joints. In vitro, Agg-CLNP suppressed costimulatory molecules and HLA class II (HLA-2) expression and upregulated CTLA-4 in human monocyte-derived DC from healthy and RA donors. In SKG mice, a T cell-driven RA model, Agg-CLNP combined with CTLA-4 IgG synergistically delayed disease onset and reduced severity. In a dexamethasone (Dex) withdrawal flare model, post-Dex Agg-CLNP treatment reduced flare severity and preserved a regulatory phenotype in DC, while suppressing local pathogenic TH17 cells. Next generation RNA sequencing of lymph node DC revealed Ctla4 upregulation and changes in other immunomodulatory genes linked to flare prevention. These findings highlight Agg-CLNP as a potential therapeutic strategy to address critical unmet needs in RA management.
-
-
In vivo experiments
-
Mus musculus (Mouse)
cGAS/STING signalling in macrophages aggravates obliterative bronchiolitis via an IFN-α-dependent mechanism after orthotopic tracheal transplantation in mice.
In Clin Transl Med on 1 May 2025 by Wan, J., Liu, H., et al.
PubMed
Our previous findings have underscored the role of innate immunity in obliterative bronchiolitis (OB). However, despite the central importance of the cyclic GMP‒AMP synthase (cGAS)/stimulator of interferon genes (STING) signalling pathway in innate immune responses, its specific contribution to OB progression remains largely unexplored.
-
-
Deficiency in the mitophagy mediator Parkin accelerates murine skin allograft rejection.
In Am J Transplant on 1 December 2024 by Wragg, K. M., Worley, M. J., et al.
PubMed
Alterations in mitochondrial function and associated quality control programs, including mitochondrial-specific autophagy, termed mitophagy, are gaining increasing recognition in the context of disease. However, the role of mitophagy in organ transplant rejection remains poorly understood. Using mice deficient in Parkin, a ubiquitin ligase that tags damaged or dysfunctional mitochondria for autophagic clearance, we assessed the impact of Parkin-dependent mitophagy on skin-graft rejection. We observed accelerated graft loss in Parkin-deficient mice across multiple skin graft models. Immune cell distributions posttransplant were largely unperturbed compared to wild-type; however, the CD8+ T cells of Parkin-deficient mice expressed more T-bet, IFNγ, and Ki67, indicating greater priming toward effector function. This was accompanied by increased circulating levels of IL-12p70 in Parkin-deficient mice. Using a mixed leukocyte reaction, we demonstrated that naïve Parkin-deficient CD4+ and CD8+ T cells exhibit enhanced activation marker expression and proliferative responses to alloantigen, which were attenuated with administration of a pharmacological mitophagy inducer (p62-mediated mitophagy inducer), known to increase mitophagy in the absence of a functional PINK1-Parkin pathway. These findings indicate a role for Parkin-dependent mitophagy in curtailing skin-graft rejection.
-
-
Cancer Research
NK Receptor Signaling Lowers TCR Activation Threshold, Enhancing Selective Recognition of Cancer Cells by TAA-Specific CTLs.
In Cancer Immunol Res on 1 October 2024 by Dong, B., Obermajer, N., et al.
PubMed
Cytotoxic CD8+ T lymphocyte (CTL) recognition of non-mutated tumor-associated antigens (TAA), present on cancer cells and also in healthy tissues, is an important element of cancer immunity, but the mechanism of its selectivity for cancer cells and opportunities for its enhancement remain elusive. In this study, we found that CTL expression of the NK receptors (NKR) DNAM1 and NKG2D was associated with the effector status of CD8+ tumor-infiltrating lymphocytes and long-term survival of patients with melanoma. Using MART1 and NY-ESO-1 as model TAAs, we demonstrated that DNAM1 and NKG2D regulate T-cell receptor (TCR) functional avidity and set the threshold for TCR activation of human TAA-specific CTLs. Superior co-stimulatory effects of DNAM1 over CD28 involved enhanced TCR signaling, CTL killer function, and polyfunctionality. Double transduction of human CTLs with TAA-specific TCR and NKRs resulted in strongly enhanced antigen sensitivity, without a reduction in antigen specificity and selectivity of killer function. In addition, the elevation of NKR ligand expression on cancer cells due to chemotherapy also increased CTL recognition of cancer cells expressing low levels of TAAs. Our data help explain the ability of self-antigens to mediate tumor rejection in the absence of autoimmunity and support the development of dual-targeting adoptive T-cell therapies that use NKRs to enhance the potency and selectivity of recognition of TAA-expressing cancer cells.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Post-immunotherapy CTLA-4 Ig treatment improves antitumor efficacy.
In Proc Natl Acad Sci U S A on 2 July 2024 by Mok, S., Ağaç Çobanoğlu, D., et al.
PubMed
Immune checkpoint therapies (ICT) improve overall survival of patients with cancer but may cause immune-related adverse events (irAEs) such as myocarditis. Cytotoxic T lymphocyte-associated antigen 4 immunoglobulin fusion protein (CTLA-4 Ig), an inhibitor of T cell costimulation through CD28, reverses irAEs in animal models. However, concerns exist about potentially compromising antitumor response of ICT. In mouse tumor models, we administered CTLA-4 Ig 1) concomitantly with ICT or 2) after ICT completion. Concomitant treatment reduced antitumor efficacy, while post-ICT administration improved efficacy without affecting frequency and function of CD8 T cells. The improved response was independent of the ICT used, whether CTLA-4 or PD-1 blockade. The frequency of Tregs was significantly decreased with CTLA-4 Ig. The resulting increased CD8/Treg ratio potentially underlies the enhanced efficacy of ICT followed by CTLA-4 Ig. This paradoxical mechanism shows that a CTLA-4 Ig regimen shown to reduce irAE severity does not compromise antitumor efficacy.
-
-
-
Rattus norvegicus (Rat)
Immunopotentiating effects of herb-partitioned moxibustion on the spleens of cyclophosphamide-induced immunosuppressed rats.
In Chin Med on 18 February 2024 by Xiong, L., Tian, Y., et al.
PubMed
To investigate the effec of the herb-partitioned moxibustion on T-lymphocyte activity in immunosuppressed rats through differential modulation of the immune checkpoint molecules CD28 and CTLA-4.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
p40 homodimers bridge ischemic tissue inflammation and heterologous alloimmunity in mice via IL-15 transpresentation.
In J Clin Invest on 25 January 2024 by Tsuda, H., Keslar, K. S., et al.
PubMed
Virus-induced memory T cells often express functional cross-reactivity, or heterologous immunity, to other viruses and to allogeneic MHC molecules that is an important component of pathogenic responses to allogeneic transplants. During immune responses, antigen-reactive naive and central memory T cells proliferate in secondary lymphoid organs to achieve sufficient cell numbers to effectively respond, whereas effector memory T cell proliferation occurs directly within the peripheral inflammatory microenvironment. Mechanisms driving heterologous memory T cell proliferation and effector function expression within peripheral tissues remain poorly understood. Here, we dissected proliferation of heterologous donor-reactive memory CD8+ T cells and their effector functions following infiltration into heart allografts with low or high intensities of ischemic inflammation. Proliferation within both ischemic conditions required p40 homodimer-induced IL-15 transpresentation by graft DCs, but expression of effector functions mediating acute allograft injury occurred only in high-ischemic allografts. Transcriptional responses of heterologous donor-reactive memory CD8+ T cells were distinct from donor antigen-primed memory CD8+ T cells during early activation in allografts and at graft rejection. Overall, the results provide insights into mechanisms driving heterologous effector memory CD8+ T cell proliferation and the separation between proliferation and effector function that is dependent on the intensity of inflammation within the tissue microenvironment.
-
-
-
Pathology
Defective LAT signalosome pathology in mice mimics human IgG4-related disease at single-cell level.
In J Exp Med on 6 November 2023 by Joachim, A., Aussel, R., et al.
PubMed
Mice with a loss-of-function mutation in the LAT adaptor (LatY136F) develop an autoimmune and type 2 inflammatory disorder called defective LAT signalosome pathology (DLSP). We analyzed via single-cell omics the trajectory leading to LatY136F DLSP and the underlying CD4+ T cell diversification. T follicular helper cells, CD4+ cytotoxic T cells, activated B cells, and plasma cells were found in LatY136F spleen and lung. Such cell constellation entailed all the cell types causative of human IgG4-related disease (IgG4-RD), an autoimmune and inflammatory condition with LatY136F DLSP-like histopathological manifestations. Most previously described T cell-mediated autoimmune manifestations require persistent TCR input. In contrast, following their first engagement by self-antigens, the autoreactive TCR expressed by LatY136F CD4+ T cells hand over their central role in T cell activation to CD28 costimulatory molecules. As a result, all subsequent LatY136F DLSP manifestations, including the production of autoantibodies, solely rely on CD28 engagement. Our findings elucidate the etiology of the LatY136F DLSP and qualify it as a model of IgG4-RD.
-
-
-
Immunology and Microbiology
Transplantation elicits a clonally diverse CD8+ T cell response that is comprised of potent CD43+ effectors.
In Cell Rep on 29 August 2023 by Cohen, G. S., Kallarakal, M. A., et al.
PubMed
CD8+ T cells mediate acute rejection of allografts, which threatens the long-term survival of transplanted organs. Using MHC class I tetramers, we find that allogeneic CD8+ T cells are present at an elevated naive precursor frequency relative to other epitopes, only modestly increase in number after grafting, and maintain high T cell receptor diversity throughout the immune response. While antigen-specific effector CD8+ T cells poorly express the canonical effector marker KLRG-1, expression of the activated glycoform of CD43 defines potent effectors after transplantation. Activated CD43+ effector T cells maintain high expression of the coreceptor induced T cell costimulator (ICOS) in the presence of CTLA-4 immunoglobulin (Ig), and dual CTLA-4 Ig/anti-ICOS treatment prolongs graft survival. These data demonstrate that graft-specific CD8+ T cells have a distinct response profile relative to anti-pathogen CD8+ T cells and that CD43 and ICOS are critical surface receptors that define potent effector CD8+ T cell populations that form after transplantation.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Localized cytotoxic T cell-associated antigen 4 and antioxidant islet encapsulation alters macrophage signaling and induces regulatory and anergic T cells to enhance allograft survival.
In Am J Transplant on 1 April 2023 by Barra, J. M., Kozlovskaya, V., et al.
PubMed
The loss of functional β-cell mass is a hallmark of type 1 diabetes. Islet transplantation represents a promising alternative approach, but immune-mediated graft destruction remains a major challenge. We sought to use islet encapsulation technologies to improve graft survival and function without systemic immunosuppression. We hypothesized islet encapsulation with nanothin coatings consisting of tannic acid (TA), an antioxidant; poly(N-vinylpyrrolidone) (PVPON), a biocompatible polymer; and cytotoxic T cell-associated antigen 4 immunoglobulin (CTLA-4-Ig), an inhibitory immune receptor, will elicit localized immunosuppression to prolong islet allograft function and suppress effector T cell responses. In the absence of systemic immunosuppression, we demonstrated (PVPON/TA/CTLA-4-Ig)-encapsulated NOD.Rag islet grafts maintain function significantly longer than control IgG-containing (PVPON/TA/IgG) and nonencapsulated controls after transplantation into diabetic C57BL/6 mice. This protection coincided with diminished proinflammatory macrophage responses mediated by signal transducer and activator of transcription 1 signaling, decreased proinflammatory T cell effector responses, and CTLA-4-Ig-specific concomitant increases in anergic CD4+ T cells and regulatory T cells. Our results provide evidence that conjugation of CTLA-4-Ig to (PVPON/TA) coatings can suppress T cell activation, enhance regulatory T cell populations, prolong islet allograft survival, and induce localized immunosuppression after transplantation.
-
-
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
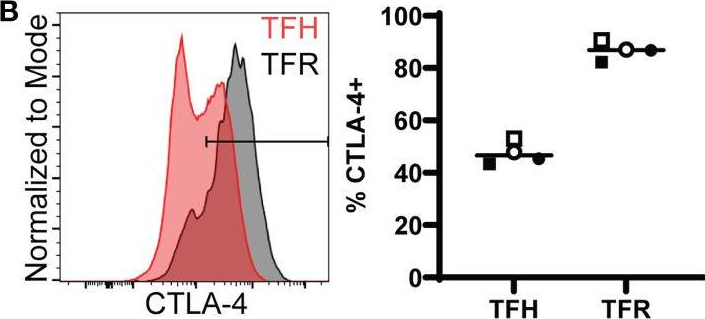
Follicular regulatory T cells eliminate HIV-1-infected follicular helper T cells in an IL-2 concentration dependent manner.
In Front Immunol on 25 November 2022 by Ollerton, M. T., Folkvord, J. M., et al.
PubMed
Follicular helper CD4+ T cells (TFH) are highly permissive to HIV and major foci of virus expression in both untreated and treated infection. Follicular regulatory CD4+ T cells (TFR) limit TFH numbers and function in vitro and in vivo. We evaluated the hypothesis that TFR suppress HIV replication in TFH using a well-established model of ex vivo HIV infection that employs tonsil cells from HIV uninfected individuals spinoculated with CXCR4- and CCR5-tropic HIV-GFP reporter viruses. Both CXCR4 and CCR5-tropic HIV replication were reduced in TFH cultured with TFR as compared to controls. Blocking antibodies to CD39, CTLA-4, IL-10, and TGF-beta failed to reverse suppression of HIV replication by TFR, and there were no sex differences in TFR suppressive activity. TFR reduced viability of TFH and even more so reduced HIV infected TFH as assessed by total and integrated HIV DNA. Exogenous IL-2 enhanced TFH viability and particularly numbers of GFP+ TFH in a concentration dependent manner. TFR reduced productively infected TFH at low and moderate IL-2 concentrations, and this was associated with decreases in extracellular IL-2. Both IL-2 expressing cells and larger numbers of FoxP3+CD4+ cells were detected in follicles and germinal centers of lymph nodes of people living with HIV. TFR may deplete TFH in vivo through restriction of IL-2 and thereby contribute to decay of HIV expressing cells in B cell follicles during HIV infection.
-
-
-
Cardiovascular biology
Low-dose IL-2 prevents murine chronic cardiac allograft rejection: Role for IL-2-induced T regulatory cells and exosomes with PD-L1 and CD73.
In Am J Transplant on 1 September 2022 by Ravichandran, R., Itabashi, Y., et al.
PubMed
To determine the effects and immunological mechanisms of low-dose interleukin-2 (IL-2) in a murine model of chronic cardiac allograft rejection (BALB/c to C57BL/6) after costimulatory blockade consisting of MR1 (250 μg/ip day 0) and CTLA4-Ig (200 μg/ip day 2), we administered low-dose IL-2 (2000 IU/day) starting on posttransplant day 14 for 3 weeks. T regulatory (Treg) cell infiltration of the grafts was determined by immunohistochemistry; circulating exosomes by western blot and aldehyde bead flow cytometry; antibodies to donor MHC by immunofluorescent staining of donor cells; and antibodies to cardiac self-antigens (myosin, vimentin) by ELISA. We demonstrated that costimulation blockade after allogeneic heart transplantation induced circulating exosomes containing cardiac self-antigens and antibodies to both donor MHC and self-antigens, leading to chronic rejection by day 45. Treatment with low-dose IL-2 prolonged allograft survival (>100 days), prevented chronic rejection, and induced splenic and graft-infiltrating CD4+ CD25+ Foxp3 Treg cells by day 45 and circulating exosomes (Foxp3+) with PD-L1 and CD73. MicroRNA 142, associated with the TGFβ pathway, was significantly downregulated in exosomes from IL-2-treated mice. In conclusion, low-dose IL-2 delays rejection in a murine model of chronic cardiac allograft rejection and also induces graft-infiltrating Tregs and circulating exosomes with immunoregulatory molecules.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
Donor Macrophages Modulate Rejection After Heart Transplantation.
In Circulation on 23 August 2022 by Kopecky, B. J., Dun, H., et al.
PubMed
Cellular rejection after heart transplantation imparts significant morbidity and mortality. Current immunosuppressive strategies are imperfect, target recipient T cells, and have adverse effects. The innate immune response plays an essential role in the recruitment and activation of T cells. Targeting the donor innate immune response would represent the earliest interventional opportunity within the immune response cascade. There is limited knowledge about donor immune cell types and functions in the setting of cardiac transplantation, and no current therapeutics exist for targeting these cell populations.
-
-
-
Immunology and Microbiology
Interferon-β acts directly on T cells to prolong allograft survival by enhancing regulatory T cell induction through Foxp3 acetylation.
In Immunity on 8 March 2022 by Fueyo-González, F., McGinty, M., et al.
PubMed
Type I interferons (IFNs) are pleiotropic cytokines with potent antiviral properties that also promote protective T cell and humoral immunity. Paradoxically, type I IFNs, including the widely expressed IFNβ, also have immunosuppressive properties, including promoting persistent viral infections and treating T-cell-driven, remitting-relapsing multiple sclerosis. Although associative evidence suggests that IFNβ mediates these immunosuppressive effects by impacting regulatory T (Treg) cells, mechanistic links remain elusive. Here, we found that IFNβ enhanced graft survival in a Treg-cell-dependent murine transplant model. Genetic conditional deletion models revealed that the extended allograft survival was Treg cell-mediated and required IFNβ signaling on T cells. Using an in silico computational model and analysis of human immune cells, we found that IFNβ directly promoted Treg cell induction via STAT1- and P300-dependent Foxp3 acetylation. These findings identify a mechanistic connection between the immunosuppressive effects of IFNβ and Treg cells, with therapeutic implications for transplantation, autoimmunity, and malignancy.
-
-
-
Immunology and Microbiology
Evaluation of immunosuppression protocols for MHC-matched allogeneic iPS cell-based transplantation using a mouse skin transplantation model.
In Inflamm Regen on 2 February 2022 by Kamatani, T., Otsuka, R., et al.
PubMed
Off-the-shelf major histocompatibility complex (MHC)-matched iPS cells (iPSC) can potentially initiate host immune responses because of the existence of numerous minor antigens. To suppress allo-immune responses, combination of immunosuppressants is usually used, but its efficacy to the allogeneic iPSC-based transplantation has not been precisely evaluated.
-
-
-
Cardiovascular biology
Donor Macrophages Modulate Rejection after Heart Transplantation
In bioRxiv on 20 September 2021 by Kopecky, B., Dun, H., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting dendritic cells with a PD-L1 based bispecific antibody rejuvenates specific anti-tumor T cells
In Research Square on 23 July 2020 by Liu, L., Chen, J., et al.
-
-
-
Cardiovascular biology
-
Immunology and Microbiology
Regulatory CD8 T cells that recognize Qa-1 expressed by CD4 T-helper cells inhibit rejection of heart allografts.
In Proc Natl Acad Sci U S A on 17 March 2020 by Choi, J. Y., Eskandari, S. K., et al.
PubMed
Induction of longstanding immunologic tolerance is essential for survival of transplanted organs and tissues. Despite recent advances in immunosuppression protocols, allograft damage inflicted by antibody specific for donor organs continues to represent a major obstacle to graft survival. Here we report that activation of regulatory CD8 T cells (CD8 Treg) that recognize the Qa-1 class Ib major histocompatibility complex (MHC), a mouse homolog of human leukocyte antigen-E (HLA-E), inhibits antibody-mediated immune rejection of heart allografts. We analyzed this response using a mouse model that harbors a point mutation in the class Ib MHC molecule Qa-1, which disrupts Qa-1 binding to the T cell receptor (TCR)-CD8 complex and impairs the CD8 Treg response. Despite administration of cytotoxic T lymphocyte antigen 4 (CTLA-4) immunoglobulin (Ig), Qa-1 mutant mice developed robust donor-specific antibody responses and accelerated heart graft rejection. We show that these allo-antibody responses reflect diminished Qa-1-restricted CD8 Treg-mediated suppression of host follicular helper T cell-dependent antibody production. These findings underscore the critical contribution of this Qa-1/HLA-E-dependent regulatory pathway to maintenance of transplanted organs and suggest therapeutic approaches to ameliorate allograft rejection.
-
-
-
Cell Biology
Circulating mitochondria in organ donors promote allograft rejection.
In Am J Transplant on 1 July 2019 by Lin, L., Xu, H., et al.
PubMed
The innate immune system is a critical regulator of the adaptive immune responses that lead to allograft rejection. It is increasingly recognized that endogenous molecules released from tissue injury and cell death are potent activators of innate immunity. Mitochondria, ancestrally related to bacteria, possess an array of endogenous innate immune-activating molecules. We have recently demonstrated that extracellular mitochondria are abundant in the circulation of deceased organ donors and that their presence correlates with early allograft dysfunction. Here we demonstrate the ability of mitochondria to activate endothelial cells (ECs), the initial barrier between a solid organ allograft and its host. We find that mitochondria exposure leads to the upregulation of EC adhesion molecules and their production of inflammatory cytokines and chemokines. Additionally, mitochondrial exposure causes dendritic cells to upregulate costimulatory molecules. Infusion of isolated mitochondria into heart donors leads to significant increase in allograft rejection in a murine heterotopic heart transplantation model. Finally, co-incubation of human peripheral blood mononuclear cells with mitochondria-treated ECs results in increased numbers of effector (IFN-γ+ , TNF-α+ ) CD8+ T cells. These data indicate that circulating extracellular mitochondria in deceased organ donors may directly activate allograft ECs and promote graft rejection in transplant recipients.
-
-
Low energy X-ray (grenz ray) treatment of purified islets prior to allotransplant markedly decreases passenger leukocyte populations.
In Islets on 4 July 2017 by Pawlick, R., Gala-Lopez, B., et al.
PubMed
Grenz rays, or minimally penetrating X-rays, are known to be an effective treatment of certain recalcitrant immune-mediated skin diseases, but their use in modulating allograft rejection has not been tested. We examined the capacity of grenz ray treatment to minimize islet immunogenicity and extend allograft survival in a mouse model. In a preliminary experiment, 1 of 3 immunologically intact animals demonstrated long-term acceptance of their grenz ray treated islet allograft. Further experiments revealed that 28.6% (2 of 7) grenz ray treated islet allografts survived >60 d. A low dose of 20Gy, was important; a 4-fold increase in radiation resulted in rapid graft failure, and transplanting a higher islet mass did not alter this outcome. To determine whether increased islet allograft survival after grenz treatment would be masked by immunosuppression, we treated the recipients with CTLA-4 Ig, and found an additive effect, whereby 17.5% more animals accepted the graft long-term versus those with CTLA-4 Ig alone. Cell viability assays verified that islet integrity was maintained after treatment with 20Gy. As well, through splenocyte infiltration analysis, donor CD4+ T cell populations 24-hours after transplant were decreased by more than16-fold in recipients receiving irradiated islets compared with control. Donor CD8+ T cell populations, although less prevalent, decreased in all treatment groups compared with control. Our results suggest that brief treatment of isolated islets with low energy grenz rays before allotransplantation can significantly reduce passenger leukocytes and promote graft survival, possibly by inducing donor dendritic cells to differentiate toward a tolerogenic phenotype.