InVivoMAb anti-mouse CD28
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | A20 cells expressing mouse CD28 and a recombinant mouse CD28-Ig fusion protein |
| Reported Applications |
in vivo T cell stimulation/activation in vitro T cell stimulation/activation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
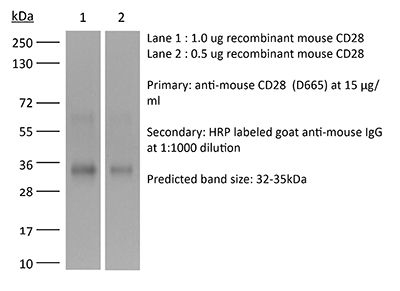
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2819055 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo T cell stimulation/activation
Schmidt, T., et al (2016). "Induction of T regulatory cells by the superagonistic anti-CD28 antibody D665 leads to decreased pathogenic IgG autoantibodies against desmoglein 3 in a HLA-transgenic mouse model of pemphigus vulgaris" Exp Dermatol 25(4):
PubMed
Pemphigus vulgaris (PV) is a potentially life-threatening autoimmune disease of the skin and mucous membranes. Its pathogenesis is based on IgG autoantibodies that target the desmosomal cadherins, desmoglein 3 (Dsg3) and desmoglein 1 (Dsg1) and induce intra-epidermal loss of adhesion. Although the PV pathogenesis is well-understood, therapeutic options are still limited to immunosuppressive drugs, particularly corticosteroids, which are associated with significant side effects. Dsg3-reactive T regulatory cells (Treg) have been previously identified in PV and healthy carriers of PV-associated HLA class II alleles. Ex vivo, Dsg3-specific Treg cells down-regulated the activation of pathogenic Dsg3-specific T-helper (Th) 2 cells. In this study, in a HLA-DRB1*04:02 transgenic mouse model of PV, peripheral Treg cells were modulated by the use of Treg-depleting or expanding monoclonal antibodies, respectively. Our findings show that, in vivo, although not statistically significant, Treg cells exert a clear down-regulatory effect on the Dsg3-driven T-cell response and, accordingly, the formation of Dsg3-specific IgG antibodies. These observations confirm the powerful immune regulatory functions of Treg cells and identify Treg cells as potential therapeutic modulators in PV.
in vivo T cell stimulation/activation
Win, S. J., et al (2016). "In vivo activation of Treg cells with a CD28 superagonist prevents and ameliorates chronic destructive arthritis in mice" Eur J Immunol 46(5): 1193-1202.
PubMed
Although regulatory T (Treg) cells are necessary to prevent autoimmune diseases, including arthritis, whether Treg cells can ameliorate established inflammatory disease is controversial. Using the glucose-6-phosphate isomerase (G6PI)-induced arthritis model in mice, we aimed to determine the therapeutic efficacy of increasing Treg cell number and function during chronic destructive arthritis. Chronic destructive arthritis was induced by transient depletion of Treg cells prior to immunization with G6PI. At different time points after disease induction, mice were treated with a CD28 superagonistic antibody (CD28SA). CD28SA treatment during the induction phase of arthritis ameliorated the acute signs of arthritis and completely prevented the development of chronic destructive arthritis. CD28SA treatment of mice with fully developed arthritis induced a significant reduction in clinical and histological signs of arthritis. When given during the chronic destructive phase of arthritis, 56 days after disease induction, CD28SA treatment resulted in a modest reduction of clinical signs of arthritis and a reduction in histopathological signs of joint inflammation. Our data show that increasing the number and activation of Treg cells by a CD28SA is therapeutically effective in experimental arthritis.
in vivo T cell stimulation/activation
Schuhmann, M. K., et al (2015). "CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke" J Cereb Blood Flow Metab 35(1): 6-10.
PubMed
While the detrimental role of non-regulatory T cells in ischemic stroke is meanwhile unequivocally recognized, there are controversies about the properties of regulatory T cells (Treg). The aim of this study was to elucidate the role of Treg by applying superagonistic anti-CD28 antibody expansion of Treg. Stroke outcome, thrombus formation, and brain-infiltrating cells were determined on day 1 after transient middle cerebral artery occlusion. Antibody-mediated expansion of Treg enhanced stroke size and worsened functional outcome. Mechanistically, Treg increased thrombus formation in the cerebral microvasculature. These findings confirm that Treg promote thrombo-inflammatory lesion growth during the acute stage of ischemic stroke.
in vitro T cell stimulation/activation
Dennehy, K. M., et al (2006). "Cutting edge: monovalency of CD28 maintains the antigen dependence of T cell costimulatory responses" J Immunol 176(10): 5725-5729.
PubMed
CD28 and CTLA-4 are the major costimulatory receptors on naive T cells. But it is not clear why CD28 is monovalent whereas CTLA-4 is bivalent for their shared ligands CD80/86. We generated bivalent CD28 constructs by fusing the extracellular domains of CTLA-4 or CD80 with the intracellular domains of CD28. Bivalent or monovalent CD28 constructs were ligated with recombinant ligands with or without TCR coligation. Monovalent CD28 ligation did not induce responses unless the TCR was coligated. By contrast, bivalent CD28 ligation induced responses in the absence of TCR engagement. To extend these findings to primary cells, we used novel superagonistic and conventional CD28 Abs. Superagonistic Ab D665, but not conventional Ab E18, predominantly ligates CD28 bivalently at low CD28/Ab ratios and induces Ag-independent T cell proliferation. Monovalency of CD28 for its natural ligands is thus essential to provide costimulation without inducing responses in the absence of TCR engagement.
Product Citations
-
-
Immunology and Microbiology
-
Endocrinology and Physiology
ZDHHC21-driven S-palmitoylation of Themis regulates the function of T cells and maintains homeostatic balance.
In Cell Commun Signal on 30 September 2025 by Meng, C., Dai, X., et al.
PubMed
-
-
-
Immunology and Microbiology
Naturalized immune responses are stable over years in a colony of laboratory mice with wild-derived microbiota.
In Immunity on 9 September 2025 by Oh, J. H., Hild, B., et al.
PubMed
Free-living mammals carry complex microbiota that co-evolved with their hosts over eons of years. The transfer of such microbiota from wild mice to genetically tractable laboratory mice has been shown to enhance modeling of human immune responses in preclinical studies. Here, we assessed the long-term stability of microbiota and immune phenotype of the first C57BL/6 mouse colony with natural microbiota (wildling mice). The bacterial gut microbiota of wildling mice maintained its increased α-diversity and richness over 5 years, with significantly greater stability than the gut microbiota of laboratory mice. Wildling mice had increased myeloid cell numbers across organs and increased activation and function of natural killer, B, and T cells, which was transferable to laboratory mice via co-housing. Immunological readouts in two preclinical models remained stable throughout the follow-up. These results demonstrate the feasibility of maintaining mouse colonies with natural, wild-derived microbiota as a sharable resource for basic and preclinical research.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
PARP inhibitors enhance antitumor immune responses by triggering pyroptosis via TNF-caspase 8-GSDMD/E axis in ovarian cancer.
In J Immunother Cancer on 4 October 2024 by Xia, Y., Huang, P., et al.
PubMed
In addition to their established action of synthetic lethality in tumor cells, poly(ADP-ribose) polymerase inhibitors (PARPis) also orchestrate tumor immune microenvironment (TIME) that contributes to suppressing tumor growth. However, it remains not fully understood whether and how PARPis trigger tumor-targeting immune responses.
-
-
-
Mus musculus (Mouse)
Contribution of Tregs to the promotion of constructive remodeling after decellularized extracellular matrix material implantation.
In Mater Today Bio on 1 August 2024 by Jiang, H., Sun, X., et al.
PubMed
Host remodeling of decellularized extracellular matrix (dECM) material through the appropriate involvement of immune cells is essential for achieving functional organ/tissue regeneration. As many studies have focused on the role of macrophages, only few have evaluated the role of regulatory T cells (Tregs) in dECM remodeling. In this study, we used a mouse model of traumatic muscle injury to determine the role of Tregs in the constructive remodeling of vascular-derived dECM. According to the results, a certain number of Tregs could be recruited after dECM implantation. Notably, using anti-CD25 to reduce the number of Tregs recruited by the dECM was significantly detrimental to material remodeling based on a significant reduction in the number of M2 macrophages. In addition, collagen and elastic fibers, which maintain the integrity and mechanical properties of the material, rapidly degraded during the early stages of implantation. In contrast, the use of CD28-SA antibodies to increase the number of Tregs recruited by dECM promoted constructive remodeling, resulting in a decreased inflammatory response at the material edge, thinning of the surrounding fibrous connective tissue, uniform infiltration of host cells, and significantly improved tissue remodeling scores. The number of M2 macrophages increased whereas that of M1 macrophages decreased. Moreover, Treg-conditioned medium further enhanced material-induced M2 macrophage polarization in vitro. Overall, Treg is an important cell type that influences constructive remodeling of the dECM. Such findings contribute to the design of next-generation biomaterials to optimize the remodeling and regeneration of dECM materials.
-
-
-
Immunology and Microbiology
Homeostatic PD-1 signaling restrains EOMES-dependent oligoclonal expansion of liver-resident CD8 T cells.
In Cell Rep on 29 August 2023 by Le Moine, M., Azouz, A., et al.
PubMed
The co-inhibitory programmed death (PD)-1 signaling pathway plays a major role in the context of tumor-specific T cell responses. Conversely, it also contributes to the maintenance of peripheral tolerance, as patients receiving anti-PD-1 treatment are prone to developing immune-related adverse events. Yet, the physiological role of the PD-1/PDL-1 axis in T cell homeostasis is still poorly understood. Herein, we show that under steady-state conditions, the absence of PD-1 signaling led to a preferential expansion of CD8+ T cells in the liver. These cells exhibit an oligoclonal T cell receptor (TCR) repertoire and a terminally differentiated exhaustion profile. The transcription factor EOMES is required for the clonal expansion and acquisition of this differentiation program. Finally, single-cell transcriptomics coupled with TCR repertoire analysis support the notion that these cells arise locally from liver-resident memory CD8+ T cells. Overall, we show a role for PD-1 signaling in liver memory T cell homeostasis.
-
-
-
In vitro experiments
-
Mus musculus (Mouse)
-
Stem Cells and Developmental Biology
Promoting Effect of L-Fucose on the Regeneration of Intestinal Stem Cells through AHR/IL-22 Pathway of Intestinal Lamina Propria Monocytes.
In Nutrients on 12 November 2022 by Tan, C., Hong, G., et al.
PubMed
The recovery of the intestinal epithelial barrier is the goal for curing various intestinal injurious diseases, especially IBD. However, there are limited therapeutics for restoring intestinal epithelial barrier function in IBD. The stemness of intestinal stem cells (ISCs) can differentiate into various mature intestinal epithelial cells, thus playing a key role in the rapid regeneration of the intestinal epithelium. IL-22 secreted by CD4+ T cells and ILC3 cells was reported to maintain the stemness of ISCs. Our previous study found that L-fucose significantly ameliorated DSS-induced colonic inflammation and intestinal epithelial injury. In this study, we discovered enhanced ISC regeneration and increased intestinal IL-22 secretion and its related transcription factor AHR in colitis mice after L-fucose treatment. Further studies showed that L-fucose promoted IL-22 release from CD4+ T cells and intestinal lamina propria monocytes (LPMCs) via activation of nuclear AHR. The coculture system of LPMCs and intestinal organoids demonstrated that L-fucose stimulated the proliferation of ISCs through an indirect manner of IL-22 from LPMCs via the IL-22R-p-STAT3 pathway, and restored TNF-α-induced organoid damage via IL-22-IL-22R signaling. These results revealed that L-fucose helped to heal the epithelial barrier by accelerating ISC proliferation, probably through the AHR/IL-22 pathway of LPMCs, which provides a novel therapy for IBD in the clinic.
-
-
-
In vitro experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
SOX-5 activates a novel RORγt enhancer to facilitate experimental autoimmune encephalomyelitis by promoting Th17 cell differentiation.
In Nat Commun on 20 January 2021 by Tian, Y., Han, C., et al.
PubMed
T helper type 17 (Th17) cells have important functions in the pathogenesis of inflammatory and autoimmune diseases. Retinoid-related orphan receptor-γt (RORγt) is necessary for Th17 cell differentiation and functions. However, the transcriptional regulation of RORγt expression, especially at the enhancer level, is still poorly understood. Here we identify a novel enhancer of RORγt gene in Th17 cells, RORCE2. RORCE2 deficiency suppresses RORγt expression and Th17 differentiation, leading to reduced severity of experimental autoimmune encephalomyelitis. Mechanistically, RORCE2 is looped to RORγt promoter through SRY-box transcription factor 5 (SOX-5) in Th17 cells, and the loss of SOX-5 binding site in RORCE abolishes RORCE2 function and affects the binding of signal transducer and activator of transcription 3 (STAT3) to the RORγt locus. Taken together, our data highlight a molecular mechanism for the regulation of Th17 differentiation and functions, which may represent a new intervening clue for Th17-related diseases.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
COVID-19
-
Immunology and Microbiology
Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform.
In Nat Commun on 30 November 2020 by Chiuppesi, F., Salazar, M. d., et al.
PubMed
Modified Vaccinia Ankara (MVA) is a highly attenuated poxvirus vector that is widely used to develop vaccines for infectious diseases and cancer. We demonstrate the construction of a vaccine platform based on a unique three-plasmid system to efficiently generate recombinant MVA vectors from chemically synthesized DNA. In response to the ongoing global pandemic caused by SARS coronavirus-2 (SARS-CoV-2), we use this vaccine platform to rapidly produce fully synthetic MVA (sMVA) vectors co-expressing SARS-CoV-2 spike and nucleocapsid antigens, two immunodominant antigens implicated in protective immunity. We show that mice immunized with these sMVA vectors develop robust SARS-CoV-2 antigen-specific humoral and cellular immune responses, including potent neutralizing antibodies. These results demonstrate the potential of a vaccine platform based on synthetic DNA to efficiently generate recombinant MVA vectors and to rapidly develop a multi-antigenic poxvirus-based SARS-CoV-2 vaccine candidate.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Acylglycerol Kinase Maintains Metabolic State and Immune Responses of CD8+ T Cells.
In Cell Metab on 6 August 2019 by Hu, Z., Qu, G., et al.
PubMed
CD8+ T cell expansions and functions rely on glycolysis, but the mechanisms underlying CD8+ T cell glycolytic metabolism remain elusive. Here, we show that acylglycerol kinase (AGK) is required for the establishment and maintenance of CD8+ T cell metabolic and functional fitness. AGK deficiency dampens CD8+ T cell antitumor functions in vivo and perturbs CD8+ T cell proliferation in vitro. Activation of phosphatidylinositol-3-OH kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling, which mediates elevated CD8+ T cell glycolysis, is tightly dependent on AGK kinase activity. Mechanistically, T cell antigen receptor (TCR)- and CD28-stimulated recruitment of PTEN to the plasma membrane facilitates AGK-PTEN interaction and AGK-triggered PTEN phosphorylation, thereby restricting PTEN phosphatase activity in CD8+ T cells. Collectively, these results demonstrate that AGK maintains CD8+ T cell metabolic and functional state by restraining PTEN activity and highlight a critical role for AGK in CD8+ T cell metabolic programming and effector function.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Laboratory mice born to wild mice have natural microbiota and model human immune responses.
In Science on 2 August 2019 by Rosshart, S. P., Herz, J., et al.
PubMed
Laboratory mouse studies are paramount for understanding basic biological phenomena but also have limitations. These include conflicting results caused by divergent microbiota and limited translational research value. To address both shortcomings, we transferred C57BL/6 embryos into wild mice, creating "wildlings." These mice have a natural microbiota and pathogens at all body sites and the tractable genetics of C57BL/6 mice. The bacterial microbiome, mycobiome, and virome of wildlings affect the immune landscape of multiple organs. Their gut microbiota outcompete laboratory microbiota and demonstrate resilience to environmental challenges. Wildlings, but not conventional laboratory mice, phenocopied human immune responses in two preclinical studies. A combined natural microbiota- and pathogen-based model may enhance the reproducibility of biomedical studies and increase the bench-to-bedside safety and success of immunological studies.
-