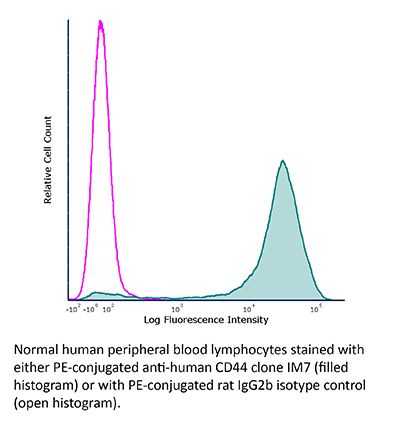
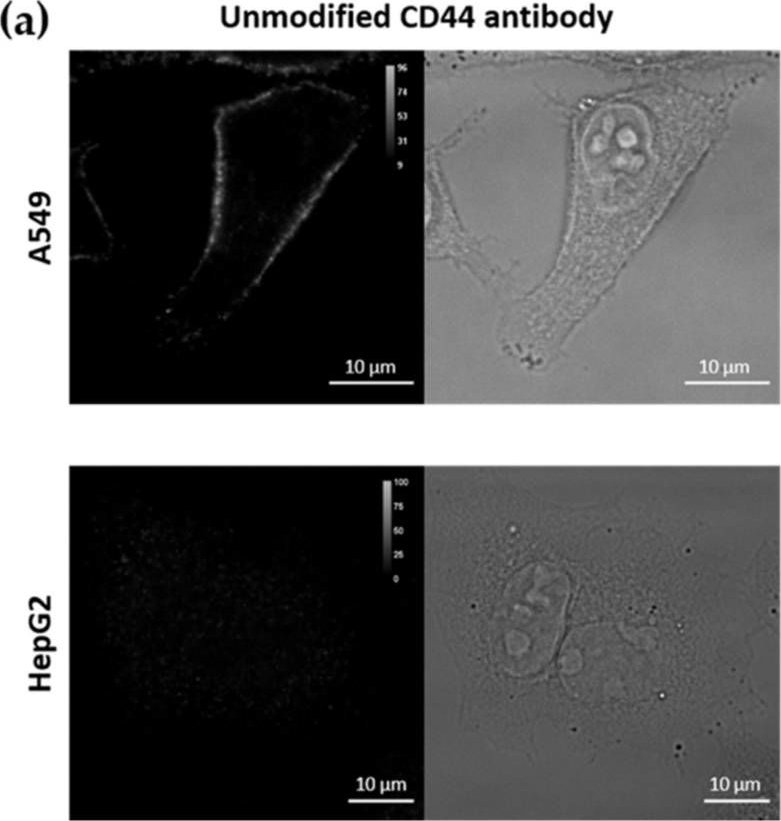
InVivoMAb anti-mouse/human CD44
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Dexamethasone-induced myeloid leukemia M1 cells |
| Reported Applications |
in vivo CD44 neutralization in vitro CD44 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107649 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD44 neutralization
Lee, S. W., et al (2020). "NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering" ACS Appl Mater Interfaces 12(4): 4285-4294.
PubMed
Recently, there has been growing interest in replacing severely damaged salivary glands with artificial salivary gland functional units created in vitro by tissue engineering approaches. Although various materials such as poly(lactic-co-glycolic acid), polylactic acid, poly(glycolic acid), and polyethylene glycol hydrogels have been used as scaffolds for salivary gland tissue engineering, none of them is effective enough to closely recapitulate the branched structural complexity and heterogeneous cell population of native salivary glands. Instead of discovering new biomaterial candidates, we synthesized hyaluronic acid-catechol (HACA) conjugates to establish a versatile hyaluronic acid coating platform named “NiCHE (nature-inspired catechol-conjugated hyaluronic acid environment)” for boosting the salivary gland tissue engineering efficacy of the previously reported biomaterials. By mimicking hyaluronic acid-rich niche in the mesenchyme of embryonic submandibular glands (eSMGs) with NiCHE coating on substrates including polycarbonate membrane, stiff agarose hydrogel, and polycaprolactone scaffold, we observed significantly enhanced cell adhesion, vascular endothelial and progenitor cell proliferation, and branching of in vitro-cultured eSMGs. High mechanical stiffness of the substrate is known to inhibit eSMG growth, but the NiCHE coating significantly reduced such stiffness-induced negative effects, leading to successful differentiation of progenitor cells to functional acinar and myoepithelial cells. These enhancement effects of the NiCHE coating were due to the increased proliferation of vascular endothelial cells via interaction between CD44 and surface-immobilized HAs. As such, our NiCHE coating platform renders any kind of material highly effective for salivary gland tissue culture by mimicking in vivo embryonic mesenchymal HA. Based on our results, we expect the NiCHE coating to expand the range of biomaterial candidates for salivary glands and other branching epithelial organs.
in vitro CD44 neutralization
Liu, S. and C. Cheng (2017). "Akt Signaling Is Sustained by a CD44 Splice Isoform-Mediated Positive Feedback Loop" Cancer Res 77(14): 3791-3801.
PubMed
Tumor cells nearly invariably evolve sustained PI3K/Akt signaling as an effective means to circumvent apoptosis and maintain survival. However, for those tumor cells that do not acquire PI3K/Akt mutations to achieve this end, the underlying mechanisms have remained obscure. Here, we describe the discovery of a splice isoform-dependent positive feedback loop that is essential to sustain PI3K/Akt signaling in breast cancer. Splice isoform CD44s promoted expression of the hyaluronan synthase HAS2 by activating the Akt signaling cascade. The HAS2 product hyaluronan further stimulated CD44s-mediated Akt signaling, creating a feed-forward signaling circuit that promoted tumor cell survival. Mechanistically, we identified FOXO1 as a bona fide transcriptional repressor of HAS2. Akt-mediated phosphorylation of FOXO1 relieved its suppression of HAS2 transcription, with FOXO1 phosphorylation status maintained by operation of the positive feedback loop. In clinical specimens of breast cancer, we established that the expression of CD44s and HAS2 was positively correlated. Our results establish a positive feedback mechanism that sustains PI3K/Akt signaling in tumor cells, further illuminating the nearly universal role of this pathway in cancer cell survival.
in vivo CD44 neutralization
Guidotti, L. G., et al (2015). "Immunosurveillance of the liver by intravascular effector CD8(+) T cells" Cell 161(3): 486-500.
PubMed
Effector CD8(+) T cells (CD8 TE) play a key role during hepatotropic viral infections. Here, we used advanced imaging in mouse models of hepatitis B virus (HBV) pathogenesis to understand the mechanisms whereby these cells home to the liver, recognize antigens, and deploy effector functions. We show that circulating CD8 TE arrest within liver sinusoids by docking onto platelets previously adhered to sinusoidal hyaluronan via CD44. After the initial arrest, CD8 TE actively crawl along liver sinusoids and probe sub-sinusoidal hepatocytes for the presence of antigens by extending cytoplasmic protrusions through endothelial fenestrae. Hepatocellular antigen recognition triggers effector functions in a diapedesis-independent manner and is inhibited by the processes of sinusoidal defenestration and capillarization that characterize liver fibrosis. These findings reveal the dynamic behavior whereby CD8 TE control hepatotropic pathogens and suggest how liver fibrosis might reduce CD8 TE immune surveillance toward infected or transformed hepatocytes.
in vivo CD44 neutralization
Mott, P. J. and A. H. Lazarus (2013). "CD44 antibodies and immune thrombocytopenia in the amelioration of murine inflammatory arthritis" PLoS One 8(6): e65805.
PubMed
Antibodies to CD44 have been used to successfully ameliorate murine models of autoimmune disease. The most often studied disease model has been murine inflammatory arthritis, where a clear mechanism for the efficacy of CD44 antibodies has not been established. We have recently shown in a murine passive-model of the autoimmune disease immune thrombocytopenia (ITP) that some CD44 antibodies themselves can induce thrombocytopenia in mice, and the CD44 antibody causing the most severe thrombocytopenia (IM7), also is known to be highly effective in ameliorating murine models of arthritis. Recent work in the K/BxN serum-induced model of arthritis demonstrated that antibody-induced thrombocytopenia reduced arthritis, causing us to question whether CD44 antibodies might primarily ameliorate arthritis through their thrombocytopenic effect. We evaluated IM7, IRAWB14.4, 5035-41.1D, KM201, KM114, and KM81, and found that while all could induce thrombocytopenia, the degree of protection against serum-induced arthritis was not closely related to the length or severity of the thrombocytopenia. CD44 antibody treatment was also able to reverse established inflammation, while thrombocytopenia induced by an anti-platelet antibody targeting the GPIIbIIIa platelet antigen, could not mediate this effect. While CD44 antibody-induced thrombocytopenia may contribute to some of its therapeutic effect against the initiation of arthritis, for established disease there are likely other mechanisms contributing to its efficacy. Humans are not known to express CD44 on platelets, and are therefore unlikely to develop thrombocytopenia after CD44 antibody treatment. An understanding of the relationship between arthritis, thrombocytopenia, and CD44 antibody treatment remains critical for continued development of CD44 antibody therapeutics.
in vivo CD44 neutralization
Hutas, G., et al (2008). "CD44-specific antibody treatment and CD44 deficiency exert distinct effects on leukocyte recruitment in experimental arthritis" Blood 112(13): 4999-5006.
PubMed
CD44, the leukocyte adhesion receptor for hyaluronan, has been considered a therapeutic target on the basis of the robust anti-inflammatory effect of CD44-specific antibodies in animal models of immune-mediated diseases. However, CD44 deficiency does not provide substantial protection against inflammation. Using intravital video microscopy in a murine model of rheumatoid arthritis, we show that CD44 deficiency and anti-CD44 antibody treatment exert disparate effects on leukocyte recruitment in inflamed joints. Leukocyte rolling, which is increased in CD44-deficient mice, is promptly abrogated in anti-CD44-treated wild-type mice. CD44-specific antibodies also trigger platelet deposition on granulocytes and subsequent depletion of this leukocyte subset in the circulation. These in vivo effects require CD44 cross-linking and are reproducible with an antibody against Gr-1, a molecule that, like CD44, is highly expressed on granulocytes. Anticoagulant pretreatment, which prevents platelet deposition, mitigates both granulocyte depletion and the suppressive effect of CD44-specific antibody on joint swelling. Our observations suggest that cross-linking of prominent cell surface molecules, such as CD44 or Gr-1, can initiate a rapid self-elimination program in granulocytes through engagement of the coagulation system. We conclude that the robust anti-inflammatory effect of CD44-specific antibodies in arthritis is primarily the result of their ability to trigger granulocyte depletion.
Product Citations
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
SPP1 + macrophages cause exhaustion of tumor-specific T cells in liver metastases.
In Nat Commun on 7 May 2025 by Trehan, R., Huang, P., et al.
PubMed
Functional tumor-specific CD8+ T cells are essential for effective anti-tumor immune response and immune checkpoint inhibitor therapy. Here we show that, compared to other organ sites, primary, metastatic liver tumors in murine models contain a higher number of tumor-specific CD8+ T cells which are also dysfunctional. High-dimensional, multi-omic analysis of patient samples reveals a higher frequency of exhausted tumor-reactive CD8+ T cells and enriched interactions between these cells and SPP1+ macrophages in profibrotic, alpha-SMA rich regions specifically in the liver. Differential pseudotime trajectory inference analysis reveals that extrahepatic signaling promotes an intermediate cell (IC) population in the liver, characterized by co-expression of VISG4, CSF1R, CD163, TGF-βR, IL-6R, and SPP1. Analysis of premetastatic adenocarcinoma patient samples reveals enrichment of this population may predict liver metastasis. These findings suggest a mechanism by which extrahepatic tumors drive liver metastasis by promoting an IC population that inhibits tumor-reactive CD8+ T cell function.
-
-
-
Cancer Research
-
Immunology and Microbiology
Malignant mesothelioma-associated inflammatory microenvironment promotes tumor progression via GPNMB.
In J Transl Med on 18 April 2025 by Belgiovine, C., Digifico, E., et al.
PubMed
Tumor-Associated Macrophages (TAMs) are the main immune component of the tumor stroma with heterogeneous functional activities, predominantly suppressing the immune response and promoting tumor progression, also via secretion of different factors. Among these, GPNMB (Glycoprotein non-metastatic B) is usually associated with disease progression in several tumor types. Malignant pleural mesothelioma (MPM) a severe neoplasia with poor prognosis, is characterized by an abundancy of TAMs, testifying the presence of a long-lasting inflammation which is pathogenetic of the disease. However, the role of GPNMB in MPM is unclear.
-
-
-
Cardiovascular biology
-
Immunology and Microbiology
ITIH5-mediated fibroblast/macrophage crosstalk exacerbates cardiac remodelling after myocardial infarction.
In J Transl Med on 24 February 2025 by Wu, Y., Meng, L., et al.
PubMed
Myocardial infarction (MI) and subsequent ischaemic cardiomyopathy (ICM) are the primary causes of heart failure. Inter-α trypsin inhibitor heavy chain 5 (ITIH5) is an extracellular matrix (ECM) protein and has been identified as a myocardial marker of ICM. However, its diagnostic value in patients with ICM and its function and molecular mechanism in regulating cardiac repair and remodelling after MI remain unknown.
-
-
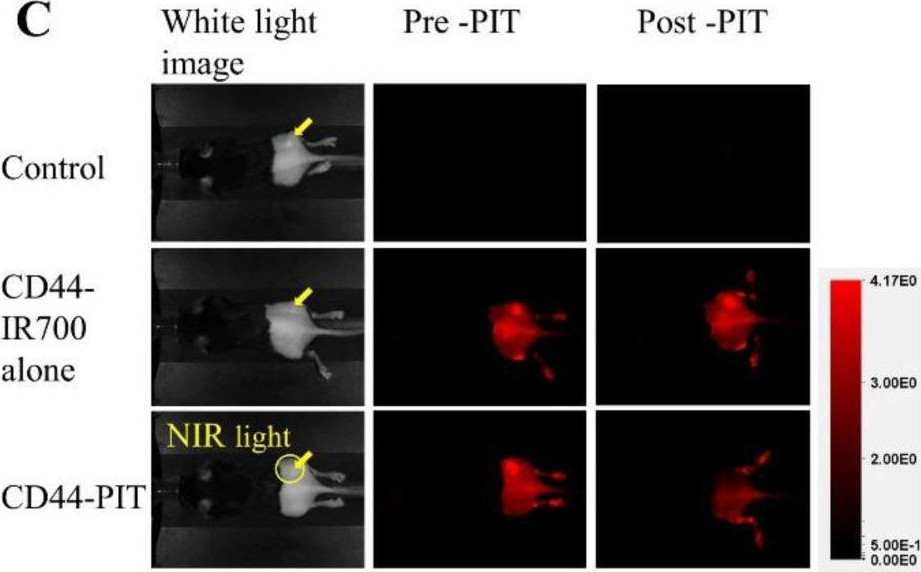
Enhancing the efficacy of near-infrared photoimmunotherapy through intratumoural delivery of CD44-targeting antibody-photoabsorber conjugates.
In EBioMedicine on 1 February 2025 by Adachi, Y., Miyake, K., et al.
PubMed
Photoimmunotherapy (PIT) is a potent modality for cancer treatment. The conventional PIT regimen involves the systemic delivery of an antibody-photoabsorber conjugate, followed by a 24-h waiting period to ensure adequate localisation on the target cells. Subsequent exposure to near-infrared (NIR) light selectively damages the target cells. We aimed to improve the efficacy of PIT in vivo by evaluating the effects of the different routes of conjugate administration on treatment outcomes.
-
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
A correctable immune niche for epithelial stem cell reprogramming and post-viral lung diseases.
In J Clin Invest on 25 July 2024 by Wu, K., Zhang, Y., et al.
PubMed
Epithelial barriers are programmed for defense and repair but are also the site of long-term structural remodeling and disease. In general, this paradigm features epithelial stem cells (ESCs) that are called on to regenerate damaged tissues but can also be reprogrammed for detrimental remodeling. Here we identified a Wfdc21-dependent monocyte-derived dendritic cell (moDC) population that functioned as an early sentinel niche for basal ESC reprogramming in mouse models of epithelial injury after respiratory viral infection. Niche function depended on moDC delivery of ligand GPNMB to the basal ESC receptor CD44 so that properly timed antibody blockade of ligand or receptor provided long-lasting correction of reprogramming and broad disease phenotypes. These same control points worked directly in mouse and human basal ESC organoids. Together, the findings identify a mechanism to explain and modify what is otherwise a stereotyped but sometimes detrimental response to epithelial injury.
-
-
-
Neuroscience
Inhibition of CD44 suppresses the formation of fibrotic scar after spinal cord injury via the JAK2/STAT3 signaling pathway.
In iScience on 16 February 2024 by Guo, J., Yang, T., et al.
PubMed
Fibrotic scar is one of the main impediments to axon regeneration following spinal cord injury (SCI). In this study, we found that CD44 was upregulated during the formation of fibrotic scar, and blocking CD44 by IM7 caused downregulation of fibrosis-related extracellular matrix proteins at both 2 and 12 weeks post-spinal cord injury. More Biotinylated dextran amine (BDA)-traced corticospinal tract axons crossed the scar area and extended into the distal region after IM7 administration. A recovery of motor and sensory function was observed based on Basso Mouse Scale (BMS) scores and tail-flick test. In vitro experiments revealed that inhibiting CD44 and JAK2/STAT3 signaling pathway decreased the proliferation, differentiation, and migration of fibroblasts induced by the inflammatory supernatant. Collectively, these findings highlight the critical role of CD44 and its downstream JAK2/STAT3 signaling pathway in fibrotic scar formation, suggesting a potential therapeutic target for SCI.
-
-
Melanocortin-4 receptor in macrophages attenuated angiotensin II-induced abdominal aortic aneurysm in mice.
In Sci Rep on 13 November 2023 by Mori, K., Okuma, H., et al.
PubMed
Obesity is recognized as an independent risk factor for abdominal aortic aneurysm (AAA). While mutations in the melanocortin-4 receptor (MC4R) gene is the most common cause of obesity caused by mutations in a single gene, the link between MC4R function and vascular disease has still remained unclear. Here, by using melanocortin-4 receptor (MC4R) deficient mice, we confirmed MC4R deficiency promotes AAA and atherosclerosis. We demonstrated the contribution of two novel factors towards vascular vulnerability in this model: leptin signaling in vascular smooth muscle cells (VSMCs) and loss of MC4R signaling in macrophages. Leptin was shown to promote vascular vulnerability via PI3K-dependent upregulation of Spp1 expression in VSMC. Additionally, Ang II-induced AAA incidence was significantly reduced when MC4R gene expression was myeloid cell-specifically rescued in MC4R deficient (MC4RTB/TB) mice. Ex vivo analysis showed a suppression in NF-κB activity in bone marrow-derived macrophages from LysM(+);MC4RTB/TB mice compared to LysM(-);MC4RTB/TB mice, which exaggerates with endogenous MC4R ligand treatment; α-MSH. These results suggest that MC4R signaling in macrophages attenuates AAA by inhibiting NF-κB activity and subsequent vascular inflammation.
-
-
In vitro experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
In vitro experiments
Important functional role of the protein osteopontin in the progression of malignant pleural mesothelioma.
In Front Immunol on 3 July 2023 by Digifico, E., Erreni, M., et al.
PubMed
Malignant Pleural Mesothelioma (MPM) is an aggressive cancer of the mesothelial lining associated with exposure to airborne non-degradable asbestos fibers. Its poor response to currently available treatments prompted us to explore the biological mechanisms involved in its progression. MPM is characterized by chronic non-resolving inflammation; in this study we investigated which inflammatory mediators are mostly expressed in biological tumor samples from MPM patients, with a focus on inflammatory cytokines, chemokines and matrix components.
-
-
-
Conjugation experiments
-
Homo sapiens (Human)
Versatile and Robust Method for Antibody Conjugation to Nanoparticles with High Targeting Efficiency.
In Pharmaceutics on 14 December 2021 by Van Zundert, I., Bravo, M., et al.
PubMed
The application of antibodies in nanomedicine is now standard practice in research since it represents an innovative approach to deliver chemotherapy agents selectively to tumors. The variety of targets or markers that are overexpressed in different types of cancers results in a high demand for antibody conjugated-nanoparticles, which are versatile and easily customizable. Considering up-scaling, the synthesis of antibody-conjugated nanoparticles should be simple and highly reproducible. Here, we developed a facile coating strategy to produce antibody-conjugated nanoparticles using 'click chemistry' and further evaluated their selectivity towards cancer cells expressing different markers. Our approach was consistently repeated for the conjugation of antibodies against CD44 and EGFR, which are prominent cancer cell markers. The functionalized particles presented excellent cell specificity towards CD44 and EGFR overexpressing cells, respectively. Our results indicated that the developed coating method is reproducible, versatile, and non-toxic, and can be used for particle functionalization with different antibodies. This grafting strategy can be applied to a wide range of nanoparticles and will contribute to the development of future targeted drug delivery systems.
-
-
Cell-Matrix Interactions Regulate Functional Extracellular Vesicle Secretion from Mesenchymal Stromal Cells.
In ACS Nano on 23 November 2021 by Lenzini, S., Debnath, K., et al.
PubMed
Extracellular vesicles (EVs) are cell-secreted particles with broad potential to treat tissue injuries by delivering cargo to program target cells. However, improving the yield of functional EVs on a per cell basis remains challenging due to an incomplete understanding of how microenvironmental cues regulate EV secretion at the nanoscale. We show that mesenchymal stromal cells (MSCs) seeded on engineered hydrogels that mimic the elasticity of soft tissues with a lower integrin ligand density secrete ∼10-fold more EVs per cell than MSCs seeded on a rigid plastic substrate, without compromising their therapeutic activity or cargo to resolve acute lung injury in mice. Mechanistically, intracellular CD63+ multivesicular bodies (MVBs) transport faster within MSCs on softer hydrogels, leading to an increased frequency of MVB fusion with the plasma membrane to secrete more EVs. Actin-related protein 2/3 complex but not myosin-II limits MVB transport and EV secretion from MSCs on hydrogels. The results provide a rational basis for biomaterial design to improve EV secretion while maintaining their functionality.
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Neuroscience
Expeditious recruitment of circulating memory CD8 T cells to the liver facilitates control of malaria.
In Cell Rep on 2 November 2021 by Lefebvre, M. N., Surette, F. A., et al.
PubMed
Circulating memory CD8 T cell trafficking and protective capacity during liver-stage malaria infection remains undefined. We find that effector memory CD8 T cells (Tem) infiltrate the liver within 6 hours after malarial or bacterial infections and mediate pathogen clearance. Tem recruitment coincides with rapid transcriptional upregulation of inflammatory genes in Plasmodium-infected livers. Recruitment requires CD8 T cell-intrinsic LFA-1 expression and the presence of liver phagocytes. Rapid Tem liver infiltration is distinct from recruitment to other non-lymphoid tissues in that it occurs both in the absence of liver tissue resident memory "sensing-and-alarm" function and ∼42 hours earlier than in lung infection by influenza virus. These data demonstrate relevance for Tem in protection against malaria and provide generalizable mechanistic insights germane to control of liver infections.
-
-
Versatile and Robust method for Antibody Conjugation to Nanoparticles with High Targeting Efficiency
In bioRxiv on 29 September 2021 by Van Zundert, I., Bravo, M., et al.
-
-
In vivo experiments
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
Serglycin induces osteoclastogenesis and promotes tumor growth in giant cell tumor of bone.
In Cell Death Dis on 23 September 2021 by He, Y., Cheng, D., et al.
PubMed
Giant cell tumor of bone (GCTB) is an aggressive osteolytic bone tumor characterized by the within-tumor presence of osteoclast-like multinucleated giant cells (MGCs), which are induced by the neoplastic stromal cells and lead to extensive bone destruction. However, the underlying mechanism of the pathological process of osteoclastogenesis in GCTB is poorly understood. Here we show that the proteoglycan Serglycin (SRGN) secreted by neoplastic stromal cells plays a crucial role in the formation of MGCs and tumorigenesis in GCTB. Upregulated SRGN expression and secretion are observed in GCTB tumor cells and patients. Stromal-derived SRGN promotes osteoclast differentiation from monocytes. SRGN knockdown in stromal cells inhibits tumor growth and bone destruction in a patient-derived orthotopic xenograft model of mice. Mechanistically SRGN interacts with CD44 on the cell surface of monocytes and thus activates focal adhesion kinase (FAK), leading to osteoclast differentiation. Importantly, blocking CD44 with a neutralizing antibody reduces the number of MGCs and suppresses tumorigenesis in vivo. Overall, our data reveal a mechanism of MGC induction in GCTB and support CD44-targeting approaches for GCTB treatment.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Cluster of Differentiation 44 Promotes Liver Fibrosis and Serves as a Biomarker in Congestive Hepatopathy.
In Hepatol Commun on 1 August 2021 by Osawa, Y., Kawai, H., et al.
PubMed
Congestive hepatopathy (CH) with chronic passive congestion is characterized by the progression of liver fibrosis without prominent inflammation and hepatocellular damage. Currently, the lack of reliable biomarkers for liver fibrosis in CH often precludes the clinical management of patients with CH. To explore fibrosis biomarkers, we performed proteome analysis on serum exosomes isolated from patients with CH after the Fontan procedure. Exosomal cluster of differentiation (CD)44 levels were increased in patients with CH compared to healthy volunteers and was accompanied by increases in serum levels of soluble CD44 and CD44 expression in the liver. To address the roles of CD44 in CH, we established a mouse model of chronic liver congestion by partial inferior vena cava ligation (pIVCL) that mimics CH by fibrosis progression with less inflammation and cellular damage. In the pIVCL mice, enhanced CD44 expression in hepatic stellate cells (HSCs) and deposition of its ligand hyaluronan were observed in the liver. Blood levels of soluble CD44 were correlated with liver fibrosis. The blockade of CD44 with specific antibody inhibited liver fibrosis in pIVCL mice and was accompanied by a reduction in S100 calcium-binding protein A4 expression following activation of HSCs. Conclusion: Chronic liver congestion promotes fibrosis through CD44. This identifies CD44 as a novel biomarker and therapeutic target of liver fibrosis in patients with CH.
-
-
-
Cancer Research
-
Immunology and Microbiology
The soluble glycoprotein NMB (GPNMB) produced by macrophages induces cancer stemness and metastasis via CD44 and IL-33.
In Cell Mol Immunol on 1 March 2021 by Liguori, M., Digifico, E., et al.
PubMed
In cancer, myeloid cells have tumor-supporting roles. We reported that the protein GPNMB (glycoprotein nonmetastatic B) was profoundly upregulated in macrophages interacting with tumor cells. Here, using mouse tumor models, we show that macrophage-derived soluble GPNMB increases tumor growth and metastasis in Gpnmb-mutant mice (DBA/2J). GPNMB triggers in the cancer cells the formation of self-renewing spheroids, which are characterized by the expression of cancer stem cell markers, prolonged cell survival and increased tumor-forming ability. Through the CD44 receptor, GPNMB mechanistically activates tumor cells to express the cytokine IL-33 and its receptor IL-1R1L. We also determined that recombinant IL-33 binding to IL-1R1L is sufficient to induce tumor spheroid formation with features of cancer stem cells. Overall, our results reveal a new paracrine axis, GPNMB and IL-33, which is activated during the cross talk of macrophages with tumor cells and eventually promotes cancer cell survival, the expansion of cancer stem cells and the acquisition of a metastatic phenotype.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Immunocytochemistry-immunofluorescence
Increased Immunogenicity of a Minimally Immunogenic Tumor after Cancer-Targeting Near Infrared Photoimmunotherapy.
In Cancers (Basel) on 12 December 2020 by Wakiyama, H., Furusawa, A., et al.
PubMed
Near-infrared photoimmunotherapy (NIR-PIT) is a highly selective cancer treatment that employs an antibody photoabsorber conjugate (APC) composed of a targeting monoclonal antibody (mAb) conjugated with a photoactivatable phthalocyanine-derivative dye. Once injected and allowed to bind to a tumor, the APC is activated by local near-infrared light which kills cancer cells and induces a strong immune response in the tumor microenvironment by unmasking of new tumor antigens emerging from damaged tumor cells. Due to its ability to incite an immune reaction, even in poorly immunogenic tumors, NIR-PIT has the potential to enhance immunogenicity in tumors especially after immune checkpoint inhibition. In this study, we employ a poorly immunogenic MOC2-luc syngeneic tumor model and evaluate the efficacy of cancer-targeting CD44-targeted NIR-PIT. Increased infiltration of CD8+ T cells observed after NIR-PIT suggested an enhanced immune environment. Next, we evaluated tumor progression and survival after the combination of CD44-targeted NIR-PIT and short-term administration of an anti-PD1 immune checkpoint inhibitor (ICI) to further activate CD8+ T cells. Additionally, in mice in which the tumors were eradicated by this combination therapy, a re-challenge with fresh MOC2-luc cells demonstrated failure of tumor implantation implying acquired long-term immunity against the cancer cells. Combination therapy decreased tumor progression and prolonged survival significantly. Therefore, we concluded that NIR-PIT was able to convert a minimally immunogenic tumor unresponsive to anti-PD-1 ICI into a highly immunogenic tumor responsive to anti-PD-1 ICI, and this therapy was capable of inducing long-term immunity against the treated cancer.
-
-
-
Immunohistochemistry-paraffin
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunohistochemistry-paraffin
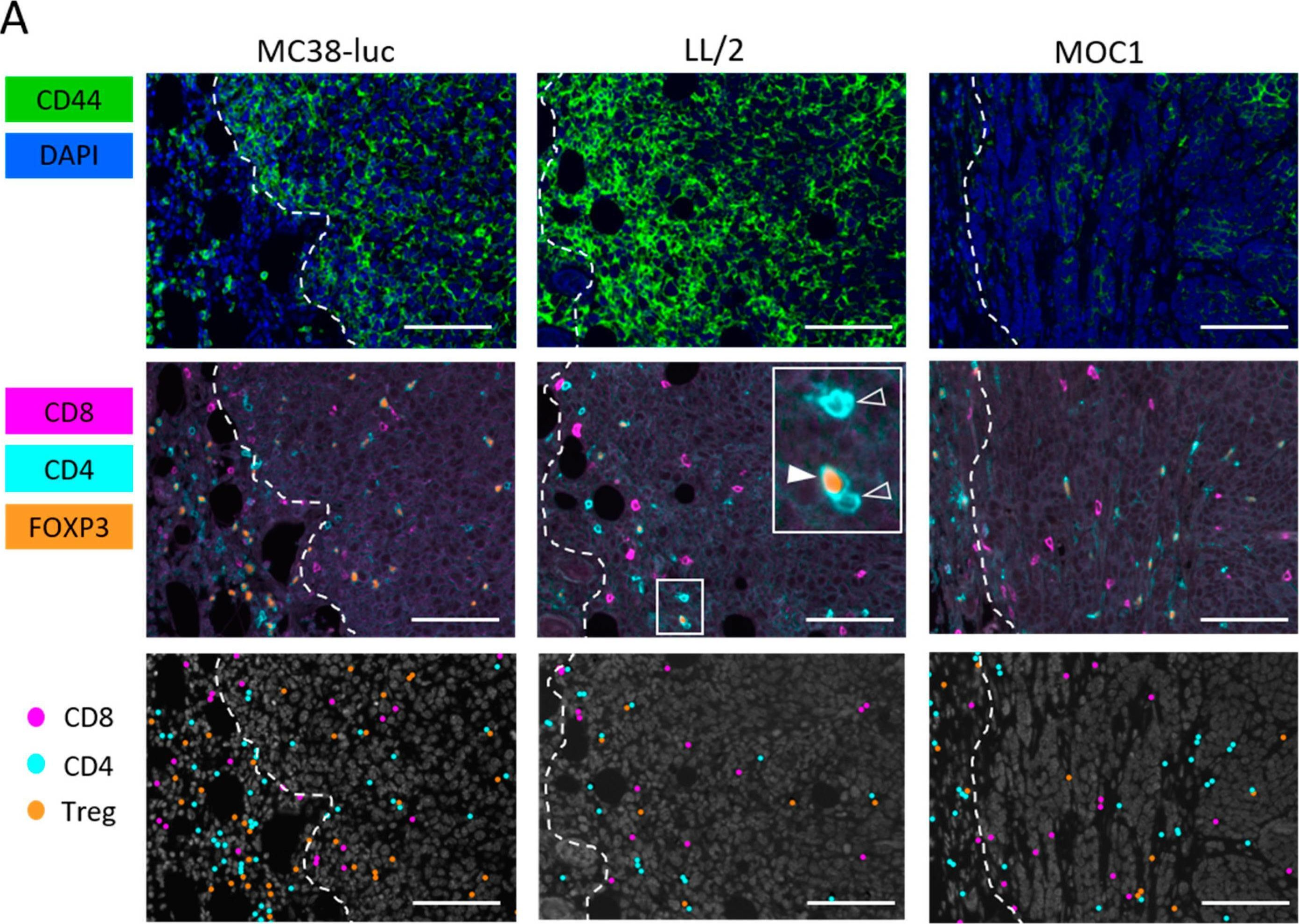
Near-Infrared Photoimmunotherapy Combined with CTLA4 Checkpoint Blockade in Syngeneic Mouse Cancer Models.
In Vaccines (Basel) on 14 September 2020 by Maruoka, Y., Furusawa, A., et al.
PubMed
Near infrared photoimmunotherapy (NIR-PIT) is a newly developed and highly selective cancer treatment that induces necrotic/immunogenic cell death. It employs a monoclonal antibody (mAb) conjugated to a photo-absorber dye, IRDye700DX, which is activated by NIR light. Tumor-targeting NIR-PIT is also at least partly mediated by a profound immune response against the tumor. Cytotoxic T-lymphocyte antigen-4 (CTLA4) is widely recognized as a major immune checkpoint protein, which inhibits the immune response against tumors and is therefore, a target for systemic blockade. We investigated the effect of combining tumor-targeted NIR-PIT against the cell-surface antigen, CD44, which is known as a cancer stem cell marker, with a systemic CTLA4 immune checkpoint inhibitor in three syngeneic tumor models (MC38-luc, LL/2, and MOC1). CD44-targeted NIR-PIT combined with CTLA4 blockade showed greater tumor growth inhibition with longer survival compared with CTLA4 blockade alone in all tumor models. NIR-PIT and CTLA4 blockade produced more complete remission in MOC1 tumors (44%) than NIR-PIT and programmed cell death protein 1 (PD-1) blockade (8%), which was reported in our previous paper. However, the combination of NIR-PIT and CTLA4 blockade was less effective in MC38-luc tumors (11%) than the combination of NIR-PIT and PD-1 blockade (70%). Nonetheless, in many cases ineffective results with NIR-PIT and PD-1 blockade were reversed with NIR-PIT and CTLA4 blockade.
-
-
-
Immunohistochemistry-immunofluorescence
-
Cancer Research
-
Immunology and Microbiology
Interleukin-15 after Near-Infrared Photoimmunotherapy (NIR-PIT) Enhances T Cell Response against Syngeneic Mouse Tumors.
In Cancers (Basel) on 10 September 2020 by Maruoka, Y., Furusawa, A., et al.
PubMed
Near infrared photoimmunotherapy (NIR-PIT) is a newly developed and highly selective cancer treatment that employs a monoclonal antibody (mAb) conjugated to a photo-absorber dye, IRDye700DX, which is activated by 690 nm light. Cancer cell-targeted NIR-PIT induces rapid necrotic/immunogenic cell death (ICD) that induces antitumor host immunity including re-priming and proliferation of T cells. Interleukin-15 (IL-15) is a cytokine that activates natural killer (NK)-, B- and T-cells while having minimal effect on regulatory T cells (Tregs) that lack the IL-15 receptor. Here, we hypothesized that IL-15 administration with cancer cell-targeted NIR-PIT could further inhibit tumor growth by increasing antitumor host immunity. Three syngeneic mouse tumor models, MC38-luc, LL/2, and MOC1, underwent combined CD44-targeted NIR-PIT and short-term IL-15 administration with appropriate controls. Comparing with the single-agent therapy, the combination therapy of IL-15 after NIR-PIT inhibited tumor growth, prolonged survival, and increased tumor infiltrating CD8+ T cells more efficiently in tumor-bearing mice. IL-15 appears to enhance the therapeutic effect of cancer-targeted NIR-PIT.
-
-
-
Blocking experiments
-
Mus musculus (Mouse)
NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering.
In ACS Appl Mater Interfaces on 29 January 2020 by Lee, S. W., Ryu, J. H., et al.
PubMed
Recently, there has been growing interest in replacing severely damaged salivary glands with artificial salivary gland functional units created in vitro by tissue engineering approaches. Although various materials such as poly(lactic-co-glycolic acid), polylactic acid, poly(glycolic acid), and polyethylene glycol hydrogels have been used as scaffolds for salivary gland tissue engineering, none of them is effective enough to closely recapitulate the branched structural complexity and heterogeneous cell population of native salivary glands. Instead of discovering new biomaterial candidates, we synthesized hyaluronic acid-catechol (HACA) conjugates to establish a versatile hyaluronic acid coating platform named "NiCHE (nature-inspired catechol-conjugated hyaluronic acid environment)" for boosting the salivary gland tissue engineering efficacy of the previously reported biomaterials. By mimicking hyaluronic acid-rich niche in the mesenchyme of embryonic submandibular glands (eSMGs) with NiCHE coating on substrates including polycarbonate membrane, stiff agarose hydrogel, and polycaprolactone scaffold, we observed significantly enhanced cell adhesion, vascular endothelial and progenitor cell proliferation, and branching of in vitro-cultured eSMGs. High mechanical stiffness of the substrate is known to inhibit eSMG growth, but the NiCHE coating significantly reduced such stiffness-induced negative effects, leading to successful differentiation of progenitor cells to functional acinar and myoepithelial cells. These enhancement effects of the NiCHE coating were due to the increased proliferation of vascular endothelial cells via interaction between CD44 and surface-immobilized HAs. As such, our NiCHE coating platform renders any kind of material highly effective for salivary gland tissue culture by mimicking in vivo embryonic mesenchymal HA. Based on our results, we expect the NiCHE coating to expand the range of biomaterial candidates for salivary glands and other branching epithelial organs.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8+ T Cells.
In Cell Metab on 6 August 2019 by Hu, Z., Qu, G., et al.
PubMed
CD8+ T cell expansions and functions rely on glycolysis, but the mechanisms underlying CD8+ T cell glycolytic metabolism remain elusive. Here, we show that acylglycerol kinase (AGK) is required for the establishment and maintenance of CD8+ T cell metabolic and functional fitness. AGK deficiency dampens CD8+ T cell antitumor functions in vivo and perturbs CD8+ T cell proliferation in vitro. Activation of phosphatidylinositol-3-OH kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling, which mediates elevated CD8+ T cell glycolysis, is tightly dependent on AGK kinase activity. Mechanistically, T cell antigen receptor (TCR)- and CD28-stimulated recruitment of PTEN to the plasma membrane facilitates AGK-PTEN interaction and AGK-triggered PTEN phosphorylation, thereby restricting PTEN phosphatase activity in CD8+ T cells. Collectively, these results demonstrate that AGK maintains CD8+ T cell metabolic and functional state by restraining PTEN activity and highlight a critical role for AGK in CD8+ T cell metabolic programming and effector function.
-