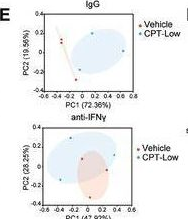
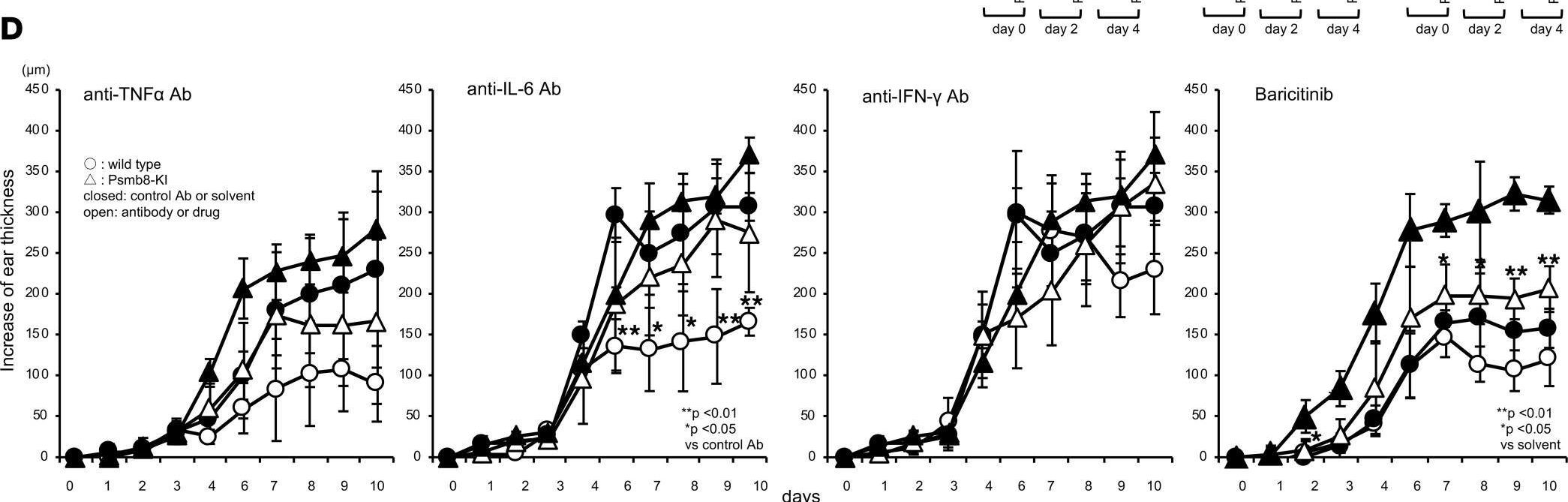
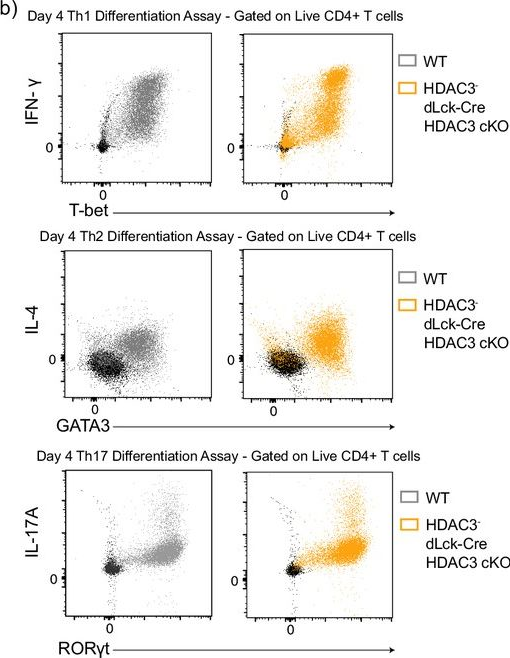
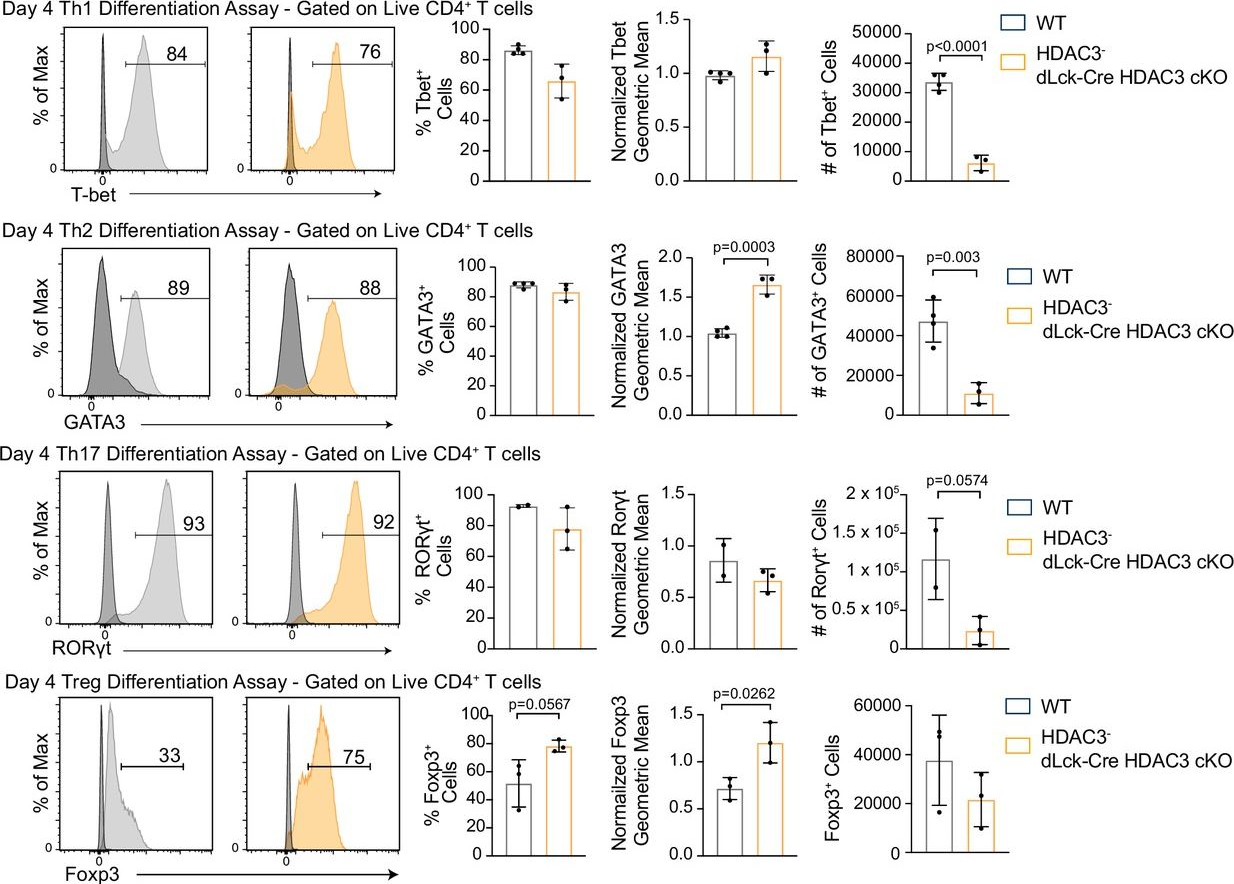
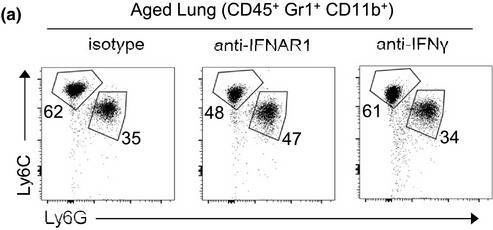
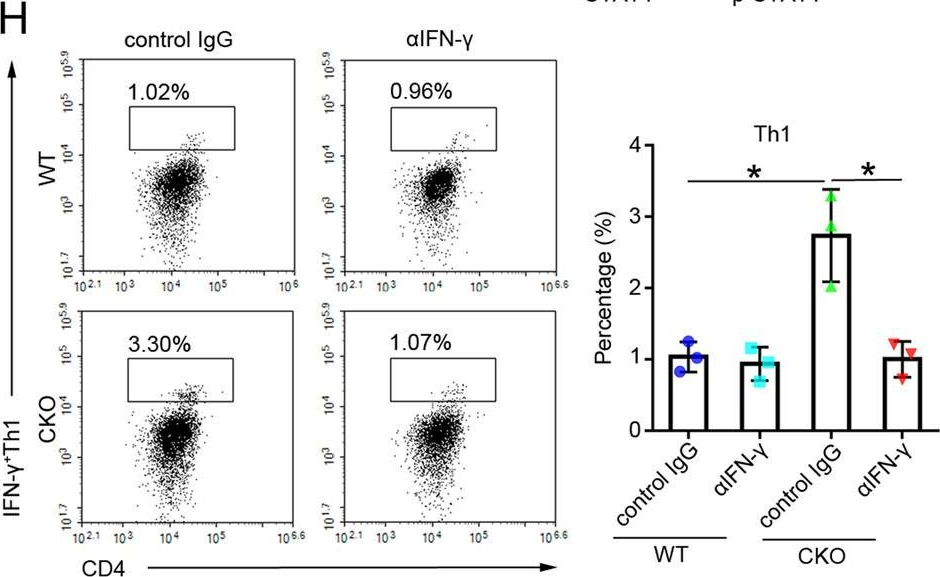
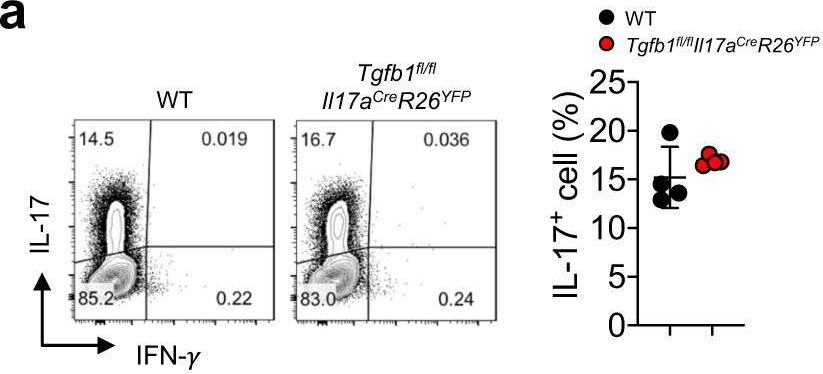
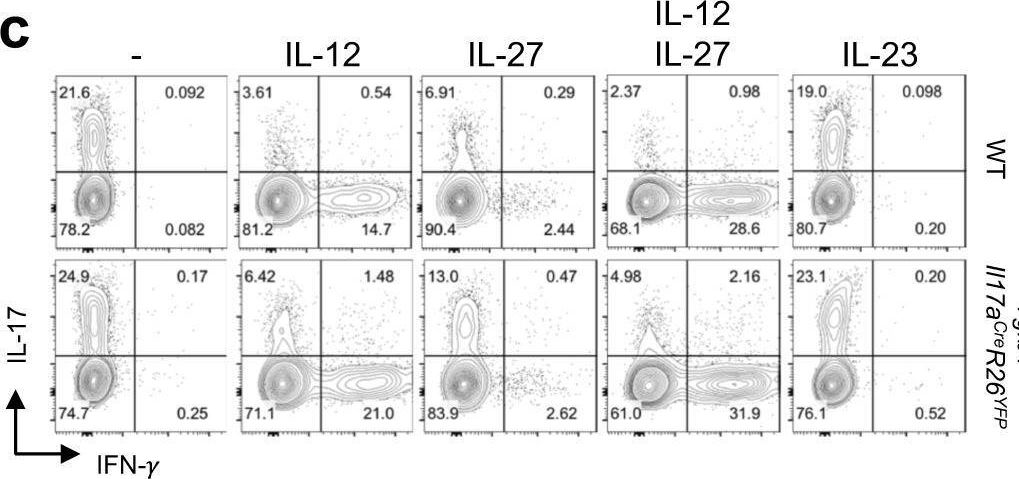
InVivoMAb anti-mouse IFNγ
Product Description
Bio X Cell is pleased to offer XMG1.2-CP058. XMG1.2-CP058 is a recombinant, chimeric version of the original XMG1.2 with identical variable domain sequences and constant region sequences converted from Rat IgG1, κ to mouse IgG1, κ for use in murine models. Species-matched chimeric antibodies exhibit regulated effector functions—including Fc receptor binding and complement activation—and cause less immunogenicity and formation of anti-drug antibodies (ADAs) than xenogenic antibodies in animal models. The highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results over hybridoma derived antibodies.
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 8.0T Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IFNγ |
| Reported Applications |
in vivo IFNγ neutralization in vitro IFNγ neutralization ELISPOT Flow cytometry Western blotin vitro Organoids/Organ-on-Chip |
| Formulation |
PBS + 0.01% Tween, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107694 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro Organoids/Organ-on-Chip
Takashima S, Sharma R, Chang W, Calafiore M, Fu YY, Jansen SA, Ito T, Egorova A, Kuttiyara J, Arnhold V, Sharrock J, Santosa E, Chaudhary O, Geiger H, Iwasaki H, Liu C, Sun J, Robine N, Mazutis L, Lindemans CA, Hanash AM (2025). "STAT1 regulates immu
PubMed
The role of the immune system in regulating tissue stem cells remains poorly understood, as does the relationship between immune-mediated tissue damage and regeneration. Graft vs. host disease (GVHD) occurring after allogeneic bone marrow transplantation (allo-BMT) involves immune-mediated damage to the intestinal epithelium and its stem cell compartment. To assess impacts of T-cell-driven injury on distinct epithelial constituents, we have performed single cell RNA sequencing on intestinal crypts following experimental BMT. Intestinal stem cells (ISCs) from GVHD mice have exhibited global transcriptomic changes associated with a substantial Interferon-γ response and upregulation of STAT1. To determine its role in crypt function, STAT1 has been deleted within murine intestinal epithelium. Following allo-BMT, STAT1 deficiency has resulted in reduced epithelial proliferation and impaired ISC recovery. Similarly, epithelial Interferon-γ receptor deletion has also attenuated proliferation and ISC recovery post-transplant. Investigating the mechanistic basis underlying this epithelial response, ISC STAT1 expression in GVHD has been found to correlate with upregulation of ISC c-Myc. Furthermore, activated T cells have stimulated Interferon-γ-dependent epithelial regeneration in co-cultured organoids, and Interferon-γ has directly induced STAT1-dependent c-Myc expression and ISC proliferation. These findings illustrate immunologic regulation of a core tissue stem cell program after damage and support a role for Interferon-γ as a direct contributor to epithelial regeneration.
in vitro Organoids/Organ-on-Chip
Takashima S, Sharma R, Chang W, Calafiore M, Fu YY, Jansen SA, Ito T, Egorova A, Kuttiyara J, Arnhold V, Sharrock J, Santosa E, Chaudhary O, Geiger H, Iwasaki H, Liu C, Sun J, Robine N, Mazutis L, Lindemans CA, Hanash AM (2025). "STAT1 regulates immu
PubMed
The role of the immune system in regulating tissue stem cells remains poorly understood, as does the relationship between immune-mediated tissue damage and regeneration. Graft vs. host disease (GVHD) occurring after allogeneic bone marrow transplantation (allo-BMT) involves immune-mediated damage to the intestinal epithelium and its stem cell compartment. To assess impacts of T-cell-driven injury on distinct epithelial constituents, we have performed single cell RNA sequencing on intestinal crypts following experimental BMT. Intestinal stem cells (ISCs) from GVHD mice have exhibited global transcriptomic changes associated with a substantial Interferon-γ response and upregulation of STAT1. To determine its role in crypt function, STAT1 has been deleted within murine intestinal epithelium. Following allo-BMT, STAT1 deficiency has resulted in reduced epithelial proliferation and impaired ISC recovery. Similarly, epithelial Interferon-γ receptor deletion has also attenuated proliferation and ISC recovery post-transplant. Investigating the mechanistic basis underlying this epithelial response, ISC STAT1 expression in GVHD has been found to correlate with upregulation of ISC c-Myc. Furthermore, activated T cells have stimulated Interferon-γ-dependent epithelial regeneration in co-cultured organoids, and Interferon-γ has directly induced STAT1-dependent c-Myc expression and ISC proliferation. These findings illustrate immunologic regulation of a core tissue stem cell program after damage and support a role for Interferon-γ as a direct contributor to epithelial regeneration.
in vitro Organoids/Organ-on-Chip
Takashima S, Sharma R, Chang W, Calafiore M, Fu YY, Jansen SA, Ito T, Egorova A, Kuttiyara J, Arnhold V, Sharrock J, Santosa E, Chaudhary O, Geiger H, Iwasaki H, Liu C, Sun J, Robine N, Mazutis L, Lindemans CA, Hanash AM (2025). "STAT1 regulates immu
PubMed
The role of the immune system in regulating tissue stem cells remains poorly understood, as does the relationship between immune-mediated tissue damage and regeneration. Graft vs. host disease (GVHD) occurring after allogeneic bone marrow transplantation (allo-BMT) involves immune-mediated damage to the intestinal epithelium and its stem cell compartment. To assess impacts of T-cell-driven injury on distinct epithelial constituents, we have performed single cell RNA sequencing on intestinal crypts following experimental BMT. Intestinal stem cells (ISCs) from GVHD mice have exhibited global transcriptomic changes associated with a substantial Interferon-γ response and upregulation of STAT1. To determine its role in crypt function, STAT1 has been deleted within murine intestinal epithelium. Following allo-BMT, STAT1 deficiency has resulted in reduced epithelial proliferation and impaired ISC recovery. Similarly, epithelial Interferon-γ receptor deletion has also attenuated proliferation and ISC recovery post-transplant. Investigating the mechanistic basis underlying this epithelial response, ISC STAT1 expression in GVHD has been found to correlate with upregulation of ISC c-Myc. Furthermore, activated T cells have stimulated Interferon-γ-dependent epithelial regeneration in co-cultured organoids, and Interferon-γ has directly induced STAT1-dependent c-Myc expression and ISC proliferation. These findings illustrate immunologic regulation of a core tissue stem cell program after damage and support a role for Interferon-γ as a direct contributor to epithelial regeneration.
in vitro Organoids/Organ-on-Chip
Wang C, Hyams B, Allen NC, Cautivo K, Monahan K, Zhou M, Dahlgren MW, Lizama CO, Matthay M, Wolters P, Molofsky AB, Peng T (2023). "Dysregulated lung stroma drives emphysema exacerbation by potentiating resident lymphocytes to suppress an epithelial
PubMed
Aberrant tissue-immune interactions are the hallmark of diverse chronic lung diseases. Here, we sought to define these interactions in emphysema, a progressive disease characterized by infectious exacerbations and loss of alveolar epithelium. Single-cell analysis of human emphysema lungs revealed the expansion of tissue-resident lymphocytes (TRLs). Murine studies identified a stromal niche for TRLs that expresses Hhip, a disease-variant gene downregulated in emphysema. Stromal-specific deletion of Hhip induced the topographic expansion of TRLs in the lung that was mediated by a hyperactive hedgehog-IL-7 axis. 3D immune-stem cell organoids and animal models of viral exacerbations demonstrated that expanded TRLs suppressed alveolar stem cell growth through interferon gamma (IFNγ). Finally, we uncovered an IFNγ-sensitive subset of human alveolar stem cells that was preferentially lost in emphysema. Thus, we delineate a stromal-lymphocyte-epithelial stem cell axis in the lung that is modified by a disease-variant gene and confers host susceptibility to emphysema.
in vitro Organoids/Organ-on-Chip
Parsa R, London M, Rezende de Castro TB, Reis B, Buissant des Amorie J, Smith JG, Mucida D (2022). "Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection" Immunity 55(7):1234-1
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus.
in vitro Organoids/Organ-on-Chip
Parsa R, London M, Rezende de Castro TB, Reis B, Buissant des Amorie J, Smith JG, Mucida D (2022). "Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection" Immunity 55(7):1234-1
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus.
in vitro Organoids/Organ-on-Chip
Parsa R, London M, Rezende de Castro TB, Reis B, Buissant des Amorie J, Smith JG, Mucida D (2022). "Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection" Immunity 55(7):1234-1
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus.
in vitro Organoids/Organ-on-Chip
Parsa R, London M, Rezende de Castro TB, Reis B, Buissant des Amorie J, Smith JG, Mucida D (2022). "Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection" Immunity 55(7):1234-1
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus.
in vitro Organoids/Organ-on-Chip
Parsa R, London M, Rezende de Castro TB, Reis B, Buissant des Amorie J, Smith JG, Mucida D (2022). "Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection" Immunity 55(7):1234-1
PubMed
The intestinal epithelium comprises the body's largest surface exposed to viruses. Additionally, the gut epithelium hosts a large population of intraepithelial T lymphocytes, or IELs, although their role in resistance against viral infections remains elusive. By fate-mapping T cells recruited to the murine intestine, we observed an accumulation of newly recruited CD4+ T cells after infection with murine norovirus CR6 and adenovirus type-2 (AdV), but not reovirus. CR6- and AdV-recruited intraepithelial CD4+ T cells co-expressed Ly6A and chemokine receptor CCR9, exhibited T helper 1 and cytotoxic profiles, and conferred protection against AdV in vivo and in an organoid model in an IFN-γ-dependent manner. Ablation of the T cell receptor (TCR) or the transcription factor ThPOK in CD4+ T cells prior to AdV infection prevented viral control, while TCR ablation during infection did not impact viral clearance. These results uncover a protective role for intraepithelial Ly6A+CCR9+CD4+ T cells against enteric adenovirus.
in vivo IFNγ neutralization
Glasner, A., et al (2018). "NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis" Immunity 48(1): 107-119 e104.
PubMed
Natural killer (NK) cells are innate lymphoid cells, and their presence within human tumors correlates with better prognosis. However, the mechanisms by which NK cells control tumors in vivo are unclear. Here, we used reflectance confocal microscopy (RCM) imaging in humans and in mice to visualize tumor architecture in vivo. We demonstrated that signaling via the NK cell receptor NKp46 (human) and Ncr1 (mouse) induced interferon-gamma (IFN-gamma) secretion from intratumoral NK cells. NKp46- and Ncr1-mediated IFN-gamma production led to the increased expression of the extracellular matrix protein fibronectin 1 (FN1) in the tumors, which altered primary tumor architecture and resulted in decreased metastases formation. Injection of IFN-gamma into tumor-bearing mice or transgenic overexpression of Ncr1 in NK cells in mice resulted in decreased metastasis formation. Thus, we have defined a mechanism of NK cell-mediated control of metastases in vivo that may help develop NK cell-dependent cancer therapies.
in vitro IFNγ neutralization
Clever, D., et al (2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche" Cell 166(5): 1117-1131 e1114.
PubMed
Cancer cells must evade immune responses at distant sites to establish metastases. The lung is a frequent site for metastasis. We hypothesized that lung-specific immunoregulatory mechanisms create an immunologically permissive environment for tumor colonization. We found that T-cell-intrinsic expression of the oxygen-sensing prolyl-hydroxylase (PHD) proteins is required to maintain local tolerance against innocuous antigens in the lung but powerfully licenses colonization by circulating tumor cells. PHD proteins limit pulmonary type helper (Th)-1 responses, promote CD4(+)-regulatory T (Treg) cell induction, and restrain CD8(+) T cell effector function. Tumor colonization is accompanied by PHD-protein-dependent induction of pulmonary Treg cells and suppression of IFN-gamma-dependent tumor clearance. T-cell-intrinsic deletion or pharmacological inhibition of PHD proteins limits tumor colonization of the lung and improves the efficacy of adoptive cell transfer immunotherapy. Collectively, PHD proteins function in T cells to coordinate distinct immunoregulatory programs within the lung that are permissive to cancer metastasis.
in vitro IFNγ neutralization
Flow Cytometry
Sell, S., et al (2015). "Control of murine cytomegalovirus infection by gammadelta T cells" PLoS Pathog 11(2): e1004481.
PubMed
Infections with cytomegalovirus (CMV) can cause severe disease in immunosuppressed patients and infected newborns. Innate as well as cellular and humoral adaptive immune effector functions contribute to the control of CMV in immunocompetent individuals. None of the innate or adaptive immune functions are essential for virus control, however. Expansion of gammadelta T cells has been observed during human CMV (HCMV) infection in the fetus and in transplant patients with HCMV reactivation but the protective function of gammadelta T cells under these conditions remains unclear. Here we show for murine CMV (MCMV) infections that mice that lack CD8 and CD4 alphabeta-T cells as well as B lymphocytes can control a MCMV infection that is lethal in RAG-1(-/-) mice lacking any T- and B-cells. gammadelta T cells, isolated from infected mice can kill MCMV infected target cells in vitro and, importantly, provide long-term protection in infected RAG-1(-/-) mice after adoptive transfer. gammadelta T cells in MCMV infected hosts undergo a prominent and long-lasting phenotypic change most compatible with the view that the majority of the gammadelta T cell population persists in an effector/memory state even after resolution of the acute phase of the infection. A clonotypically focused Vgamma1 and Vgamma2 repertoire was observed at later stages of the infection in the organs where MCMV persists. These findings add gammadelta T cells as yet another protective component to the anti-CMV immune response. Our data provide clear evidence that gammadelta T cells can provide an effective control mechanism of acute CMV infections, particularly when conventional adaptive immune mechanisms are insufficient or absent, like in transplant patient or in the developing immune system in utero. The findings have implications in the stem cell transplant setting, as antigen recognition by gammadelta T cells is not MHC-restricted and dual reactivity against CMV and tumors has been described.
in vitro IFNγ neutralization
Flow Cytometry
Hou, L., et al (2015). "The protease cathepsin L regulates Th17 cell differentiation" J Autoimmun. S 0896-8411(15): 30024-X.
PubMed
Previously we reported that IL-17+ T cells, primarily IL-17+ gammadelta cells, are increased in mice lacking the protease inhibitor serpinB1 (serpinb1-/- mice). Here we show that serpinB1-deficient CD4 cells exhibit a cell-autonomous and selective deficiency in suppressing T helper 17 (Th17) cell differentiation. This suggested an opposing role for one or more protease in promoting Th17 differentiation. We found that several SerpinB1-inhibitable cysteine cathepsins are induced in Th17 cells, most prominently cathepsin L (catL); this was verified by peptidase assays, active site labeling and Western blots. Moreover, Th17 differentiation was suppressed by both broad cathepsin inhibitors and catL selective inhibitors. CatL is present in Th17 cells as single chain (SC)- and two-chain (TC)-forms. Inhibiting asparagine endopeptidase (AEP) blocked conversion of SC-catL to TC-catL and increased generation of serpinb1-/- Th17 cells, but not wild-type Th17 cells. These findings suggest that SC-catL is biologically active in promoting Th17 generation and is counter-regulated by serpinB1 and secondarily by AEP. Thus, in addition to regulation by cytokines and transcription factors, differentiation of CD4 cells to Th17 cells is actively regulated by a catL-serpinB1-AEP module. Targeting this protease regulatory module could be an approach to treating Th17 cell-driven autoimmune disorders.
in vitro IFNγ neutralization
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro IFNγ neutralization
Cao, A. T., et al (2015). "Interleukin (IL)-21 promotes intestinal IgA response to microbiota" Mucosal Immunol 8(5): 1072-1082.
PubMed
Commensal microbiota-specific T helper type 17 (Th17) cells are enriched in the intestines, which can convert into T follicular helper (Tfh) in Peyer’s patches, and are crucial for production of intestinal immunoglobulin A (IgA) against microbiota; however, the role of Th17 and Tfh cytokines in regulating the mucosal IgA response to enteric microbiota is still not completely known. In this study, we found that intestinal IgA was impaired in mice deficient in interleukin (IL)-17 or IL-21 signaling. IL-21, but not IL-17, is able to augment B-cell differentiation to IgA(+) cells as mediated by transforming growth factor beta1 (TGFbeta1) and accelerate IgA class switch recombination (CSR). IL-21 and retinoic acid (RA) induce IgA(+) B-cell development and IgA production and drives autocrine TGFbeta1 production to initiate IgA CSR. Repletion of T-cell-deficient TCRbetaxdelta(-/-) mice with Th17 cells specific for commensal bacterial antigen increased the levels of IgA(+) B cells and IgA production in the intestine, which was blocked by neutralizing IL-21. Thus IL-21 functions to strongly augment IgA production under intestinal environment. Furthermore, IL-21 promotes intestinal B-cell homing through alpha4beta7 expression, alone or with TGFbeta and RA. Together, IL-21 from microbiota-specific Th17 and/or Tfh cells contributes to robust intestinal IgA levels by enhancing IgA(+) CSR, IgA production and B-cell trafficking into the intestine.
in vitro IFNγ neutralization
Flow Cytometry
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vivo IFNγ neutralization
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vitro IFNγ neutralization
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vitro IFNγ neutralization
Flow Cytometry
Rabenstein, H., et al (2014). "Differential kinetics of antigen dependency of CD4+ and CD8+ T cells" J Immunol 192(8): 3507-3517.
PubMed
Ag recognition via the TCR is necessary for the expansion of specific T cells that then contribute to adaptive immunity as effector and memory cells. Because CD4+ and CD8+ T cells differ in terms of their priming APCs and MHC ligands we compared their requirements of Ag persistence during their expansion phase side by side. Proliferation and effector differentiation of TCR transgenic and polyclonal mouse T cells were thus analyzed after transient and continuous TCR signals. Following equally strong stimulation, CD4+ T cell proliferation depended on prolonged Ag presence, whereas CD8+ T cells were able to divide and differentiate into effector cells despite discontinued Ag presentation. CD4+ T cell proliferation was neither affected by Th lineage or memory differentiation nor blocked by coinhibitory signals or missing inflammatory stimuli. Continued CD8+ T cell proliferation was truly independent of self-peptide/MHC-derived signals. The subset divergence was also illustrated by surprisingly broad transcriptional differences supporting a stronger propensity of CD8+ T cells to programmed expansion. These T cell data indicate an intrinsic difference between CD4+ and CD8+ T cells regarding the processing of TCR signals for proliferation. We also found that the presentation of a MHC class II-restricted peptide is more efficiently prolonged by dendritic cell activation in vivo than a class I bound one. In summary, our data demonstrate that CD4+ T cells require continuous stimulation for clonal expansion, whereas CD8+ T cells can divide following a much shorter TCR signal.
in vivo IFNγ neutralization
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vitro IFNγ neutralization
ELISPOT
Deng, L., et al (2014). "Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice" J Clin Invest 124(2): 687-695.
PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
in vivo IFNγ neutralization
Flow Cytometry
Simons, D. M., et al (2013). "Autoreactive Th1 cells activate monocytes to support regional Th17 responses in inflammatory arthritis" J Immunol 190(7): 3134-3141.
PubMed
We have examined mechanisms underlying the formation of pathologic Th17 cells using a transgenic mouse model in which autoreactive CD4(+) T cells recognize influenza virus hemagglutinin (HA) as a ubiquitously expressed self-Ag and induce inflammatory arthritis. The lymph nodes of arthritic mice contain elevated numbers of inflammatory monocytes (iMO) with an enhanced capacity to promote CD4(+) Th17 cell differentiation, and a regional inflammatory response develops in the paw-draining lymph nodes by an IL-17-dependent mechanism. The activation of these Th17-trophic iMO precedes arthritis development and occurs in the context of an autoreactive CD4(+) Th1 cell response. Adoptive transfer of HA-specific CD4(+) T cells into nonarthritic mice expressing HA as a self-Ag similarly led to the formation of Th1 cells and of iMO that could support Th17 cell formation, and, notably, the accumulation of these iMO in the lymph nodes was blocked by IFN-gamma neutralization. These studies show that autoreactive CD4(+) Th1 cells directed to a systemically distributed self-Ag can promote the development of a regional Th17 cell inflammatory response by driving the recruitment of Th17-trophic iMO to the lymph nodes.
in vivo IFNγ neutralization
Flow Cytometry
Yu, X., et al (2013). "A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity" Cancer Res 73(7): 2093-2103.
PubMed
Converting the immunosuppressive tumor environment into one that is favorable to the induction of antitumor immunity is indispensable for effective cancer immunotherapy. Here, we strategically incorporate a pathogen (i.e., flagellin)-derived, NF-kappaB-stimulating “danger” signal into the large stress protein or chaperone Grp170 (HYOU1/ORP150) that was previously shown to facilitate antigen crosspresentation. This engineered chimeric molecule (i.e., Flagrp170) is capable of transporting tumor antigens and concurrently inducing functional activation of dendritic cells (DC). Intratumoral administration of adenoviruses expressing Flagrp170 induces a superior antitumor response against B16 melanoma and its distant lung metastasis compared with unmodified Grp170 and flagellin. The enhanced tumor destruction is accompanied with significantly increased tumor infiltration by CD8(+) cells as well as elevation of IFN-gamma and interleukin (IL)-12 levels in the tumor sites. In situ Ad.Flagrp170 therapy provokes systemic activation of CTLs that recognize several antigens naturally expressing in melanoma (e.g., gp100/PMEL and TRP2/DCT). The mechanistic studies using CD11c-DTR transgenic mice and Batf3-deficient mice reveal that CD8alpha(+) DCs are required for the improved T-cell crosspriming. Antibody neutralization assays show that IL-12 and IFN-gamma are essential for the Flagrp170-elicited antitumor response, which also involves CD8(+) T cells and natural killer cells. The therapeutic efficacy of Flagrp170 and its immunostimulating activity are also confirmed in mouse prostate cancer and colon carcinoma. Together, targeting the tumor microenvironment with this chimeric chaperone is highly effective in mobilizing or restoring antitumor immunity, supporting the potential therapeutic use of this novel immunomodulator in the treatment of metastatic diseases.
in vivo IFNγ neutralization
Flow Cytometry
Kugler, D. G., et al (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927.
PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo IFNγ neutralization
Flow Cytometry
Mohr, E., et al (2010). "IFN-{gamma} produced by CD8 T cells induces T-bet-dependent and -independent class switching in B cells in responses to alum-precipitated protein vaccine" Proc Natl Acad Sci U S A 107(40): 17292-17297.
PubMed
Alum-precipitated protein (alum protein) vaccines elicit long-lasting neutralizing antibody responses that prevent bacterial exotoxins and viruses from entering cells. Typically, these vaccines induce CD4 T cells to become T helper 2 (Th2) cells that induce Ig class switching to IgG1. We now report that CD8 T cells also respond to alum proteins, proliferating extensively and producing IFN-gamma, a key Th1 cytokine. These findings led us to question whether adoptive transfer of antigen-specific CD8 T cells alters the characteristic CD4 Th2 response to alum proteins and the switching pattern in responding B cells. To this end, WT mice given transgenic ovalbumin (OVA)-specific CD4 (OTII) or CD8 (OTI) T cells, or both, were immunized with alum-precipitated OVA. Cotransfer of antigen-specific CD8 T cells skewed switching patterns in responding B cells from IgG1 to IgG2a and IgG2b. Blocking with anti-IFN-gamma antibody largely inhibited this altered B-cell switching pattern. The transcription factor T-bet is required in B cells for IFN-gamma-dependent switching to IgG2a. By contrast, we show that this transcription factor is dispensable in B cells both for IFN-gamma-induced switching to IgG2b and for inhibition of switching to IgG1. Thus, T-bet dependence identifies distinct transcriptional pathways in B cells that regulate IFN-gamma-induced switching to different IgG isotypes.
in vivo IFNγ neutralization
Flow Cytometry
Quezada, S. A., et al (2010). "Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts" J Exp Med 207(3): 637-650.
PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
Product Citations
-
Intratumoral natural killer cells show reduced effector and cytolytic properties and control the differentiation of effector Th1 cells.
In Oncoimmunology on 6 February 2017 by Paul, S., Kulkarni, N., et al.
PubMed
Natural killer (NK) cells are known to have effector and cytolytic properties to kill virus infected or tumor cells spontaneously. Due to these properties, NK cells have been used as an adoptive cellular therapy to control tumor growth in various clinical trials but have shown limited clinical benefits. This indicates that our knowledge about phenotypic and functional differences in NK cells within the tumor microenvironment and secondary lymphoid tissues is incomplete. In this work, we report that B16F10 cell-induced melanoma recruits the CD11b+CD27+ subset of NK cells at a very early stage during tumor progression. These intratumoral NK cells showed increased expression of CD69, reduced inhibitory receptor KLRG1, and decreased proliferative ability. As compared to splenic NK cells, intratumoral NK cells showed decreased expression of activating receptors NKG2D, Ly49D and Ly49H; increased inhibitory receptors, NKG2A and Ly49A; decreased cytokines IFNγ and GM-CSF; decreased cytokine receptors IL-21R, IL-6Rα, and CD122 expression. Depletion of NK cells led to decrease peripheral as well as intratumoral effector CD4+T-bet+ cells (Th1), and increased tumor growth. Furthermore, purified NK cells showed increased differentiation of Th1 cells in an IFNγ-dependent manner. Anti-NKG2D in the culture promoted differentiation of effector Th1 cells. Collectively, these observations suggest that intratumoral NK cells possess several inhibitory functions that can be partly reversed by signaling through the NKG2D receptor or by cytokine stimulation, which then leads to increased differentiation of effector Th1 cells.
-
-
Immunology and Microbiology
Harnessing the dual immunomodulatory function of myeloid-derived suppressor cells to reshape the inflammatory microenvironment for osteoarthritis therapy.
In Mater Today Bio on 1 December 2025 by Guo, Z., Chen, T., et al.
PubMed
Osteoarthritis (OA) pathogenesis is profoundly influenced by dysregulated immune dynamics, where persistent interleukin-17 (IL-17)/T helper 17 (Th17) cell mediated inflammation coordinates with failed regenerative processes to perpetuate joint destruction. Here, we unveil the role of myeloid-derived suppressor cells (MDSCs) as dual-phase regulators that paradoxically orchestrate both inflammatory escalation and tissue repair in OA progression. Intra-articular administration of MDSCs in OA mice amplified IL-17 dependent inflammatory cascades and chemokine-driven leukocyte recruitment, revealing a context-dependent pro-inflammatory phenotype. Unexpectedly, MDSC depletion failed to attenuate joint damage, implying their indispensable yet multifaceted role in OA pathogenesis. Mechanistically, MDSCs exhibited functional plasticity by upregulating arginase-1 to polarize M2 macrophages, fostering a regenerative niche alongside their inflammatory activity. To resolve this duality, we developed a bio-responsive hydrogel-microsphere system integrating transforming growth factor β1 (TGF-β1) and interleukin-1 β1 antibody (anti-IL-1β) loaded mesoporous silica nanoparticles (MSNs). This spatiotemporally controlled platform selectively suppressed MDSC-mediated Th17 cell expansion while harnessing their intrinsic capacity to drive M2 macrophage polarization and chondrogenesis. The resultant shift from a pro-inflammatory to pro-regenerative microenvironment significantly attenuated cartilage erosion and restored joint integrity in OA models. Our findings redefine MDSCs as bifunctional immune orchestrators in OA and establish precision biomaterial guided immune decoding as a paradigm-shifting therapeutic strategy. By engineering MDSCs plasticity through antagonistic cytokine delivery, this work provides a blueprint for microenvironment remodeling in degenerative joint diseases.
-
-
-
Cell Biology
-
Cancer Research
-
Biochemistry and Molecular biology
The transcription factor ZEB2 mediates the antitumor efficacy of tumor-infiltrating lymphocytes in non-small cell lung cancer.
In Cell Death Dis on 7 November 2025 by Wang, J., Liu, F., et al.
PubMed
Immune checkpoint blockade (ICB) offers an in vivo approach to activate CD8+ tumor-infiltrating lymphocytes (CD8+TILs) in cases of advanced non-small cell lung cancer (NSCLC). A large fraction of NSCLC patients is unresponsive to ICBs and relapse due to the development of dysfunctional CD8+TILs with impaired cytotoxicity. Therefore, an improved understanding of regulator(s) that favor the development of cytotoxic Teff cells over dysfunctional CD8+TILs is required for the success of ICB therapy in NSCLC patients. Here, our metaVIPER-based scRNA-seq analysis of deep CD8+ cell scRNA-seq data from 14 treatment-naïve NSCLC patients revealed that the master regulon ZEB2 may drive CD8+ differentiation along the cytotoxic effector trajectory in NSCLC tumors. In vitro, ZEB2 acts downstream of T-bet to stimulate lung tumor-reactive Teff cell differentiation. This T-bet/ZEB2 axis displays immunotherapeutic effects on KP.SIY lung tumors independent of ICB therapy and mediates the therapeutic effects of murine serum albumin-fused IL-2 + IL-12 combination immunotherapy (IL2-MSA + IL12-MSA) in mice. IL2-MSA + IL12-MSA operates through a parallel STAT4/FOXO1-mediated mechanism that promotes CD8+TIL T-bet/ZEB2 expression and lung tumor-reactive Teff cell differentiation. In conclusion, immunotherapeutic regimens that support ZEB2 activity in CD8+ cells may show promise in NSCLC patients.
-
-
-
Immunology and Microbiology
Salmonella-superspreader hosts require gut regulatory T cells to maintain a disease-tolerant state.
In J Exp Med on 3 November 2025 by Di Luccia, B., Massis, L. M., et al.
PubMed
Host-pathogen interactions involve two critical strategies: resistance, whereby hosts clear invading microbes, and tolerance, whereby hosts carry high pathogen burden asymptomatically. Here, we investigate mechanisms by which Salmonella-superspreader (SSP) hosts maintain an asymptomatic state during chronic infection. We found that regulatory T cells (Tregs) are essential for this disease-tolerant state, limiting intestinal immunopathology and enabling SSP hosts to thrive, while facilitating Salmonella transmission. Treg depletion in SSP mice resulted in decreased survival, heightened gut inflammation, and impairment of the intestinal barrier, without affecting Salmonella persistence. Colonic Tregs from SSP mice exhibited a unique transcriptomic profile characterized by the upregulation of type 1 inflammatory genes, including the transcription factor T-bet. In the absence of Tregs, we observed robust expansion of cytotoxic CD4+ T cells, with CD4+ T cell depletion restoring homeostasis. These results uncover a critical host strategy to establish disease tolerance during chronic enteric infection, providing novel insights into mucosal responses to persistent pathogens and chronic intestinal inflammation.
-
-
-
Cancer Research
-
Immunology and Microbiology
αTIGIT-IL2 achieves tumor regression by promoting tumor-infiltrating regulatory T cell fragility in mouse models.
In Nat Commun on 17 October 2025 by Wang, T., Xu, Y., et al.
PubMed
Administration of IL-2 may promote the suppressive function and proliferation of Treg cells that cause immune tolerance in patients with cancer, which causes low-dose IL-2 to fail in achieving an optimal anti-tumor effect. Here, we designed an immunocytokine by fusing IL-2 and an anti-TIGIT monoclonal antibody, named αTIGIT-IL2, that targets Treg cells and promotes their fragility in the tumor milieu. These fragile-like Treg cells show impaired suppressive function and high IFN-γ production, triggering an immune-reactive tumor microenvironment. Such inflammation leads to the recruitment and functional reprogramming of intratumoral neutrophils, improving cross-talk between neutrophils and CD8+ T cells and enhancing the antitumor ability of CD8+ T cells. Combination therapy with αTIGIT-IL2 and PD-1 blocker could eliminate triple-negative breast cancer (TNBC) tumors resistant to immune checkpoint blockade (ICB) therapy. These findings provide the basis for developing a new generation of immunocytokines that target Treg cells and promote their fragility in the tumor milieu, resulting in robust antitumor immunity.
-
-
-
Immunology and Microbiology
A recombinant Cedar virus preclinical model that recapitulates neurological features of henipavirus disease.
In iScience on 17 October 2025 by Huaman, C., Clouse, C., et al.
PubMed
Nipah virus (NiV) and Hendra virus (HeV) are members of the henipavirus genus that cause severe respiratory and/or neurological disease in humans. Because NiV and HeV can only be handled under BSL-4 containment, there are significant practical barriers to the study of pathogenicity and the evaluation of therapeutic countermeasures. However, Cedar virus (CedV) is a non-pathogenic henipavirus that can be used in a BSL-2 setting. Here, we demonstrate that recombinant CedVs that express the F and G glycoproteins of NiV or HeV display an in vivo tissue tropism that better emulates authentic NiV and HeV. Moreover, by severely impairing interferon signaling through the use of STAT1-deficient mice, we show that rCedVs expressing NiV/HeV F and G cause neurological disease signs and mortality in most animals. Thus, this BSL-2 mouse model represents a powerful tool for pre-clinical investigation of candidate therapeutics and studies of henipavirus pathogenesis mechanisms.
-
-
-
Immunology and Microbiology
Memory Regulatory T Cells Reprogram into Protective Tfh-like Effectors in Recurrent Malaria
In bioRxiv on 15 October 2025 by Charles-Chess, N. A. E., Ruberto, A. A., et al.
-
-
-
Immunology and Microbiology
-
Cancer Research
The fibroblast-driven melanoma/Treg vitiligo mouse model is effectively suppressed by IFNγ blocking antibody.
In Front Med (Lausanne) on 13 October 2025 by Xie, Y., Cui, J., et al.
PubMed
The use of immunosuppressive drugs for vitiligo treatment carries a potential risk of increased infection, whereas IFNγ bispecific antibodies represent an alternative therapeutic strategy. Researchers employing the melanoma/Treg vitiligo mouse model identified a critical role of dermal fibroblasts during the progressive phase of vitiligo. These fibroblasts respond to IFNγ and recruit CD8 + T cells, a mechanism that also contributes to the bilateral symmetrical depigmentation commonly observed in vitiligo patients. To evaluate the suitability of this model as a platform for developing fibroblast-targeted IFNγ bispecific antibodies, mice were administered regular injections of IFNγ monoclonal antibodies. The IFNγ monoclonal antibody treatment significantly suppressed vitiligo progression in the melanoma/Treg model. Owing to its advantages-including straightforward induction, multi-antigen specificity, and the demonstrated efficacy of IFNγ blocking antibodies, the melanoma/Treg vitiligo mouse model is a robust platform for advancing the development of fibroblast-targeted IFNγ bispecific antibodies.
-
-
Macrophages orchestrate elimination of Shigella from the intestinal epithelial cell niche via TLR-induced IL-12 and IFN-γ.
In Cell Host Microbe on 10 September 2025 by Eislmayr, K. D., Nichols, C. A., et al.
PubMed
Bacteria of the genus Shigella replicate in intestinal epithelial cells and cause shigellosis, a severe diarrheal disease that resolves spontaneously in most healthy individuals. During shigellosis, neutrophils are abundantly recruited to the gut and have long been thought to be central to Shigella control and pathogenesis. However, how shigellosis resolves remains poorly understood due to the longstanding lack of a tractable and physiological animal model. Here, using our newly developed Nlrc4-/-Casp11-/- mouse model of shigellosis, we unexpectedly find no major role for neutrophils in limiting Shigella or in disease pathogenesis. Instead, we uncover an essential role for macrophages in the host control of Shigella. Macrophages respond to Shigella via Toll-like receptors (TLRs) to produce IL-12, which then induces IFN-γ, a cytokine that is essential to control Shigella replication in intestinal epithelial cells. Collectively, our findings reshape our understanding of the innate immune response to Shigella.
-
-
Immunology and Microbiology
OCA-B promotes pathogenic maturation of stem-like CD4+ T cells and autoimmune demyelination.
In J Clin Invest on 1 July 2025 by Hughes, E. P., Syage, A. R., et al.
PubMed
Stem-like T cells selectively contribute to autoimmunity, but the activities that promote their pathogenicity are incompletely understood. Here, we identify the transcription coregulator OCA-B as a driver of the pathogenic maturation of stem-like CD4+ T cells to promote autoimmune demyelination. Using 2 human multiple sclerosis (MS) datasets, we show that POU2AF1, the gene encoding OCA-B, is elevated in CD4+ T cells from patients with MS. We show that T cell-intrinsic OCA-B loss protects mice from experimental autoimmune encephalomyelitis (EAE) while preserving responses to viral CNS infection. In EAE models driven by antigen re-encounter, OCA-B deletion nearly eliminates CNS infiltration, proinflammatory cytokine production, and clinical disease. OCA-B-expressing CD4+ T cells of mice primed with autoantigen express an encephalitogenic gene program and preferentially confer disease. In a relapsing-remitting EAE model, OCA-B loss protects mice specifically at relapse. During remission, OCA-B promotes the expression of Tcf7, Slamf6, and Sell in proliferating CNS T cell populations. At relapse time points, OCA-B loss results in both the accumulation of an immunomodulatory CD4+ T cell population expressing Ccr9 and Bach2, and loss of proinflammatory gene expression from Th17 cells. These results identify OCA-B as a driver of pathogenic CD4+ T cells.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Cancer Research
Orchestrating intratumoral DC-T cell immunity for enhanced tumor control via radiotherapy-activated TLR7/8 prodrugs in mice.
In Nat Commun on 1 July 2025 by Yin, X., Ding, Z., et al.
PubMed
Optimizing intratumoral dendritic cell (DC)-T cell responses is pivotal for effective cancer immunotherapy. However, the mechanistic governing these dynamics within the tumor microenvironment (TME) remains unclear, and strategies to improve their therapeutic potential are underexplored. Here, we show that precise radiotherapy activates the pro-TLR7/8 agonist imidazoquinoline (IMDQ) locally in preclinical tumor models, stimulating DCs to elicit T cell immunity without the need for further recruitment or causing systemic toxicity. Mechanistically, this synergistic approach triggers type I interferon via STING and MyD88 signaling pathways, strengthening local immune responses. Importantly, we reveal that fractionated, low-dose radiotherapy can effectively optimize local DC-T cell dynamics to control the irradiated tumor, while also promoting abscopal effect. Thus, our findings underscore the critical role of harnessing intratumoral DCs to reinvigorate pre-existing T cell immunity and provide mechanistic insights into improving both local and distal tumor control, opening new avenues for advancing cancer immunotherapy.
-
-
-
Cancer Research
Fc-optimized anti-CTLA-4 antibodies increase tumor-associated high endothelial venules and sensitize refractory tumors to PD-1 blockade.
In Cell Rep Med on 17 June 2025 by Blanchard, L., Vina, E., et al.
PubMed
The lack of T cells in tumors is a major hurdle to successful immune checkpoint therapy (ICT). Therefore, therapeutic strategies promoting T cell recruitment into tumors are warranted to improve the treatment efficacy. Here, we report that Fc-optimized anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies are potent remodelers of tumor vasculature that increase tumor-associated high endothelial venules (TA-HEVs), specialized blood vessels supporting lymphocyte entry into tumors. Mechanistically, this effect is dependent on the Fc domain of anti-CTLA-4 antibodies and CD4+ T cells and involves interferon gamma (IFNγ). Unexpectedly, we find that the human anti-CTLA-4 antibody ipilimumab fails to increase TA-HEVs in a humanized mouse model. However, increasing its Fc effector function rescues the modulation of TA-HEVs, promotes CD4+ and CD8+ T cell infiltration into tumors, and sensitizes recalcitrant tumors to programmed cell death protein 1 (PD-1) blockade. Our findings suggest that Fc-optimized anti-CTLA-4 antibodies could be used to reprogram tumor vasculature in poorly immunogenic cold tumors and improve the efficacy of ICT.
-
-
-
Immunology and Microbiology
Intermittent fasting exacerbates colon inflammation by promoting Th17 cell differentiation through inhibition of gut microbiota-derived indoleacrylic acid.
In World J Gastroenterol on 14 June 2025 by Fu, R., Zhang, P., et al.
PubMed
Intermittent fasting (IF), particularly time-restricted feeding (TRF), is increasingly popular has gained popularity for weight loss, yet management, but its effects impact on gut health remain unclear. Remains inadequately understood. This study explores how investigated the effects of TRF effects on intestinal health and explored the underlying mechanisms.
-
-
-
Immunology and Microbiology
IGF1R Promotes Th17/Treg Cell Development in Experimental Autoimmune Prostatitis.
In J Inflamm Res on 5 May 2025 by Guan, Y., Yue, S., et al.
PubMed
Chronic prostatitis is a common urological disorder in young and middle-aged men, characterized by frequent relapses and an unknown etiology. We investigated the potential function of insulin-like growth factor 1 (IGF1) -related ligands in chronic prostatitis in the current study.
-
-
-
Immunology and Microbiology
-
Cancer Research
Antitumor CD4+ T Helper 1 Cells Target and Control the Outgrowth of Disseminated Cancer Cells.
In Cancer Immunol Res on 2 May 2025 by Ganesan, R., Lee, M. C., et al.
PubMed
Detection of disseminated cancer cells (DCC) in the bone marrow (BM) of patients with breast cancer is a critical predictor of late recurrence and distant metastasis. Conventional therapies often fail to completely eradicate DCCs in patients. In this study, we demonstrate that intratumoral priming of antitumor CD4+ T helper 1 (Th1) cells was able to eliminate the DCC burden in distant organs and prevent overt metastasis, independent of CD8+ T cells. Intratumoral priming of tumor antigen-specific CD4+ Th1 cells enhanced their migration to the BM and distant metastatic site to selectively target DCC burden. The majority of these intratumorally activated CD4+ T cells were CD4+PD1- T cells, supporting their nonexhaustion stage. Phenotypic characterization revealed enhanced infiltration of memory CD4+ T cells and effector CD4+ T cells in the primary tumor, tumor-draining lymph node, and DCC-driven metastasis site. A robust migration of CD4+CCR7+CXCR3+ Th1 cells and CD4+CCR7-CXCR3+ Th1 cells into distant organs further revealed their potential role in eradicating DCC-driven metastasis. The intratumoral priming of antitumor CD4+ Th1 cells failed to eradicate DCC-driven metastasis in CD4- or IFN-γ knockout mice. Moreover, antitumor CD4+ Th1 cells, by increasing IFN-γ production, inhibited various molecular aspects and increased classical and nonclassical MHC molecule expression in DCCs. This reduced stemness and self-renewal while increasing immune recognition in DCCs of patients with breast cancer. These results unveil an immune basis for antitumor CD4+ Th1 cells that modulate DCC tumorigenesis to prevent recurrence and metastasis in patients.
-
-
-
Immunology and Microbiology
Inhibition of Sphingosine-1-Phosphate Receptor 2 (S1P2) Attenuates Imiquimod-Induced Psoriasis-Like Skin Inflammation in BALB/c Mice.
In Biomol Ther (Seoul) on 1 May 2025 by Lee, J. H., Im, D. S., et al.
PubMed
Serum and epidermal levels of sphingosine 1-phosphate (S1P) are higher in patients with psoriasis than healthy subjects. Although roles of type 1 S1P receptor, S1P1, in the development of psoriasis has intensively been investigated, roles of S1P2 have not been elucidated. We aim to investigate whether blockage of S1P2 reduce imiquimod-induced psoriasis-like dermatitis using an S1P2 antagonist, JTE-013, in combination with S1pr2 wild-type (WT) and knock-out (KO) BALB/c mice. Imiquimod induced increase of erythematous papules and plaques with silver scaling, whereas administration of JTE-013 significantly suppressed those increases in S1pr2 WT mice. Deficiency of S1pr2 gene reduced the imiquimod-induced symptoms. Imiquimod increased mRNA expression levels of pro-inflammatory Th1/Th17 cytokines, whereas JTE-013 significantly suppressed those increases in S1pr2 WT mice. Deficiency of S1pr2 gene also suppressed the imiquimod-induced pro-inflammatory cytokine expression. Imiquimod induced enlargement of lymph nodes and spleens, whereas JTE-013 suppressed them in S1pr2 WT mice. Imiquimod induced increase of pro-inflammatory Th1/Th17 cytokine levels and Th17 cell numbers in lymph nodes and spleens, whereas JTE-013 suppressed them in S1pr2 WT mice. In summary, the present results suggest that blockage of S1P2 could suppress the characteristics of psoriasis-form dermatitis and be a therapeutic strategy.
-
-
-
Immunology and Microbiology
T-cell-derived IFN-γ suppresses T follicular helper cell differentiation and antibody responses.
In EMBO J on 1 May 2025 by Sala, E., Nelli, M., et al.
PubMed
CD4+ T cells play a critical role in antiviral humoral and cellular immune responses. We have previously reported that subcutaneous lymphocytic choriomeningitis virus (s.c. LCMV) infection is characterized by a stark compartmentalization of CD4+ T cells, leading to strong TH1 cell polarization but virtually absent T follicular helper (TFH) cells, key drivers of humoral immunity. Here, we investigate the mechanisms responsible for this impaired TFH differentiation. We show that T-bet+ cells induced by LCMV infection encompass a TH1 cell subset expressing granzyme B (GzmB), and a Tcf-1+ cell subset that retains the potential for TFH differentiation without expressing mature TFH markers. Notably, IFN-γ blockade enables full differentiation of Tcf-1+ cells into TFH cells, formation of germinal centers, and increased antibody production. Suppression of TFH cells by IFN-γ is not directly mediated by CD4+ T cells but rather involves another cell type, likely dendritic cells (DCs). Our study provides novel insights into the mechanisms underlying early CD4+ T-cell polarization and humoral responses to viruses, with the potential to facilitate the development of effective vaccine strategies.
-
-
-
Immunology and Microbiology
Systemic 4-1BB stimulation augments extrafollicular memory B cell formation and recall responses during Plasmodium infection.
In Cell Rep on 22 April 2025 by Calôba, C., Sturtz, A. J., et al.
PubMed
T-dependent germinal center (GC) output, comprising plasma cells and memory B cells (MBCs), is crucial for clearance of Plasmodium infection and protection against reinfection. In this study, we examine the effect of an agonistic antibody targeting 4-1BB (CD137) during experimental malaria. We show that exogenous 4-1BB stimulation, despite delaying the effector GC response, surprisingly enhances humoral memory recall and protection from reinfection. Single-cell RNA and assay for transposase-accessible chromatin (ATAC) sequencing of MBCs from mice receiving 4-1BB stimulation reveal populations with distinct transcriptional signatures and a chromatin landscape indicative of superior recall and proliferative potential. Importantly, our results indicate that the effects of 4-1BB stimulation are dependent on interleukin (IL)-9R signaling in B cells but independent of parasite load during primary infection. Our study proposes an immunomodulatory approach to enhance the quality of the MBC pool, providing superior protection during infection and vaccination, particularly in the context of malaria.
-
-
-
Immunology and Microbiology
Synergizing adaptive immunity and regenerative signals to enhance osteochondral defects repair.
In Bioact Mater on 1 April 2025 by Lin, C., Ying, C., et al.
PubMed
In clinical practice, repairing osteochondral defects (OCDs) is challenging because of the complex cartilage/subchondral bone structure and intricate immunological microenvironment. Here, we identify the crucial role of adaptive immunity dysfunction by revealing that an increase of T helper 17 (Th17) cells exacerbated osteochondral tissue degradation via its pro-inflammatory cytokine interleukin-17 (IL-17) in the early-stage OCDs. Next, we leveraged this adaptive immunity mechanism and combined it with regenerative signals to develop a multifunctional hydrogel system capable of simultaneously tackling immune dysfunction and regenerative deficiency. Rapid IL-4 release from the methacrylated hyaluronic acid (HAMA) hydrogel exerts a potent immunomodulatory effect by inhibiting the differentiation and function of Th17 cells. Moreover, transforming growth factor-beta1 anchored on methacrylated hyaluronic acid and heparin (HAMA@HepMA) microparticles provides sustained regenerative signals, which synergistically transform the pro-inflammatory microenvironment into a pro-regenerative niche for enhanced OCDs healing. Our study suggests that targeting specific immune pathways can significantly enhance the efficacy of regenerative strategies, paving the way for innovative treatments in orthopedic medicine.
-
-
Targeted labeling and depletion of alveolar macrophages using VeDTR mouse technology.
In iScience on 21 March 2025 by Nakayama, Y., Sasai, M., et al.
PubMed
Alveolar macrophages (AMs) are essential for maintaining lung homeostasis. However, their roles in respiratory infections have been controversial because the methods of depleting them have often suffered from poor cell selectivity. To resolve this problem, we here used VeDTR technology to generate a transgenic mouse line in which AMs can be specifically depleted using diphtheria toxin. When various respiratory infections were examined using this system, we found that AMs prevented the proliferation of Mycobacterium abscessus. This result differed from previous findings using clodronate liposomes to deplete macrophages. We also revealed that the disappearance of AMs contributes to the reduction of bacterial load in the lungs and that AMs are indispensable for GM-CSF-mediated defense against M. abscessus infection. Taken together, the development of an AM-specific depletion system has provided an opportunity to study the roles of AMs in various respiratory infections from a different perspective.